GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
Franelyne Pataueg Casuga,Agnes Llamasares Castillo,Mary Jho-Anne Tolentino Corpuz
1The Graduate School,University of Santo Tomas,Espaˊna,Manila,Philippines
2Faculty of Pharmacy,University of Santo Tomas,Espaˊna,Manila,Philippines
3Research Center for Natural and Applied Sciences,University of Santo Tomas,Espaˊna,Manila,Philippines
GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
Franelyne Pataueg Casuga1,2*,Agnes Llamasares Castillo1,2,3,Mary Jho-Anne Tolentino Corpuz1,2,3
1The Graduate School,University of Santo Tomas,Espaˊna,Manila,Philippines
2Faculty of Pharmacy,University of Santo Tomas,Espaˊna,Manila,Philippines
3Research Center for Natural and Applied Sciences,University of Santo Tomas,Espaˊna,Manila,Philippines
ARTICLE INFO
Article history:
Accepted 22 Aug 2016
Available online 20 Sep 2016
Broussonetia luzonica(Blanco)
Selective sequential extraction
GC-MS analysis
Endemic
Percolation
Objective:To investigate and characterize the chemical composition of the different crudeextractsfrom the leavesofBroussonetia luzonica(Blanco)(Moraceae)(B.luzonica),an endemic plant in the Philippines.
Methods:The air dried leaves were powdered and subjected to selective sequential extraction using solvents of increasing polarity through percolation,namely,n-hexane,ethyl acetate and methanol to obtain three different extracts.Then,each of the extracts was further subjected to gas chromatography-mass spectrometry.
Results:Qualitative determination of the different biologically active compounds from crude extracts of B.luzonica using gas chromatography-mass spectrometry revealed different types of high and low molecular weight chemical entities with varying quantities present in each of the extracts.These chemical compounds are considered biologically and pharmacologically important.Furthermore,the three different extracts possess unique physicochemical characteristics which may be attributed to the compounds naturally present in significant quantities in the leaves of B.luzonica.
Conclusions:The three extracts possess major bioactive compounds that were identified and characterized spectroscopically.Thus,identification of different biologically active compounds in the extracts of B.luzonica leaves warrants further biological and pharmacological studies.
1.Introduction
Plants play a significant role in the prevention and treatment of diseases and can even prevent and reduce the adverse effects of conventional treatments[1].They can be a source of chemical compounds of biological and pharmacological importance. History reveals that plants are sources of successful drugs,and will continuously be important for screening of new lead compounds[2].An essential part in the investigation of plant is the identification of the biologically active compounds present in plant leading to further biological and pharmacological studies[3-5].Broussonetia luzonica(Blanco)(Moraceae)(B.luzonica),a tree endemic in the Philippines,is commonly found in thicketsand forests at low and medium altitudes.For Filipinos especially those from the northern part of Luzon,the leaves and flowers are edible and used in vegetable recipes such as pinakbet and bulanglang[6,7].Compoundspresentin otherspeciesof Broussonetia exhibited remarkable biological activities. Broussonetia papyrifera exhibited potent antiproliferative effects on estrogen receptor-positive MCF-7 breast cancer cells in vitro[5];furthermore,its methanolic extract exhibited cytotoxicity against HeLa cell lines and HepG2 cell lines[8].Plants under the same genus might also exhibit the same biological activities because of similar active principles present in them[9]. Identification of bioactive compounds present in the leaves of B.luzonica is conducted for further reference of future studies on one of the edible and endemic plants of the Philippines.There are no published literaturesthatdetermine the bioactive compounds present in the different extracts of B.luzonica leaves by gas chromatography-mass spectrometry(GC-MS)analysis.
Tel:+63 27112419,+63 9399042476
E-mail:fran_casuga@yahoo.com
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
This study aimed to investigate and characterize the bioactive compounds in the different crude extracts of B.luzonica leaves.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
2.Materials and methods
2.1.Plant sample
Fresh leaves of the endemic plant B.luzonica were collected from Solano,Nueva Vizcaya,Philippines in June 9-10,2015. Sample of plant was identified by a botanist and herbarium curator of Research Center of Natural and Applied Sciences,University of Santo Tomas,Manila and assigned with herbarium accession number of 012978.
2.2.Extraction of crude extracts
The leaves of the plant were air dried at room temperature for about 3 days.The dried leaves were ground using Wiley Mill Model No.2(Arthur H.Thomas Co.,Philadelphia,USA).Dried and powdered samples were subjected to selective sequential extraction using solvents of increasing polarity,namely,n-hexane,ethyl acetate and methanol[10].Then the extract was evaporated to dryness using Heidolph Rotavapor(Germany).
2.3.The GC-MS analysis
The GC-MS analysis of bioactive compounds from the different extracts of the leaves of B.luzonica was done using Agilent Technologies GC systems with GC-7890A/MS-5975C model(Agilent Technologies,Santa Clara,CA,USA)equipped withHP-5MScolumn(30m inlength×250μm in diameter×0.25μm in thickness of film).Spectroscopic detection by GC-MS involved an electron ionization system which utilized high energy electrons(70 eV).Pure helium gas(99.995%)was used as the carrier gas with flow rate of 1 mL/min.The initial temperaturewassetat50-150°Cwithincreasingrateof3°C/min and holding time of about 10 min.Finally,the temperature was increased to 300°C at 10°C/min.One microliter of the prepared 1%of the extracts diluted with respective solvents was injected in an splitless mode.Relative quantity of the chemical compounds present in each of the extracts of B.luzonica was expressed as percentage based on peak area produced in the chromatogram.
2.4.Identification of chemical constituents
Bioactive compounds extracted from different extracts of B.luzonica were identified based on GC retention time on HP-5MS column and matching of the spectra with computer software data of standards(Replib and Mainlab data of GC-MS systems).
3.Results
3.1.Percentage yield
From three batches of approximately 1 kg of air dried powdered leaves,mean percentage yields of 3.64%(SD=0.33)of n-hexane extract,1.62%(SD=0.05)of ethyl acetate extract and 6.34% (SD=0.32)of methanol extract were obtained. Methanol extract gave the highest percentage yield.Most of the constituents were polar in nature.
3.2.Physical properties
The different crude extracts from B.luzonica leaves possessed unique physical characteristics.The n-hexane extract was yellow to yellow orange in color with agreeable odor;the pH was 5.79 in 10%aqueous solution and specific gravity was 1.02.The ethyl acetate extract was brown to dark brown in color with ammoniacal odor;the pH was 5.22 in 10%aqueous solution and specific gravity was1.03.Themethanol extract was green to dark greenin color with sweetened agreeable odor;the pH was 4.07 in 10% aqueous solution having specific gravity of 2.4.
3.3.Bioactive compounds present in the extracts
The bioactive compounds present in methanol,n-hexane and ethyl acetate extracts obtained from B.luzonica leaves are shown in Tables 1-3.Their identification and characterization were based on their elution order in a HP-5MS column.Theelution time,molecular formula and the amount of these bioactive compounds were also presented.Based on abundance,the top three major compounds present in the methanolic extract were lupeol(21.973%),tritriacontane(16.670%)andγ-sitosterol(14.754%).The n-hexane crude extract contained squalene(29.028%)followed by tetracosane(8.626%)and triacontane(7.341%).The ethyl acetate crude extract had phytol(20.288%),1,2,3-propanetriol,monoacetate(21.211%)and squalene(6.8%)as the top three major compounds.The GC chromatograms of the three extracts presented in Figures 1-3 show the retentiontime in the column and the detected peaks which correspond to the bioactive compounds present in the extract.
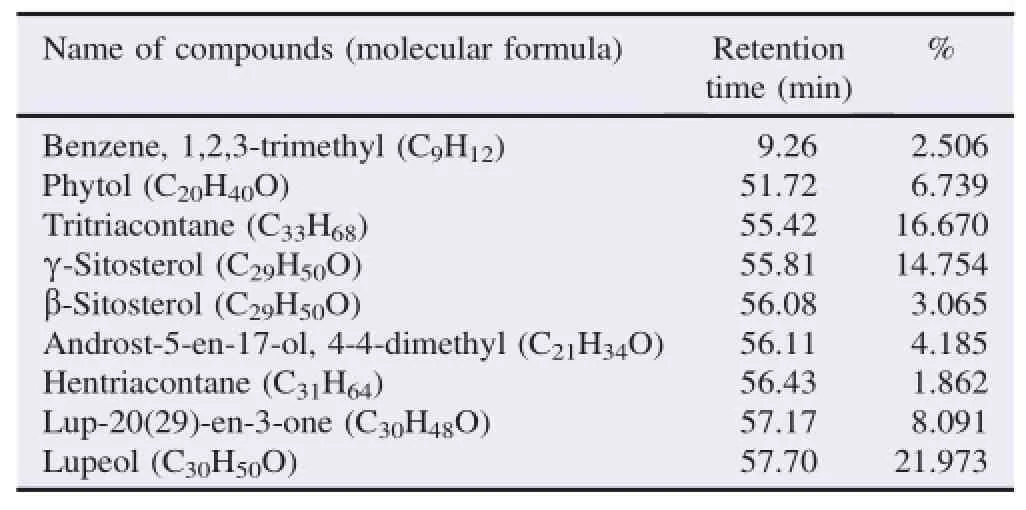
Table 1Biologically active chemical compounds of methanol extract from B.luzonica leaves.
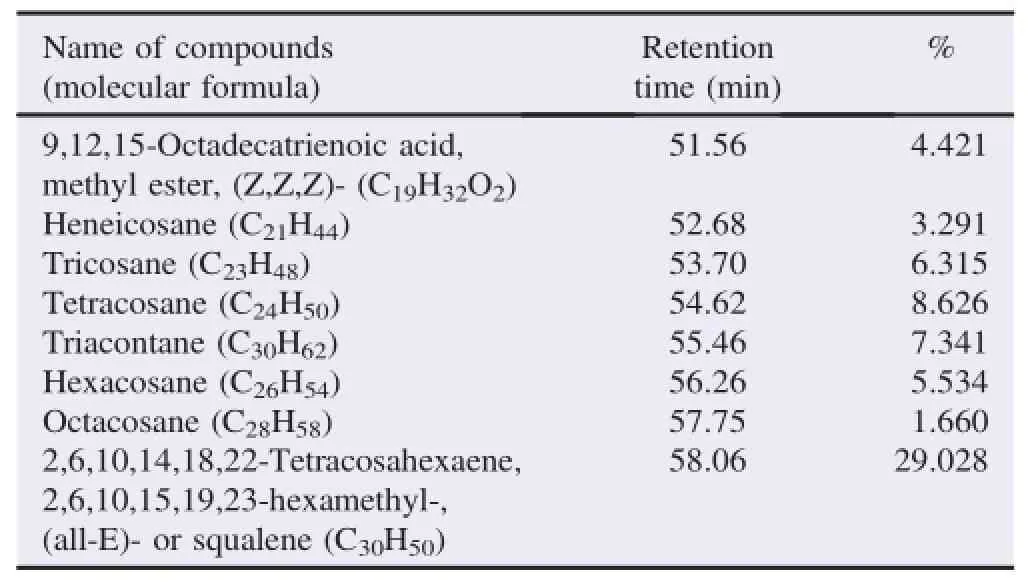
Table 2Biologically active chemical compounds of n-hexane extract from B.luzonica leaves.
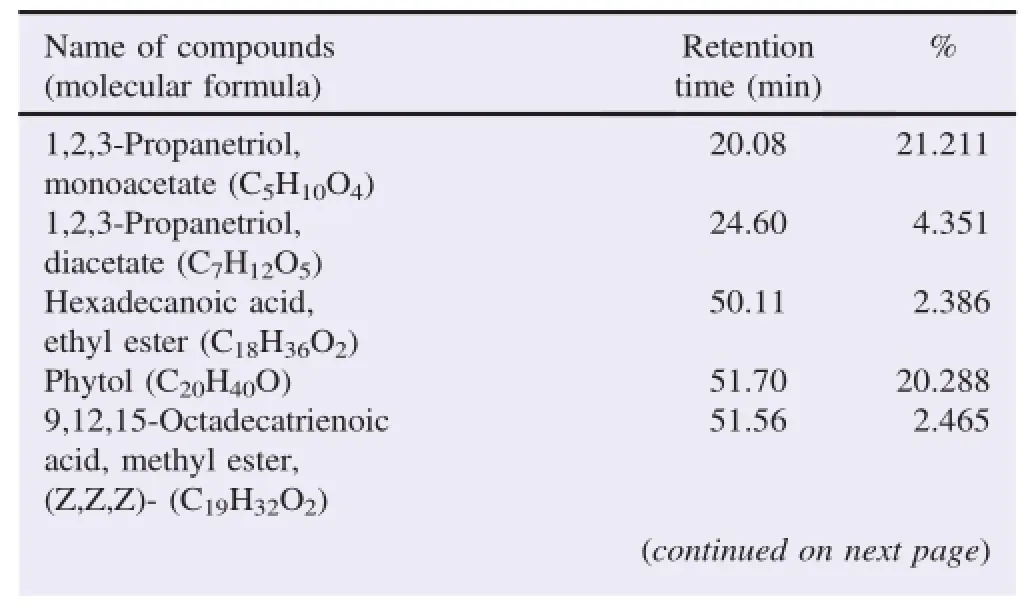
Table 3Biologically active chemical compounds of ethyl acetate extract from B.luzonica leaves.
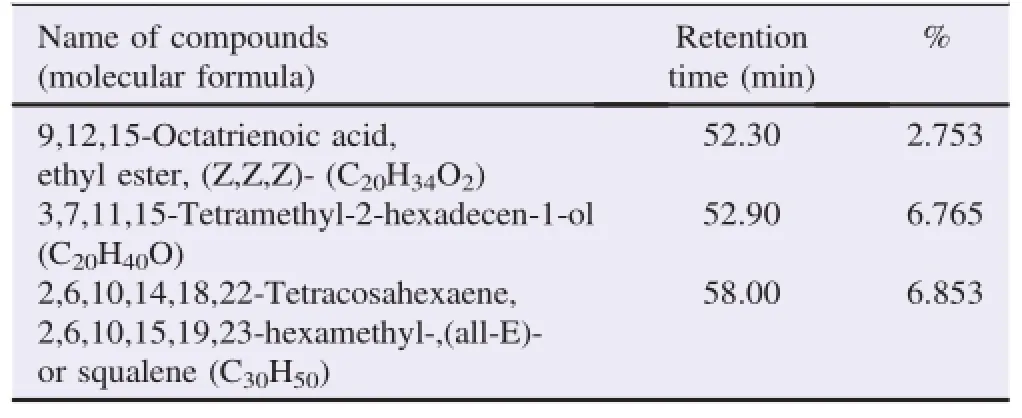
Table 3(continued)
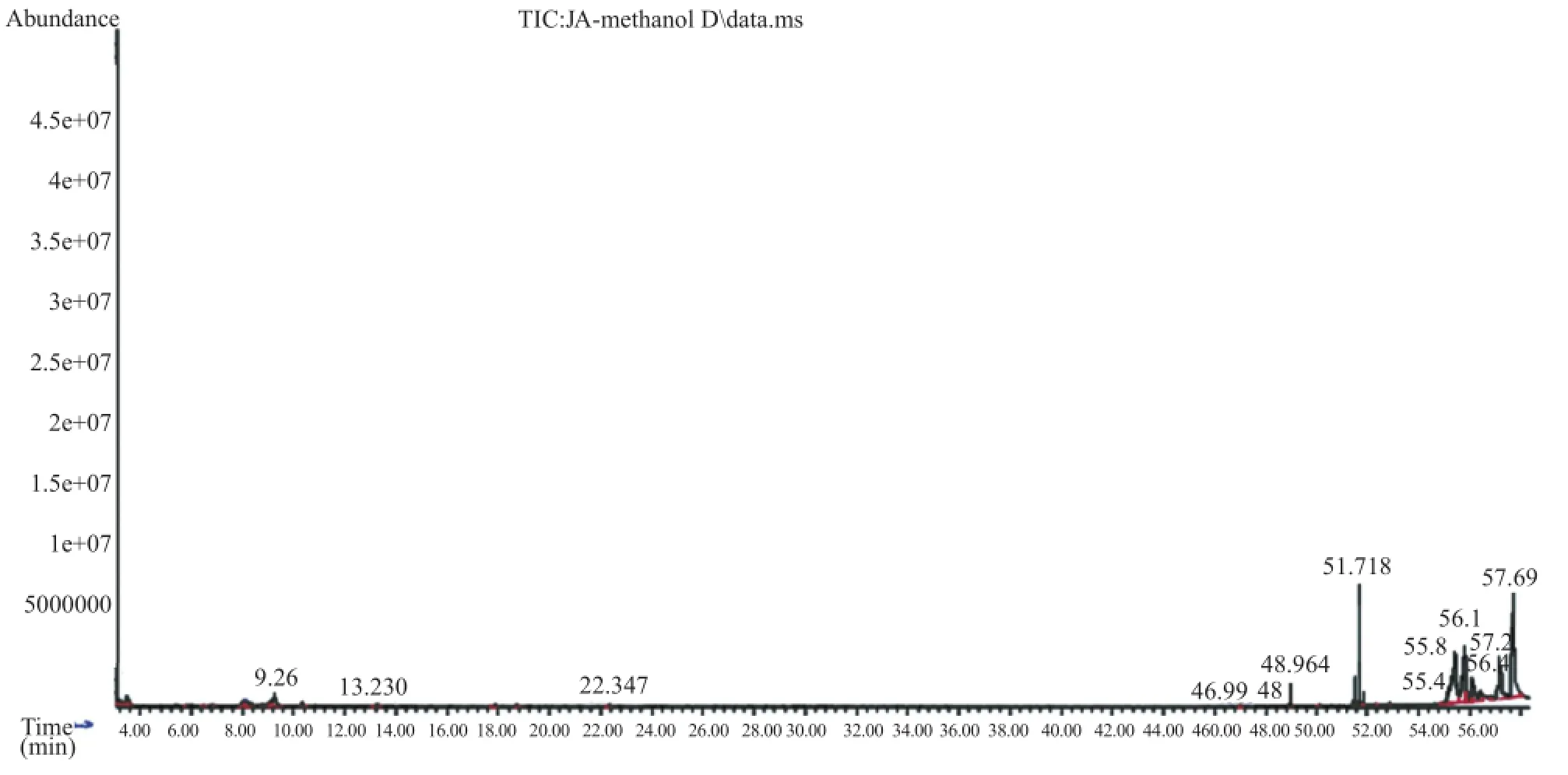
Figure 1.A typical chromatogram of the bioactive compounds present in methanol crude extract.
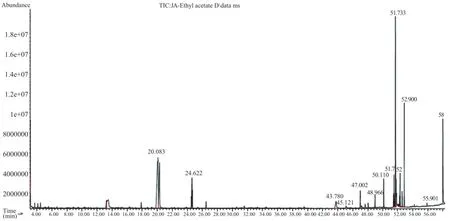
Figure 2.A typical chromatogram of the bioactive compounds present in ethyl acetate crude extract.
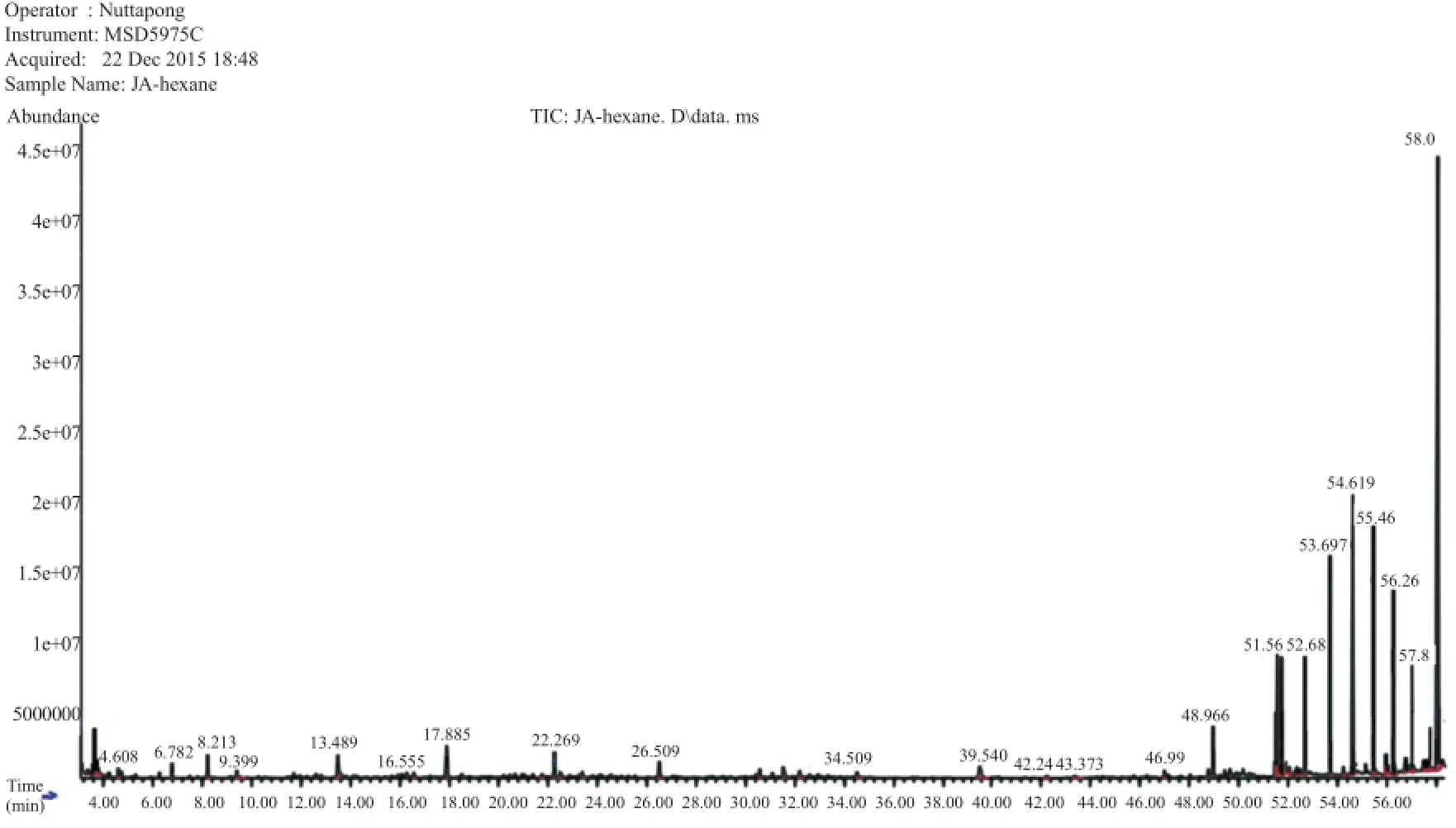
Figure 3.A typical chromatogram of the bioactive compounds present in n-hexane crude extract.
4.Discussion
Different crude extracts were obtained from the leaves of B.luzonica through selective sequential extraction with solvents of increasing polarity,namely,n-hexane,ethyl acetate and methanol.GC-MS analysis of the n-hexane,ethyl acetate and methanol extracts revealed the presence of various bioactive compounds.Phytol is present both in methanol(6.739%)and ethyl acetate extracts(20.288%)but in different quantities.In addition,squalene and 9,12,15-octadecatrienoic acid,methyl ester,(Z,Z,Z)-are both present in n-hexane and ethyl acetate extracts.The three crude extracts did not reveal a common major compound in them.Based on studies,some of the constituents revealed by GC-MS are biologically active compounds.They were proven to possess pharmacologic activities which may contribute to the healing potential of the plant.Phytol was proven to exhibit antioxidant and antinociceptive effects[11,12]. Phytol,precursor of synthetic vitamin E and vitamin K,was found to be cytotoxic against breast cancer cell lines(MCF7)[13,14].Propanetriol monoacetate was confirmed to be present in other plants exhibiting antimicrobial,anti-inflammatory,diuretic and anticancer effects[15].Propanetriol monoacetate was proven to be a precursor of tricetin(antifungal),but may also serve as a prodrug and vehicle for anticancer agents[16].
In addition,phytosterols such asα-sitosterol andβ-sitosterol were proven to prevent cancer through targeting different mechanisms leading to cancer.They were able to prevent angiogenesis by increasing antioxidant enzymes and inhibiting reactive oxygen species production and oxidative stress.These sterols even blocked inflammatory cytokines and induced apoptosis[17-19].
Other studies revealed that lupeol,squalene,tetracosane and triacontane possess various pharmacological properties.Lupeol exhibited marked anti-inflammatory and anticancer properties[20]. Several investigations revealed that lupeol blocks tumorigenesis by affecting molecular growth pathways which are involved in cell proliferation and cell death[21,22].In vivo and in vitro tests revealed potent anti-mutagenic property of lupeol[23-25].Squalene has antioxidant,chemopreventive,antitumor and hypocholesterolemic activities[26,27].Tetracosane showed cytotoxicity against AGS,MDA-MB-231,HT29 and NIH 3T3 cells[28].Triacontane possesses antibacterial,antidiabetic and antitumor activities[29,30].
These biologicalactivities ofcompounds presentin B.luzonica leaf extract support the medicinal application of the plant.The study revealed major bioactive compounds present in all of the extracts.Identification of these compounds in the plant serves as the basis in determining the possible health benefits of the plant leading to further biologic and pharmacologic studies.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was supported by Faculty of Pharmacy and Office of Grants,Endowments and Partnerships in Higher Education;University of Santo Tomas,Manila,Philippines;Nanocast Lab,DepartmentofChemistry,MahidolUniversity,Bangkok,Thailand;Department of Science and Technology,Philippines;and Commission on Higher Education,Philippines.
References
[1]Bachrach ZY.Contribution of selected medicinal plants for cancer prevention and therapy.Acta Fac Medicae Naissensis 2012;29(3): 117-23.
[2]Atanasov AG,Waltenberger B,Pferschy-Wenzig EM,Linder T,Wawrosch C,Uhrin P,et al.Discovery and resupply of pharmacologically active plant-derived natural products:a review.Biotechnol Adv 2015;3(8):1582-614.
[3]Momin MA,Bellah SF,Rahman SM,Rahman AA,Murshid GM,Emran TB.Phytopharmacological evaluation ofethanol extract of Sida cordifolia L.roots.Asian Pac J Trop Biomed 2014;4(1):18-24.
[4]Farid MM,Hussein SR,Ibrahim LF,Desouky MA,Elsayed AM,Oqlah AA,et al.Cytotoxic activity and phytochemical analysis of Arum palaestinum Boiss.Asian Pac J Trop Biomed 2015;5(11): 944-7.
[5]Guo F,Feng L,Huang C,Ding H,Zhang X,Wang Z,et al. Phenylflavone derivatives from Broussonetia papyrifera inhibit the growth of breast cancer cells in vitro and in vivo.Phytochem Lett 2013;6(3):331-6.
[6]Paz-Alberto AM,Tamayo-Galvez C.Handbook on trees.Quezon City:Rex Book Store Inc.;2004,p.106.
[7]Snelder DJ,Lasco RD,editors.Smallholder tree growing for rural development and environmental services.Heidelberg:Springer Science and Business Media B.V.;2008,p.48-52.
[8]Kumar NN,Ramakrishnaiah H,Krishna V,Radhika M.Cytotoxic activity or Broussonetia papyrifera(L.)Vent.on MCF-7,Hela and HepG-2 cell lines.Int J Pharm Pharm Sci 2014;6(5):339-42.
[9]Sousa EO,Costa JG.Genus Lantana:chemical aspects and biological activities.Rev Bras Farmacogn 2012;22(5):1115-80.
[10]Buss AD,Butler MS,editors.Natural product chemistry for drug discovery.Cambridge:The Royal Society of Chemistry;2010,p.153.
[11]Santos CC,Salvadori MS,Mota VG,Costa LM,de Almeida AA,de Oliveira GA,et al.Antinociceptive and antioxidant activities of phytol in vivo and in vitro models.Neurosci J 2013;2013: 949452.
[12]Pejin B,Savic A,Sokovic M,Glamoclija J,Ciric A,Nikolic M,et al.Further in vitro evaluation of antiradical and antimicrobial activities of phytol.Nat Prod Res 2014;28(6):372-6.
[13]Ogunlesi M,Okiei W,Ofor E,Osibete AE.Analysis of the essential oil from the dried leaves of Euphorbia hirta Linn(Euphorbiaceae),a potential medication for asthma.Afr J Biotechnol 2009;8(24):7042-50.
[14]Satyal P,Dosoky NS,Poudol A,Setzer WN.Essential oil constituents and their biologic activities from the leaves of Cassia fistula growing in Nepal.Open Access J Med Aromat Plants 2012;3(2):1-4.
[15]Foo LW,Salleh E,Mamat SNH.Extraction and qualitative analysis of Piper betle leaves for antimicrobial activities.Int J Eng Technol Sci Res 2:1-8.
[16]Juneious CE.Molecular biological determination of PKC inhibitory effects of 1,2,3-propanetriol monoacetate produced form marine sponge associated bacteria.In:3rd International Conference on Clinical Microbioloby&Microbial Genomics;2014 Sep 24-26;Valencia,Spain.
[17]Woyengo TA,Ramprasath VR,Jones PJ.Anticancer effects of phytosterols.Eur J Clin Nutr 2009;63(7):813-20.
[18]Imanaka H,Koide H,Shimizu K,Asai T,Kinouchi Shimizu N,Ishikado A,et al.Chemoprevention of tumor metastasis by liposomal beta-sitosterol intake.Biol Pharm Bull 2008;31(3):400-4.
[19]Rathee P,Rathee D,Rathee D,Rathee S.In vitro cytotoxic activity ofβ-sitosterol triacontenate isolated from Capparis decidua(Forsk.)Edgew.Asian Pac J Trop Med 2012;5(3):225-30.
[20]Saleem M.Lupeol,a novel anti-inflammatory and anti-cancer dietary triterpene.Cancer Lett 2009;285(2):109-15.
[21]Murtaza I,Saleem M,Adhami VM,Hafeez BB,Mukhtar H. Suppression of cFLIP by lupeol,a dietary triterpene,is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells.Cancer Res 2009;69(3): 1156-65.
[22]Saleem M,Maddodi N,Abu Zaid M,Khan N,bin Hafeez B,Asim M,et al.Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis.Clin Cancer Res 2008;14(7):2119-27.
[23]Lee TK,Poon RT,Wo JY,Ma S,Guan XY,Myers JN,et al. Lupeol suppresses cisplatin-induced nuclear factor-kappaB activation in head and neck squamous cell carcinoma and inhibits local invasion and nodal metastasis in an orthotopic nude mouse model. Cancer Res 2007;67(18):8800-9.
[24]Lira Wde M,dos Santos FV,Sannomiya M,Rodrigues CM,Vilegas W,Varanda EA.Modulatory effect of Byrsonima basiloba extracts on the mutagenicity of certain direct and indirect-acting mutagens in Salmonella typhimurium assays.J Med Food 2008;11(1):111-9.
[25]You YJ,Nam NH,Kim Y,Bae KH,Ahn BZ.Antiangiogenic activity of lupeol from Bombax ceiba.Phytother Res 2003;17(4): 341-4.
[26]Spanova M,Drum G.Squalene-biochemistry,molecular biology,press biotechnology and applications.Eur J Lipid Sci Technol 2011;113(11):1299-320.
[27]Das B,Antoon R,Tsuchida P,Lofti S,Mirozova O,Farhat W,et al.Squalene selectively protects mouse bone marrow progenitors against cisplatin and carboplatin-induced cytotoxicity in vivo without protecting tumor growth.Neoplasia 2008;10(10):1105-19.
[28]Uddin SJ,Grice D,Tiralongo E.Evaluation of cytotoxic activity of patriscabratine,tetracosane and various flavonoids isolated from the Bangladeshi medicinal plant Acrostichum aureum.Pharm Biol 2012;50(10):1276-80.
[29]Mallick SS,Dighe VV.Detection and estimation of alpha-amyrin,beta-sitosterol,lupeol and n-tricontane in two medicinal plants by high performance thin layer chromatography.Adv Chem 2014;2014:143948.
[30]Mammen D,Daniel M,Sane RT.Seasonal and geographical variations in chemical constituents of Leptadenia reticulata.Int J Pharm Sci Rev Res 2010;4(2):111-6.
18 Jan 2016
in revised form 13 Jul 2016
Original article http://dx.doi.org/10.1016/j.apjtb.2016.08.015
Franelyne Pataueg Casuga,Graduate School,University of Santo Tomas,Espaˊna,Manila,Philippines.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
- Antiacanthamoebic properties of natural and marketed honey in Pakistan
- Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
- Anthelmintic activity of Saba senegalensis(A.DC.)Pichon(Apocynaceae)extract against adult worms and eggs of Haemonchus contortus
