Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract
Idir Moualek,Ghenima Iratni Aiche,Nadjet Mestar Guechaoui,Souad Lahcene,Karim Houali
Laboratory Analytical Biochemistry&Biotechnology Research(LABAB),Faculty of Biological Sciences and Agricultural Sciences,Mouloud Mammeri University of Tizi-Ouzou,Tizi Ouzou,Algeria
Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract
Idir Moualek*,Ghenima Iratni Aiche,Nadjet Mestar Guechaoui,Souad Lahcene,Karim Houali
Laboratory Analytical Biochemistry&Biotechnology Research(LABAB),Faculty of Biological Sciences and Agricultural Sciences,Mouloud Mammeri University of Tizi-Ouzou,Tizi Ouzou,Algeria
ARTICLE INFO
Article history:
revised form 9 Apr 2016
Accepted 10 Jul 2016
Available online 20 Sep 2016
Arbutus unedo
Antioxidant
Anti-inflammatory
Anti-hemolytic activity
Objective:To evaluate the antioxidant and anti-inflammatory activities of aqueous extract of Arbutus unedo(A.unedo)leaves.
Methods:In this context,the in vitro antioxidant activity was demonstrated by 2,2-diphenyl-1-picrylhydrazyl,hydroxyl radical and H2O2radical scavenging,ferrous ion chelating,ferric reducing power,total antioxidant capacity and by the protection against peroxidation ofβ-carotene-linoleic acid in emulsion.The anti-inflammatory activity was evaluated first by studying the membrane of human red blood cells against different hypotonic concentrations of NaCl and against heat,inhibiting the denaturation of albumin.
Results:Total phenolic and flavonoid content were found respectively[(207.84±15.03)mg gallic acid equivalent/g,and(13.070±0.096)mg quercetin equivalent/g].The extract displayed significant scavenging activity of some radicals such as 2,2-diphenyl-1-picrylhydrazyl[IC50at(7.956±0.278)μg/mL],·OH[IC50=(1015.74±46.35μg/ mL)],H2O2[IC50=(114.77±16.86)μg/mL]and showed a good antioxidant activity through ferrous ion chelating activity[IC50=(1014.30±36.21)μg/mL],ferric reducing power[IC50=(156.55±17.40)μg/mL],total antioxidant capacity[IC50=(461.67 ±4.16)μg/mL]andβ-carotene-linoleic acid protection against peroxidation[I %=(87.04±1.21)%at 1 000μg/mL].
Conclusions:A.unedo showed in vitro anti-inflammatory activity by inhibiting the heat induced albumin denaturation and red blood cells membrane stabilization.Our results show that aqueous leaf extract of A.unedo has good antioxidant activity and interesting anti-inflammatory properties.A.unedo aqueous extract can be used to prevent oxidative and inflammatory processes.
1.Introduction
Medicinal plants are considered as an important source of new molecules with high antioxidant potential.Polyphenols,commonly referred to as antioxidant compounds,play a major role in safeguarding health,and a protection against diseases like cancer,has recently been shown[1].
Tel:+213 775595914
E-mail:moualek_idir@yahoo.fr
The study protocol was performed according to the Helsinki declaration and approved by Scientific Committee of the Faculty of Biology(CSFB).Informed written consent was obtained from Hospital Department of Hematology(University Hospital Nedir Mohamed of Tizi-Ouzou).
Foundation Project:Supported by University Hospital Nedir Mohamed of Tizi-Ouzou(Grant No.cnp-um/8819-136/2014)and Laboratory Analytical Biochemistry &Biotechnology Research(LABAB),Faculty of Biological Sciences and Agricultural Sciences,Mouloud Mammeri University of Tizi-Ouzou,Algeria(Grant No.bn-001375-14-993).
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
Freeradicalsaregeneratedbyvariousexogenouschemicalsand several endogenous metabolic processes oxidize the biomolecules leading to cell death and tissue damage.The organism must keep freeradicals atrelatively low concentrations usingvarious defense systems and antioxidant molecules such as glutathione[2].
Poorly absorbed and extensively metabolized polyphenols cannot act a direct antioxidant activity,but they have the ability to interact with target proteins to regulate various cellular and molecular processes,giving them biological activity[3].
Drug that plays a role in the stabilization of erythrocyte membrane against osmotic lysis and heat indicates their potential to maintain the integrity of biological membrane.Knowing that erythrocyte membrane resembles to lysosomal membrane,the stabilization of erythrocyte can be extrapolated to the stabilization of lysosomal membrane.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Arbutus unedo L.(Ericaceae family)(A.unedo),commonly called strawberry tree,is an evergreen shrub endemic to Mediterranean region[4].It is widely used in traditional medicine,and nowadays many of its virtues have been scientifically proven as antioxidant,antihypertensive,anti-hyperglycemic and antiinflammatory[5-7].
The objective of this work is to deepen the knowledge of antioxidant and anti-inflammatory capacities of A.unedo leaves.
2.Materials and methods
2.1.Plant collection
A.unedo leaves were collected in mid-December 2014 from Tizi-Ouzou,Algeria.The plant was identified by Doctor Mahmoud Laribi,botanist at Mouloud Mammeri University of Tizi-Ouzou department of vegetal biology,where a voucher specimen was deposited(FSBSA/MK/2105).
The sample was dried and then ground to obtain a powder that was stored at room temperature and in the dark until extraction.
2.2.Extract preparation
Twenty grams of powder were dissolved in 200 mL of distilled water.After 24 h of maceration at room temperature,the filtrate was lyophilized.
2.3.Determination of total phenolic content
The concentration of phenolic compounds in plant extract was determined using the Folin-Ciocalteu spectrophotometric method[8].
The reaction mixture was prepared by mixing 200μL of extract(40μg/mL)with 1 mL of Folin-Ciocalteu reagent(diluted ten times)and 800μL of sodium carbonate(75 mg/mL). The mixture was incubated for 45 min at room temperature and the absorbance was measured against a blank at 760 nm.
The same procedure was repeated for the standard solution of gallic acid and the calibration curve was constructed.The results are expressed in mg of gallic acid equivalent per gram of extract.
2.4.Determination of total flavonoid content
The total content of flavonoids in leaves extract was determined using the aluminum chloride spectrophotometric assay[9,10].The plant extract(1 mL)was mixed with the same volume of a methanolic solution containing 2%of aluminum trichloride. After incubation for 10 min,the absorbance of the reaction mixture was measured at 430 nm against a methanol blank.
A standard curve of quercetin was drawn and the results were expressed as mg of quercetin equivalent per gram of extract.
2.5.Determination of 2,2-diphenyl-1-picrylhydrazyl(DPPH)radicals scavenging activity
The free radical scavenging activity of the extract was measured using the stable free radical DPPH test according to the method described before[11,12].A total of 250μL of 0.8 mmol/L DPPH in ethanol was mixed with 3.75 mL of the extract.The reaction was carried out in triplicate and the absorbance was measured at 517 nm after 30 min in dark.LAscorbic acid was used as reference standard.
The percent of scavenging activity was calculated using the following equation.
Scavenging activity(%)=[(Ac-As)/Ac]×100
where,Ac stands for the absorbance of the control and As stands for absorbance of the sample.
2.6.β-Carotene bleaching assay
Antioxidant activity of the extract of leaves of the strawberry treeand levelofbutylated hydroxytoluene(BHT)were measured according to the published method[13,14].The emulsion mixture was prepared in 50 mL round-bottom flask containing 1 mL of chloroform(high performance liquid chromatography grade),0.5 mgβ-carotene,25μL linoleic acid and 200 mg of Tween 40.Chloroform was completely evaporated using a vacuum evaporator at 40°C for 10 min.
After evaporation,the mixture was immediately diluted in 100 mL of distilled water saturated with oxygen.The ethanolic stock solution of the extract(350μL,concentrations were 0.2,0.4,0.6,0.8,and 1.0 mg/mL)was mixed with 2.5 mL of emulsion.The same procedure was repeated with positive control BHT.
Absorbance of the mixtures was measured at 470 nm immediately after their preparation(t=0 min)and at incubation time t=120 min against the blank.
The percentage of inhibition was calculated with the following equation:
Inhibition(%)=[(Aa120-Ac120)/(Ac0-Ac120)]×100
where,Aa120is the absorbance of the antioxidant at t=120 min,Ac120is the absorbance of the control at t=120 min and Ac0is the absorbance of the control at t=0 min.
2.7.Hydroxyl radical scavenging assay
Scavenging activity of hydroxyl radical of the extract was measured according to the method of Rajamanikandan et al.[15]. Threemilliliters ofthefinalreactionsolutionconsistedofaliquots(500μL)of various concentrations of the extract,1 mL FeSO4(1.5 mmol/L),0.7 mL hydrogen peroxide(6 mmol/L)and 0.3 mL sodium salicylate(20 mmol/L).The reaction mixture was incubated for 1 h at 37°C.L-Ascorbic acid was used as the standard.
The color development was measured at 560 nm against a blank.
2.8.Hydrogen peroxide radical scavenging activity
The scavenging ability of the water extract of A.unedo on hydrogen peroxide was determined according to the method of Serteser et al.[16].A solution of hydrogen peroxide(40 mmol/L)was prepared in phosphate buffer(pH 7.4).The extract in distilled water(3.4 mL)was added to a hydrogen peroxide solution(0.6 mL,40 mmol/L).Absorbance of hydrogen peroxide at 230 nm was measured 10 min later against a blank solution containing the phosphate buffer without hydrogen peroxide.
The percentage of hydrogen peroxide scavenging by the extract and standard(L-ascorbic acid)was calculated using the following equation:
Scavenged H2O2(%)=(1-As/Ac)×100
where,Ac is the absorbance of the control(without the extract)and As is absorbance in the presence of the extract.The experiment was repeated in triplicate.
2.9.Ferrous ion chelating activity
Ferrous ion chelating activity was determined by inhibition of the formation of iron(II)-ferrozine complex,following the previous published method[17,18].Briefly,100μL of 0.6 mmol/ L FeCl2was added to 500μL of different concentrations of the extract or ethylenediamine tetraacetic acid(EDTA)(positive control).The reaction mixture was adjusted to a final volume of 1.5 mL with methanol,and then 100μL of 5 mmol/L ferrozine solution were added.
The mixture was shaken vigorously and left to stand at room temperature for 5 min.Absorbance was determined at 562 nm and percent of chelation was calculated using the following equation:
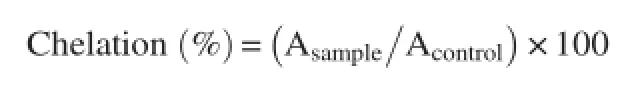
where,Asampleis the absorbance of the sample and Acontrolis the absorbance of the control.
2.10.Ferric reducing power assay
Reducing power was determined by the method described by Oyaizu and Hazra et al.[19,20].Different concentrations of the extract were mixed with 1.25 mL of 0.2 mol/L,pH 6.6 sodium phosphate buffer and 1.25 mL of potassium ferricyanide(1%). The mixture was incubated at 50°C for 20 min.
After incubation,the reaction mixture was acidified with 1.25 mL of trichloroacetic acid(10%)and centrifuged at 3 000 r/ min for 10 min.Finally,0.5 mL of freshly prepared FeCl3(0.1%)was added to this solution,and the absorbance was measured at 700 nm.Ascorbic acid at various concentrations was used as standard.
2.11.Total antioxidant capacity
Total antioxidant capacity was estimated by phosphomolybdenum assay[21,22].The tubes containing the extract and reagent solution(0.6 mol/L sulfuric acid,28 mmol/L sodium phosphate and 4 mmol/L ammonium molybdate)were incubated at 95°C for 90 min.Then the solution was cooled to room temperature and the absorbance was read at 695 nm.Ascorbic acid was used as standard.
2.12.Antihemolytic activity
2.12.1.Red blood cell suspension
Blood was obtained by venipuncture from healthy volunteers collected in heparinized tubes and centrifuged at 2 000 r/min for 10 min at 4°C.After removing the plasma,red blood cells(RBCs)were washed for three successive times using phosphate buffer saline(PBS)(0.9%NaCl).The study protocol was performed according to the Helsinki declaration and approved by Scientific Committee of the Faculty of Biology(CSFB). Informed written consent was obtained from Hospital Department of Hematology(University Hospital Nedir Mohamed of Tizi-Ouzou).
2.12.2.Hypotonic solution induced hemolysis
Membrane stabilizing activity of the extract was assessed using hypotonic solution induced hemolysis,and the method was described by de Freitas et al.[23].In hypotonic solution,the test sample consisted of washed stock erythrocyte(RBC)suspension(40μL)with 1 mL of hypotonic solution(0.1%,0.3%,0.5%,0.7%,0.9%NaCl)in sodium PBS(pH 7.4)containing either of the different concentrations of aqueous extract.
The mixture was incubated for 30 min at 37°C under gentle stirring,centrifuged for 10 min at 2 000 r/min and the absorbance of the supernate was measured at 540 nm.
Inhibition of hemolysis(%)=[(OD1-OD2)/OD1]×100
where,OD1 is the optical density of hypotonic-buffered saline solution alone(control)and OD2 is the optical density of test sample in hypotonic solution.
2.12.3.Heat induced hemolysis
Different concentrations of the extract(μg/mL)or aspirin dissolved in isotonic PBS(pH 7.4)was mixed with 1 mL of 2% RBCs suspension.The reaction mixture was incubated in a water bath at 56°C for 30 min.After incubation,the tubes were cooled under running tap water,then centrifuged at 2 000 r/min for 10 min and the absorbance of the supernatants was estimated at 560 nm[24].
The percentage of protection against heat induced hemolysis was calculated by using the following equation:
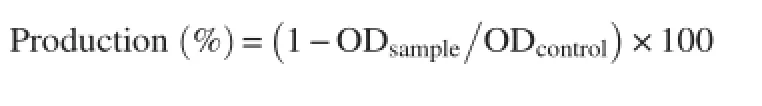
2.12.4.Oxidant induced hemolysis
One milliliter of RBC suspension(5%)in PBS(pH 7.4)was incubated for 15 nm at 37°C with 1 mL of the extract at different concentrations.After pre-incubation,the mixture was centrifuged at 2 000 r/min for 10 min at 4°C,the supernatant was removed and packed RBCs were resuspended with 0.5 mmol/L HOCl in PBS.After this,the incubation was performed as previously described.The absorbance was determined at 540 nm[25,26].
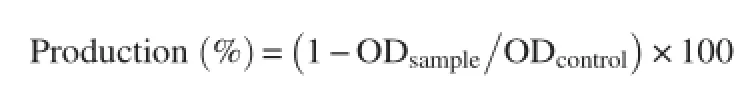
2.12.5.Inhibition of albumin denaturation
A solution of 0.2%(w/v)of egg albumin was prepared in a PBS(pH 6.4).A volume of 50μL of the extract or standard at different concentrations was added to 5 mL of this stock solution.
The test tubes were heated at 72°C for 5 min and then cooled.The absorbance of these solutions was determined at 660 nm[27].
3.Results
3.1.Phenolic content
Many studies report that phenolic compounds play an important role in human health due to their antioxidant activity.
The total phenols of A.unedo aqueous extract[(207.84 ±15.03)mg gallic acid equivalent/g of extract]was calculated according to the equation y=0.006x+0.027(R2=0.990).
3.2.Flavonoid content
After construction of the calibration curve[y=0.022x+0.182(R2=0.994)],collected data clearly showed a good amount of flavonoid content in the aqueous extract[(13.070±0.096)mg quercetin equivalent/g of extract].
3.3.DPPH scavenging activity
The DPPH radical scavenging activity was recorded in terms of inhibition percent as shown in Figure 1.
The parameterused to comparethe radical scavenging activity of the extract and ascorbic acid is IC50value,defined as the concentration of antioxidant required for 50%scavenging of DPPH radicals.The IC50value for ascorbic acid was(2.359±0.091)μg/ mL,which was comparatively lower than the IC50[(7.956± 0.278)μg/mL]of aqueous extract.
3.4.β-Carotene bleaching
In the evaluation of the antioxidant activity,β-carotene bleaching method was used to measure the ability of the extract to inhibit lipid peroxidation.The antioxidant activity was expressed as percent inhibition.Figure 2 shows that the antioxidant activity increases with the increasing concentrations of the extract used.
Almost similar results were obtained for BHT[%Inhibition=96.88±0.34(positive control)]and A.unedo(%Inhibition=87.04±1.21)at 1 000μg/mL,which indicates a high potential antioxidant activity of the extract.
3.5.Hydroxyl radical scavenging
The hydroxyl scavenging activity of A.unedo aqueous extract was evaluated by its ability to compete with salicylic acid for hydroxyl radicals.

Figure 1.DPPH radical scavenging activity of ascorbic acid and aqueous leaf extract of A.unedo.
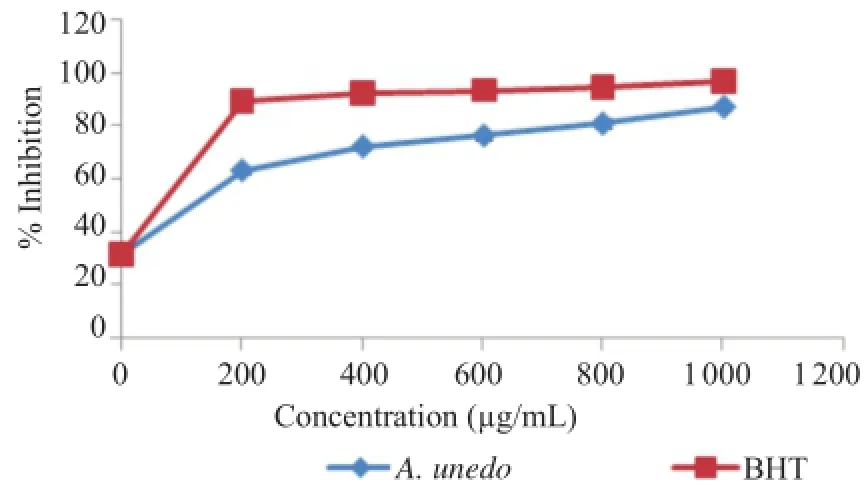
Figure 2.β-Carotene bleaching inhibition by BHT and aqueous extract of A.unedo.
As shown in Figure 3,hydroxyl radical scavenging increased with increase in concentrations.The ascorbic acid[IC50=(758.83 ±7.40)μg/mL]showed moreeffective scavenging ability when compared to that of aqueous extract[IC50=(1 015.74±46.35)μg/mL].
Themaximum scavenging activity wasfound to be(97.80±0.18)%for ascorbic acid and(79.23±1.14)%for aqueous extract at 2 mg/mL.
3.6.Hydrogen peroxide radical scavenging activity
Scavenging activity of the extract and ascorbic acid as reference compound against hydrogen peroxide in terms of effective concentration was remarkably different and they were shown to be 83.14%(600μg)and 96.3%(90μg),respectively(Figure 4).

Figure 3.Hydroxyl radical scavenging activity of ascorbic acid and aqueous extract of A.unedo.

Figure 4.Hydrogen peroxide radical scavenging activity of ascorbic acid and aqueous extract of A.unedo.
According to the results,A.unedo showed an activity dependent on the concentration and the H2O2scavenging IC50was(114.77±16.86)μg/mL,which indicates a less effective scavenging potentialreferring to ascorbic acid[IC50=(49.19±2.70)μg/mL].
3.7.Ferrous ion chelating activity
Antioxidant action may be of secondary type.One of the most important mechanisms is the chelating of pro-oxidant metals such as iron.Ferrozine forms a complex with Fe2+with a characteristic red color,but in the presence of chelating agent,the complex formation is disrupted and the red color is decreased.Measurement of color reduction,therefore,allows the estimation of the chelating activity of the plant extract.
The metal chelating effect of investigated extract and EDTA were dependent on concentrations(Figure 5).
EDTA[IC50=(57.21±0.44)μg/mL]in this assay demonstrated relatively high activity in comparison to the extract[IC50=(1 014.30±36.21)μg/mL].
3.8.Ferric reducing power
The extract showed concentration-dependent reducing power.However,its reducing power[IC50=(156.55±17.40)μg/ mL]was lower than that of ascorbic acid[IC50=(56.72± 2.79)μg/mL](Figure 6).
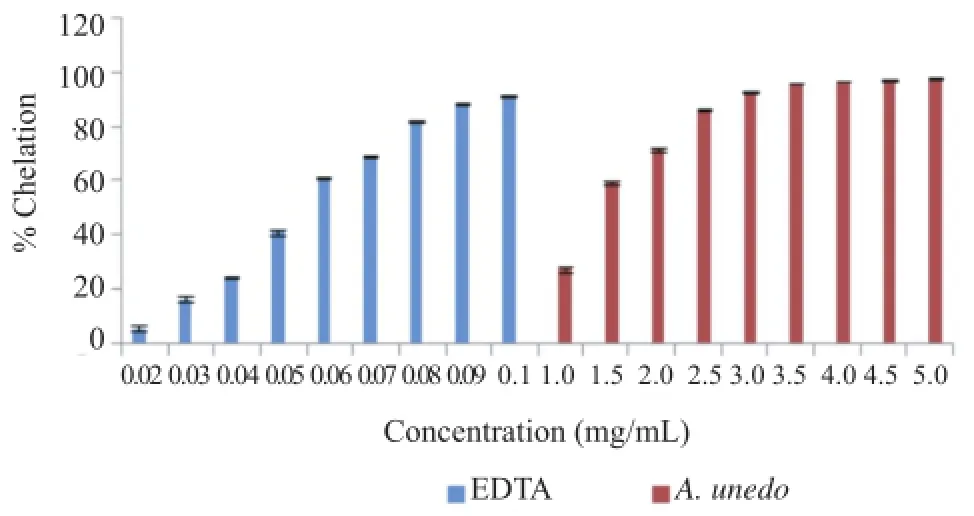
Figure 5.Ferrous ion chelating activity of EDTA and aqueous extract of A.unedo.
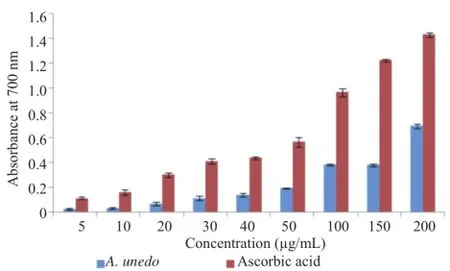
Figure 6.Ferric reducing power of ascorbic acid and aqueous extract of A.unedo.
3.9.Total antioxidant capacity
This test was based on the reduction of Mo(VI)to Mo(V)by the extract and formation at acid pH of green phosphate/Mo(V)complex.
Results showed the antioxidant activity of the extract and ascorbic acid in a dose dependent manner at concentrations 100-500μg/mL.The IC50value of antioxidant capacity for the ascorbic acid[(292±7.54)μg/mL]was greater than the extract IC50[(461.67±4.16)μg/mL)(Figure 7).
3.10.Membrane stabilizing activity
As shown in Figures 8 and 9,the extract prevented the erythrocyte membrane against lysis induced either by hypotonic solution and heat.
For hypotonic solution induced hemolysis,at concentration range of 0.250-1.500 mg/mL,the extract showed significant inhibitory effect against RBCs hemolysis[(46.15±0.70)%,(79.53±0.43)%,(70.78±1.38)%,(71.95±2.26)%and(76.46±1.80)%respectively for 0.1%,0.3%,0.5%,0.7%and 0.9%of NaCl when the concentration of the extract was 1.500 mg/mL].
In heat induced hemolysis,the extract inhibited lysis of erythrocyte membrane in the range of(45.70±1.30)%-(79.66 ±1.92)%at concentration range of 0.025-0.500 mg/mL.Aspirin demonstrated protection in the range of(25.65±1.57)%-(58.49 ±1.23)%.
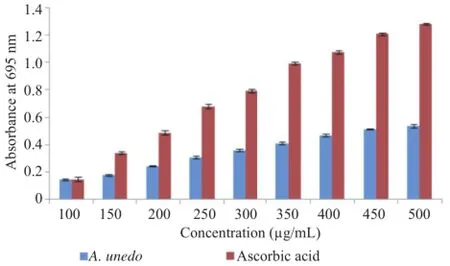
Figure 7.Total antioxidant capacity of ascorbic acid and aqueous extract of A.unedo.
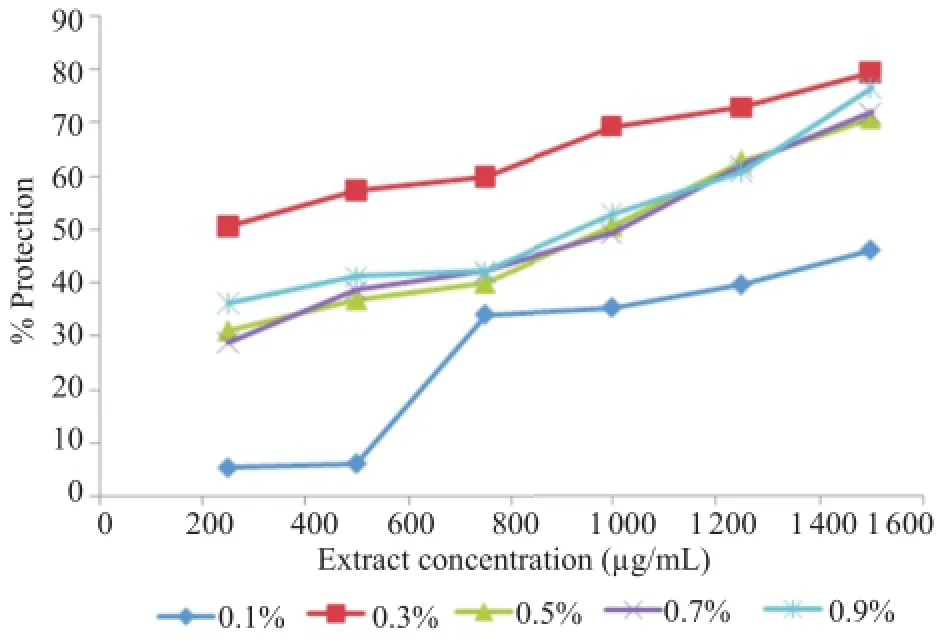
Figure 8.Effect of A.unedo aqueous extract on hypotonicity-induced hemolysis.
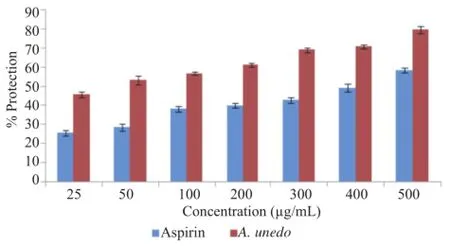
Figure 9.Effect of A.unedo aqueous extract on heat-induced hemolysis.
3.11.Oxidant induced hemolysis
As shown in Figure 10,protective effect of the extract against HOCl induced hemolysis was dose dependent.In fact,the hemolysis ratio gradually decreased with the increasing dose of the extract.Protection was already evident at 1 mg/mL of the extract with(73.90±2.08)%protection.
3.12.Inhibition of albumin denaturation
Protein denaturation is involved in inflammation and plant extracts showing inhibition of denaturation are often tested for anti-inflammatory activity.
For inhibiting thermally induced denaturation of albumin,the extract showed an astonishingly effect at different concentrations as shown in Figure 11.
A maximum inhibition of(74.28±0.86)%was observed at 500μg/mL for the extract,and(92.23±0.32)%at 500μg/mL for aspirin,the anti-inflammatory standard.
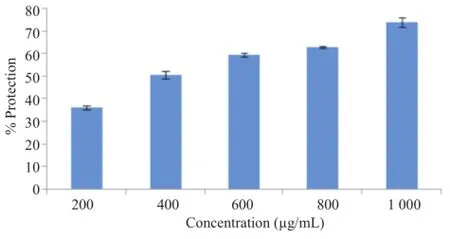
Figure 10.Effect of A.unedo aqueous extract on HOCl induced hemolysis.
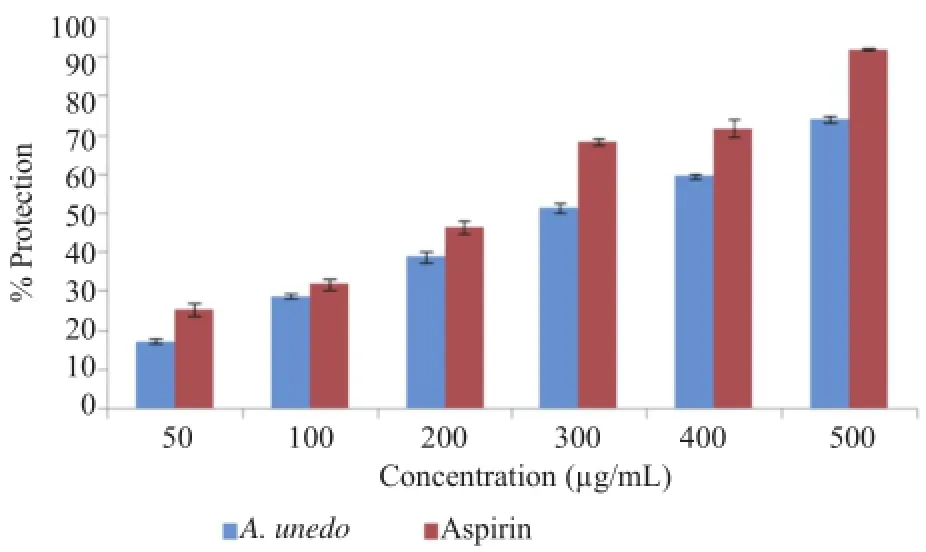
Figure 11.Effect of A.unedo aqueous extract on albumin denaturation.
4.Discussion
Antioxidants are substances that prevent various pathologic changes in living cell by protecting oxidation of its major constituents(proteins,lipids,carbohydrates and DNA)[28].In plant extracts,the antioxidant activity is performed by polyphenols and is correlated positively with their concentration.From the obtained results,we can see a great antioxidant potential of the studied extract when compared to other species described in the literature.
So,the phenolic content of the studied extract exceed that reported by Mendes et al.[29]with(170.3±1.4)mg GAE/g and Oliveira et al.[30]with(172.21±6.29)mg GAE/g,that of Malva parviflora L.with(0.83±0.063)mg/g[31],Anacardium excelsum with(1.49±0.03)mg/g and Piper putumayoense with(10.20±0.03)mg/g[32].
In the first part of our study,we focused on the antioxidant activity of the aqueous extract of A.unedo.
The large amount of polyphenols in this plant can explain the scavenging and antioxidant activity,due to their loss of proton properties,chelate formation,dismutation of radicals and giving up hydrogen atoms from their hydroxyl groups with radicals to form stable phenoxyl radicals[33].
Monitored in our study by several tests,this potential was confirmed in comparison to other works.
So,DPPHscavengingactivity,incomparisontootherworkson theaqueous extract ofA.unedo[487.2μg/mL[34],(73.7±6.3)μg/ mL[30]and(87±7)μg/mL[29]],oursamplepresentedalowerIC50which revealed a higher scavenging activity.
The hydroxyl radical,the representative reactive oxygen species generated in biological systems,can be formed from superoxide anion and hydrogen peroxide in the presence of metal ions.
Considered as the most reactive free radical,hydroxyl radical is most often implicated in the pathology of free radical because of its ability to interact with intracellular targets such as DNA,thus causing significant damage.
The extract was found to be a less effective scavenger of hydroxyl radical compared to reported results[(80.160± 0.015)%at 1 mg/mL]for Quisqualis indica[35].
Hydrogen peroxide itself is not very reactive,but it can sometimes be toxic to cell because it can give rise to hydroxyl radical in the cells[36,37].Thus,the removal of H2O2is very important for antioxidant defense in cell systems or food[38].
Compared to the scavenging effect reported by Kumar and Pandey[39]with(26.02±1.91)%at 250μg/mL,our extract showed a better scavenging activity with(60.53±1.18)%at the same concentration.
The ability of the extract to slow down theβ-carotene bleaching is used in the evaluation of its antioxidant activity and its ability to inhibit lipid peroxidation.
The inhibition percentage for A.unedo extract at 1 mg/mL,was significantly important compared to those reported by Norhaiza et al.[40]for Labisia pumila var.alata with(89.72 ±0.95)%andLabisiapumila var.pumila with(59.09±2.24)%at 40 mg/mL.
These data lead us to believe the great biological activity of the extract such as anti-cancer activity,for which one of the causes of its occurrence is lipid peroxidation.
A.unedo showed a stronger chelating activity ferrous ion chelating activity(IC50)compared to reported data for aqueous extract of Smilax excelsa with(1.55±0.06)mg/mL][41].
This chelating potential indicated a significant protective activity of the extract against oxidative damage by sequestering iron(II)ions that may turn into catalyst for Fenton-type reactions or participate in metal-catalyzed hydroperoxide decomposition reactions[42].
Ferric reducing power is a simple test of antioxidant capacity,and often used as indicator of antioxidant potential for a plant extract.In this test,antioxidant electron donation leads to the neutralization of the free radical[43].
An increase in absorbance corresponds to an increase of the reducing power of the extract tested[44,45].
A.unedo water extract was characterized by higher ferric reducing power than other data reported for the same water extract of the plant[IC50=(287.7±15.6)μg/mL][30].
Electron donating capacity and antioxidant activity are two related concepts and reflecting reducing power of a plant extract. The antioxidant molecules present in the extract play a reductant role and cause the reduction of the Fe3+/ferricyanide complex to the ferrous form.
A.unedo leaves extract showed greater total antioxidant capacity compared to Pistacia lentiscus[IC50=(500.0±22.3)μg/ mL][46].This result suggests an important electron donating ability of the extract and so a great antioxidant capacity.
In the second partof ourstudy,we explored antiinflammatory activity of A.unedo aqueous extract through the study of its ability to stabilize RBCs membrane.
For hypotonic solution induced hemolysis,compared to 24.5%produced by Momordica charantia aqueous extract at 2.0 mg/mL[47],the studied extract presented stronger protection.
In heat induced hemolysis,compared to Murraya paniculata[(33.49±0.51)%at 2 mg/mL][48],the plant extract showed greater protection.
While the protection percentage against HOCl induced hemolysis was lower than that recorded for the aqueous extract of Rhus typhina[(61.06±2.53)%at the concentration of 20μg/ mL][49].
These results provide evidence of anti-inflammatory activity of the extract which showed a good protective effect of RBCs against heat,oxidant and hypotonic solution induced hemolysis.This feature can be explained by the ability of the extract to edit the calcium influx in erythrocytes[50].
Knowing that the erythrocyte membrane resembles to lysosomal membrane and as such,the effect of extracts on the stabilizationoferythrocytecouldbeextrapolated tothestabilizationof lysosomalmembrane[51].Theanti-inflammatoryactivitycanalso be explained by the inhibition of release of lysosomal content at the site of inflammation[52].
During the investigation of the activity of the plant extract on albumin denaturation we observed that A.unedo extract showed a greater protection comparatively to data observed for Erythrina indica[(65.21±1.77)%at 800μg/mL][53].
According to the fact that proteins denaturation is the cause of inflammation and rheumatoid arthritis,the protection of albumin denaturation confirms and contributes to anti-inflammatory activity of A.unedo extract.
This study demonstrated in vitro antioxidant and antiinflammatory activities of A.unedo.Aqueous leaves extract,through scavenging,chelating and reducing activities indicated in the performed tests,showed a good antioxidant activity. Furthermore,the protection of RBCs indicated a membrane stabilizing effect of the extract.
These results lead to the conclusion that A.unedo aqueous extract has antioxidant and anti-inflammatory potential.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by University Hospital Nedir Mohamed of Tizi-Ouzou(Grant No.cnp-um/8819-136/2014)and Laboratory Analytical Biochemistry&Biotechnology Research,Faculty of Biological Sciences and Agricultural Sciences,Mouloud Mammeri University of Tizi-Ouzou,Algeria(Grant No.bn-001375-14-993).
References
[1]Rodríguez ML,Estrela JM,Ortega´AL.Natural polyphenols and apoptosis induction in cancer therapy.J Carcinog Mutagn 2013;S6:004.
[2]Bhattacharyya A,Chattopadhyay R,Mitra S,Crowe SE.Oxidative stress:an essential factor in the pathogenesis of gastrointestinal mucosal diseases.Physiol Rev 2014;94(2):329-54.
[3]Amiot MJ,Riollet C,Landrier JF.Polyphenols and metabolic syndrome.M´edecine des Maladies M´etaboliques 2009;3(5): 476-82.
[4]Sulusoglu M,Cavusoglu ES.Arbutus unedo L.(Strawberry tree)selection in Turkey Samanli mountain locations.J Med Plants Res 2011;5(15):3545-51.
[5]Afkir S,Nguelefack TB,Aziz M,Zoheir J,Cuisinaud G,Bnouham M,et al.Arbutus unedo prevents cardiovascular and morphological alterations in L-NAME-induced hypertensive rats Part I:cardiovascular and renal hemodynamic effects of Arbutus unedo in L-NAME-induced hypertensive rats.J Ethnopharmacol 2008;116(2):288-95.
[6]Medjdoub H,Selles C,Tabti B.Preliminary phytochemical screening of Arbutus unedo L.and anti-hyperglycemic effect of the root aqueous extract on streptozotocin-induced diabetic Wistar rats. J Chem Pharm Res 2014;6(11):195-9.
[7]Mariotto S,Esposito E,Di Paola R,Ciampa A,Mazzon E,de Prati AC,et al.Protective effect of Arbutus unedo aqueous extract in carrageenan-induced lung inflammation in mice.Pharmacol Res 2008;57:110-24.
[8]Li HB,Wong CC,Cheng KW,Chen F.Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants.LWT-Food Sci Technol 2008;41(3):385-90.
[9]Quettier-Deleu C,Gressier B,Vasseur J,Dine T,Brunet C,Luyckx M,et al.Phenolic compounds and antioxidant activities of buckwheat(Fagopyrum esculentum Moench)hulls and flour. J Ethnopharmacol 2000;72(1):35-42.
[10]Akrout A,Gonzalez LA,El Jani H,Madrid PC.Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia.Food Chem Toxicol 2011;49(2): 342-7.
[11]Santos SA,Pinto PC,Silvestre AJ,Neto CP.Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L.Ind Crops Prod 2010;31(3):521-6.
[12]Sharma OP,Bhat TK.DPPH antioxidant assay revisited.Food Chem 2009;113(4):1202-5.
[13]Aslan A,G¨ull¨uce M,S¨okmen M,Adɩg¨uzel A,Sahin F,¨Ozkan H. Antioxidant and antimicrobial properties of the Lichens Cladonia foliacea.,Dermatocarpon miniatum.,Everinia divaricata.,Evernia prunastri.,and Neofuscella pulla.Pharm Biol 2006;44(4):247-52.
[14]Dawidowicz AL,Olszowy M.Influence of some experimental variables and matrix components in the determination of antioxidant properties byβ-carotene bleaching assay:experiments withBHT used as standard antioxidant.Eur Food Res Technol 2010;231(6):835-40.
[15]RajamanikandanS,SindhuT,DurgapriyaD,SophiaD,RagavendranP,Gopalakrishnan VK.Radical scavenging and antioxidant activity of ethanolic extract of Mollugo nudicaulis by in vitro assays.Indian J Pharm Educ Res 2011;45(4):310-6.
[16]Serteser A,Kargıoˇglu M,G¨ok V,Baˇgci Y,¨Ozcan MM,Arslan D. Antioxidant properties of some plants growing wild in Turkey. Grasas y Aceites 2009;60(2):147-54.
[17]Dinis TC,Maderia VM,Almeida LM.Action of phenolic derivatives(acetaminophen,salicylate,and 5-aminosalicylate)as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers.Arch Biochem Biophys 1994;315(1):161-9.
[18]Nabavi SF,Nabavi SM,Hellio C,Alinezhad H,Zare M,Azimi R,et al.Antioxidant and antihemolytic activities of methanol extract of Hyssopusangustifolius.JApplBotFood Qual2013;85(2):198-201.
[19]Oyaizu M.Studies on product of browning reaction prepared from glucoseamine.Jpn J Nutr 1986;44(6):307-15.
[20]Hazra B,Biswas S,Mandal N.Antioxidant and free radical scavenging activity of Spondias pinnata.BMC Complement Altern Med 2008;8:63.
[21]Prieto P,Pineda M,Aguilar M.Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex:specific application to the determination of vitamin E.Anal Biochem 1999;269(2):337-41.
[22]Rao AS,Reddy SG,Babu PP,Reddy AR.The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran.BMC Complement Altern Med 2010;10:4.
[23]de Freitas MV,Netto Rde C,da Costa Huss JC,de Souza TM,Costa JO,Firmino CB,et al.Influence of aqueous crude extracts of medicinal plants on the osmotic stability of human erythrocytes. Toxicol In Vitro 2008;22(1):219-24.
[24]Sakat S,Juvekar AR,Gambhire MN.In vitro antioxidant and antiinflammatory activity of methanol extract of Oxalis corniculata Linn.Int J Pharm Pharm Sci 2010;2(1):146-55.
[25]Suwalsky M,Orellana P,Avello M,Villena F.Protective effect of Ugni molinae Turcz against oxidative damage of human erythrocytes.Food Chem Toxicol 2007;45(1):130-5.
[26]Chandler JD,Min E,Huang J,Nichols DP,Day BJ.Nebulized thiocyanate improves lung infection outcomes in mice.Br J Pharmacol 2013;169(5):1166-77.
[27]Karthik K,Kumar BR,Priya VR,Kumar SK,Rathore RS.Evaluation of anti-inflammatory activity of Canthium parviflorum by invitro method.Indian J Res Pharm Biotechnol 2013;1(5):729-30.
[28]Halliwell B,Whiteman M.Measuring reactive species and oxidative damage in vivo and in cell culture:how should you do it and what do the results mean?Br J Pharmacol 2004;142(2):231-55.
[29]Mendes L,de Freitas V,Baptista P,Carvalho M.Comparative antihemolytic and radical scavenging activities of strawberry tree(Arbutus unedo L.)leaf and fruit.Food Chem Toxicol 2011;49(9): 2285-91.
[30]Oliveira I,Coelho V,Baltasar R,Pereira JA,Baptista P.Scavenging capacity of strawberry tree(Arbutus unedo L.)leaves on free radicals.Food Chem Toxicol 2009;47(7):1507-11.
[31]Farhan H,Rammal H,Hijazi A,Hamad H,Daher A,Reda M,et al. In vitro antioxidant activity of ethanolic and aqueous extracts from crude Malva parviflora L.grown in Lebanon.Asian J Pharm Clin Res 2012;5(3):234-8.
[32]Lizcano LJ,Bakkali F,Ruiz-Larrea MB,Ruiz-Sanz JI.Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use.Food Chem 2010;119(4):1566-70.
[33]Aksoy L,Kolay E,Aˇgıl¨on¨u Y,Aslan Z,Kargıoˇglu M.Free radical scavenging activity,total phenolic content,total antioxidant status,and total oxidant status of endemic Thermopsis turcica.Saudi J Biol Sci 2013;20(3):235-9.
[34]Orak HH,Yagar H,Isbilir SS,Demirci AS¸,G¨um¨u T,Ekinci N. Evaluation of antioxidant and antimicrobial potential of strawberry tree(Arbutus unedo L.)leaf.Food Sci Biotechnol 2011;20(5): 1249-56.
[35]Bose A,Bose S,Maji S,Chakraborty P.Free radical scavenging property of Quisqualis indica.Int J Biomed Pharm Sci 2009;3(1): 1-4.
[36]Halliwell B.Reactive oxygen species in living systems:source,biochemistry,and role in human disease.Am J Med 1991;91(3C): 14S-22S.
[37]Kumar RS,Rajkapoor B,Perumal P.Antioxidant activities of Indigofera cassioides Rottl.Ex.DC.using various in vitro assay models.Asian Pac J Trop Biomed 2012;2(4):256-61.
[38]Turkoglu S,Turkoglu I,Kahyaoglu M,Celık S.Determination of antimicrobial and antioxidant activities of Turkish endemic Ajuga chamaepitys(L.)Schreber subsp.euphratica P.H.Davis(Lamiaceae).J Med Plants Res 2010;4(13):1260-8.
[39]Kumar S,Pandey AK.Chemistry and biological activities of flavonoids:an overview.ScientificWorldJournal 2013;2013:162750.
[40]Norhaiza M,Maziah M,Hakiman M.Antioxidative properties of leaf extracts of a popular Malaysian herb,Labisia pumila.J Med Plant Res 2009;3:217-23.
[41]Ozsoy N,Can A,Yanardag R,Akev N.Antioxidant activity of Smilax excelsa L.leaf extracts.Food Chem 2008;110(3):571-83.
[42]Adefegha SA,Oboh G.Water extractable phytochemicals from some Nigerian spices inhibit Fe2+-induced lipid peroxidation in rat's brain-in vitro.J Food Process Technol 2011;2(1):104.
[43]Nishaa S,Vishnupriya M,Sasikumar JM,Hephzibah PC,Gopalakrishnan VK.Antioxidant activity of ethanolic extract of Maranta arundinacea L.tuberous rhizomes.Asian J Pharm Clin Res 2012;5(4):85-8.
[44]Singleton VL,Rossi JA.Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents.Am J Enol Vitic 1965;16(3):144-58.
[45]Goudjil MB,Ladjel S,Bencheikh SE,Zighmi S,Hamada D.Study of the chemical composition,antibacterial and antioxidant activities of the essential oil extracted from the leaves of Algerian Laurus nobilis Lauraceae.J Chem Pharm Res 2015;7(1):379-85.
[46]GhenimaAI,IdirM,NadjetMG,SamiaMA,MihoubZM,KarimH. In vitro evaluation of biological activities of Pistacia lentiscus aqueous extract.Int J Pharm Pharm Sci 2015;7(11):133-9.
[47]Umukoro S,Ashorobi RB.Evaluation of anti-inflammatory and membrane stabilizing property ofaqueousleafextractof Momordica charantia in rats.Afr J Biomed Res 2006;9(2):119-24.
[48]Laboni FR,Afsari M,Howlader MS,Labu ZK,Julie AS. Thrombolytic and membrane stabilizing activities of ethanolic extract of local medicinal plant Murraya paniculata.(Family: Rutaceae).J Pharmacogn Phytochem 2015;4(2):17-20.
[49]Olchowik E,Lotkowski K,Mavlyanov S,Abdullajanova N,Ionov M,Bryszewska M,et al.Stabilization of erythrocytes against oxidative and hypotonic stress by tannins isolated from sumac leaves(Rhus typhina L.)and grape seeds(Vitis vinifera L.).Cell Mol Biol Lett 2012;17(3):333-48.
[50]Chopade AR,Somade PM,Sayyad FJ.Membrane stabilizing activity and protein denaturation:a possible mechanism of action for the anti-inflammatory activity of Phyllanthus amarus.JKIMSU 2012;1(1):67-72.
[51]Omale J,Okafor PN.Comparative antioxidant capacity,membrane stabilization,polyphenol composition and cytotoxicity of the leaf and stem of Cissus multistriata.Afr J Biotechnol 2008;7(17): 3129-33.
[52]Govindappa M,Naga Sravya S,Poojashri MN,Sadananda TS,Chandrappa CP,Santoyo G,et al.Antimicrobial,antioxidant and in vitro anti-inflammatory activity and phytochemical screening of water extract of Wedelia trilobata(L.)Hitchc.J Med Plants Res 2011;5(24):5718-29.
[53]Sakat S,Tupe PN,Juvekar AR.In-vitro anti-inflammatory activity of aqueous and methanol extracts of Erythrina indica Lam leaves. Pharmacologyonline 2009;3:221-9.
14 Mar 2016
in revised form 4 Apr,2nd
Original article http://dx.doi.org/10.1016/j.apjtb.2016.09.002
Idir Moualek,Laboratory Analytical Biochemistry& Biotechnology Research(LABAB),Faculty of Biological Sciences and Agricultural Sciences,Mouloud Mammeri University of Tizi-Ouzou,Tizi Ouzou,Algeria.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
- Antiacanthamoebic properties of natural and marketed honey in Pakistan
- GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
- Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
