Larvicidal activities of hydro-ethanolic extracts of three Cameroonian medicinal plants against Aedes albopictus
Tankeu Nzufo Francine,Biapa Nya Prosper Cabral,Pieme Constant Anatole,Moukette Moukette Bruno,Nanfack Pauline,Ngogang Yonkeu Jeanne
1Laboratory of Biochemistry,Department of Biochemistry and Physiological Sciences,Faculty of Medicine and Biomedical Sciences,University of Yaound´e I,PO Box 1364,Yaound´e,Cameroon
2Laboratory of Medicinal Plant Biochemistry,Food Science and Nutrition,Department of Biochemistry,Faculty of Science,University of Dschang,PO Box 67,Dschang,Cameroon
3Laboratory of Sustainable Management of the Agro-ecosystems,ENEA-Italian National Agency for New Technologies,Energy and Sustainable Economic Development,Via Anguillarese,301,00123,Rome,Italy
Larvicidal activities of hydro-ethanolic extracts of three Cameroonian medicinal plants against Aedes albopictus
Tankeu Nzufo Francine1,3,Biapa Nya Prosper Cabral2*,Pieme Constant Anatole1,Moukette Moukette Bruno1,Nanfack Pauline1,Ngogang Yonkeu Jeanne1
1Laboratory of Biochemistry,Department of Biochemistry and Physiological Sciences,Faculty of Medicine and Biomedical Sciences,University of Yaound´e I,PO Box 1364,Yaound´e,Cameroon
2Laboratory of Medicinal Plant Biochemistry,Food Science and Nutrition,Department of Biochemistry,Faculty of Science,University of Dschang,PO Box 67,Dschang,Cameroon
3Laboratory of Sustainable Management of the Agro-ecosystems,ENEA-Italian National Agency for New Technologies,Energy and Sustainable Economic Development,Via Anguillarese,301,00123,Rome,Italy
ARTICLE INFO
Article history:
Accepted 26 Nov 2015
Available online 20 Sep 2016
Aedes albopictus
Larvicides
Plant extracts
Syzygium guineense
Zanthoxylum heitzii
Objective:To investigate the larvicidal activity of Syzygium guineense(Myrtaceae)(S.guineense),Monodora myristica and Zanthoxylum heitzii(Rutaceae)(Z.heitzii)extracts against Aedes albopictus(Ae.albopictus).
Methods:The larvicidal activity of the hydro-ethanolic extracts from these plant species was assessed at three different concentrations(50,100 and 200 mg/L)on first-instar of Ae.albopictus larvae in comparison with untreated controls.Mortality rate was recorded daily for a period of 12 days.The values of LC50and lethal time killing 50%of the tested individuals(LT50)were calculated using the log-probit analysis.
Results:The root extract of S.guineense exhibited the best activity with 100%mortality after 8 days of treatment at 200 mg/L,followed by the fruit extract of Z.heitzii with 83.33%mortality at the same concentration.Nonetheless,larvae were most susceptible to the fruit extract of Z.heitzii both in terms of LC50(39.89 mg/L)and LT50(145.68 h).A statistically significant difference between the control and the group treated at 200 mg/L was noticed in all the extracts.
Conclusions:The present study shows that the hydro-ethanolic extracts of S.guineense,Monodora myristica and Z.heitzii tested have significant larvicidal activity.These preliminary results are of great interest and some of these plant species can be proposed for the formulation of new bioinsecticides to control Ae.albopictus populations.
1.Introduction
Mosquitoes are known vectors of several disease-causing pathogens.Aedes albopictus(Ae.albopictus)(Diptera:Culicidae)is known as a mosquito species with an invasive behavior and a competent vector of various viruses highly dangerous to human health[1].It is just second to Aedes aegypti as vector of dengue fever which is endemic in large areas of Africa,America and South East Asia[2,3].Moreover,Ae.albopictus has gained the position of the most important public health vector species in Southern Europe.In fact,it was responsible for the recent occurrence of autochthonous epidemics of chikungunya and dengue viruses in this region[4].Today,about two fifths of the world's population are at risk of dengue with cases reported in more than 100 countries[4].Increased urbanization,migration,poor environmental sanitation,low investment in prevention and community participation and persistent use of control methods having limited efficacy are some of the major causes of emergence and re-emergence of vector-borne diseases in developing countries[5].At present,since there are no effective vaccines against dengue and chikungunya[6],the most recommended strategy to interrupt pathogen transmission cyclesrelieson controlstrategies focusing on the mosquito vectors.
Tel:+237 75965283
E-mail:prbiapa@yahoo.fr
Peer review under responsibility of Hainan Medical University.The journal implements double-blind peer review practiced by specially invited international editorial board members.
2221-1691/Copyright©2016 Hainan Medical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
The current mosquito control methods are mainly based on synthetic insecticides usually considered as the first line of action owing to their quick action.However,repeated application of chemical control often results in an unintended artificialselection of resistantmutants within the vector population[5,7].In addition,many cases of lethal and sublethal pesticide poisoning of humans have occurred[8].Besides leading to a constant increase in the cost of application due to development of genetic resistance,the continuous use of synthetic insecticides also causes ecological imbalance manifested by pollution of the environment and destruction of non-target organisms[9].
In addition to the different approaches proposed for adult mosquito control[10],developing an efficient strategy targeting larval stage is considered a key objective as part of the integrated mosquito management[11].
Plants may be an alternative source of agents for the ecosustainable control of mosquitoes at the larval stages.The use of plant extracts has several appealing features since they are less hazardous,safer for non-target organisms,rich in bioactive chemicals,and biodegradable[12-15].In the last two decades,a great attention has been given by scientists on the larvicidal properties of plant extracts and essential oils[16],but very few studies have been carried out on Ae.albopictus over a long period of treatment[17-20].
Syzygium guineense(Wild.)(S.guineense)is widely distributed in tropical regions and is used in African traditional medicine.This plant is used to treat epilepsy,stomachache,diarrhea,malaria,coughs,broken bones,wounds,asthma,sore throat,intercostal pain and as a tonic.The powdered bark is used as an antispasmodic,purgative[21].The antibacterial properties of the aqueous extract of S.guineense have been demonstrated against different strains of bacteria responsible for diarrhea[22]. Ethanol extracts of the stem barks of S.guineense showed molluscicidal activities and cardiovascular properties,mainly due to the reduction of blood pressure[23].Antibacterial activity of triterpenes isolated from S.guineense has been demonstrated[24].Other biological properties such as antiinflammatory,analgesic and immunologicalactivities of different parts of S.guineense have been reported[25].The chemical composition of essential oil from S.guineense was also investigated[26].A recent study demonstrated that leaves,stem barks and roots of S.guineense had antioxidant properties and were rich in polyphenols[27].
Zanthoxylum heitzii(Aubr´ev.&Pellegr.)(Z.heitzii)is one of the cultivar of Fagara zanthoxyloides found in the West region of Cameroon.Its fruit is used as a spice for the preparation of“nkui”and“Nah poh”,two traditional dishes of Cameroon[28].It is also used as medicinal plant in Central Africa for the treatment of many diseases such as cancer,syphilis,malaria and other urogenital infections[29].The bark of Z.heitzii is used as insecticides and revealed some activities againstcardiac infections[30,31].Fagaricine,an aqueous extract formulation from root of Z.heitzii,was demonstrated as an immune-restorative phytomedicine to treat immunodeficiency as well as an effective antioxidant and in vitro antisickling agent[32,33].A recent study demonstrated that fruit and bark extracts of Zanthoxylum induced mitochondrial apoptosis and G0/G1 phase arrest in human leukemia HL-60 cells[34].
Several molecules were isolated from the stem bark of Z.heitzii.Among them,two amides(heitziamide A and heitziamide B)and two phenylethanoids(heitziethanoid A and heitziethanoid B)and trans-fagaramide,arnottianamide,iso-γ fagarine,iso-skimmianine,arctigenin methyl ether,savinin,(+)-eudesmin,(+)-sesamin,lupeol,lupeone,β-sitosterol,stigmasterol and stigmasterol-3-O-β-D-glucopyranoside[35].
Monodora myristica(Dunal)(M.myristica)is widely used especially to relieve toothache as well as in the treatment of dysentery.When roasted and ground,the seeds are rubbed on the skin for skin diseases[36].
Despite the important biological properties described,these plants have never been investigated as potential source of larvicidal molecules effective against mosquitoes.The objective of this study was to evaluate the insecticide effectiveness of the Cameroonian species S.guineense,M.myristica and Z.heitzii against Ae.albopictus larvae.
2.Materials and methods
2.1.Plant material and extract preparation
S.guineense,Z.hertzii and M.myristica were collected respectively in Bafia(centre region),Bachingou(west region)and Mount Kalla(centre region),identified at the National Herbarium of Cameroon by Mr.NANA and referenced under the following numbers:S.guineense(roots and leaves)(20899/ SRF/Cam),Z.heitzii(fruits,leaves and barks)(1441/SRF)and M.myristica(fruit)(27690/SRF/Cam).The collected plant materials were air-dried and ground into fine powder.One hundred grams of each powder was macerated in 1 L of aqueous-ethanolic mixture 70:30(v/v)for 72 h at room temperature and the obtained solution was filtered with Whatman No.1 filter paper. The residue was further macerated twice under the same conditions.The filtrates obtained from each extraction were mixed and concentrated under vacuum.The obtained extracts were kept at 4°C until use.
2.2.Ae.albopictus rearing
Ae.albopictus colony were derived from individuals collected in Central Italy and reared at the Laboratory of Sustainable Management of the Agro-ecosystems of Italian National Agency for New Technologies,Energy and Sustainable Economic Development(ENEA;Rome,Italy).Colonies were maintained in the laboratory under standard conditions at(27±1)°C;(75±10)%relative humidity,and with a 14:10 h photoperiod.Larvae were reared at a density of one larva per 5 mL water,in plastic trays containing dechlorinated water,provided with an aeration tube and supplemented with a powder obtained by crushing dry cat food(Friskies®adults).Adults were maintained in rearing cages(40 cm×40 cm×40 cm)and offered 10%sucrose.Females were provided with fresh defibrinated bovine blood using a thermostatic apparatus[37].Eggs were oviposited on paper towels in cups partially filled with water.Strips of towel paper were submerged in a hatching broth for 5 h to obtain the first-instar larvae.
2.3.Larvicidal bioassay
Each crude extract was tested at 200,100 and 50 mg/L by mixing 20 first instar Ae.albopictus in 25 mL of each mixture ofextract solution.As control,20 larvae were exposed to a 1% acetone solution because this solution had been used to dissolve the extract.Larvae were fed at regular intervals of 24 h.Each container was daily monitored for larval mortality over a period of 12 days.The experiment was replicated three times and the mean of one triplicate was recorded in this study.
2.4.Statistical analysis
Kruskal-Wallis non parametric test(P<0.05)followed by a post hoc Dunnett C was used for testing the larvicidal activity of each extract at various concentrations and times of exposure. Log-probit regression analysis was used to determine LC50and lethal time(LT50)killing 50%of the tested individuals.The interaction between concentration and time was studied using the Univariate General Linear Model test and Fisher test.Analyses were performed using the Statistical Software SPSS version 16 for Windows.
3.Results
Larval mortality varied with the extract concentration,the plant part and the time of exposure.In comparison with the control,all of the tested extracts were active against Ae. albopictus larvae but showed different levels of toxicity. Figure 1 present the percentages of mortality induced by each tested extract depending on the exposure time and concentration.Among the extracts(Figure 1A,E and F),significant differences(P<0.05)were observed from 192 h(8th day)when using the following extracts:roots and leaves of S.guineense,barks of Z.heitzii and leaves of M.myristica(Kruskal-Wallis analysis with Dunnett C as post hoc).The percentages of mortality were significantly different(P<0.05)when the time of exposure of the larvae to the extracts reached 192 h,240 h and 288 h.A statistically significant difference was always found between control and the extracts at 200 mg/L concentration.Moreover,only the root extract of S.guineense showed a statistically significant difference of induced mortality when comparing 50 and 200 mg/L concentrations(Figure 1A). Overall,the root extract of S.guineense was the most potent as it induced 100%mortality at 200 mg/L within the 8th day of exposure(Figure 1A),followed by the bark extract of Z.heitzii(Figure 1E)inducing 83.33%mortality at the same concentration.In general,all of the tested extracts exhibited a strong significant correlation(P<0.001)with a coefficient higher than 0.90.The plots resulting from the Univariate General Linear Model test checking the effect of the interaction of time and concentration on the percentage of induced mortality are also shown in Figure 1.Since this kind of diagrams generally inform about the progression due to the interaction,they may show two forms of lines,parallel or non parallel,which indicate a significant or not significant interaction respectively. Then,we can assume that between 144 h and 192 h,the interaction could be significant with all the tested extracts.This was confirmed by Fisher test that showed a significant difference between 144 h and 192 h with high coefficients with respect to root and leaf extracts of S.guineense(Fisher:9.54,0.01;10.80,0.01),fruit,leaf and bark extract of Z.heitzii(Fisher:257.39,0.01;5.66,0.01;7.96,0.01),leaf extract of M.myristica(Fisher:11.34,0.01).
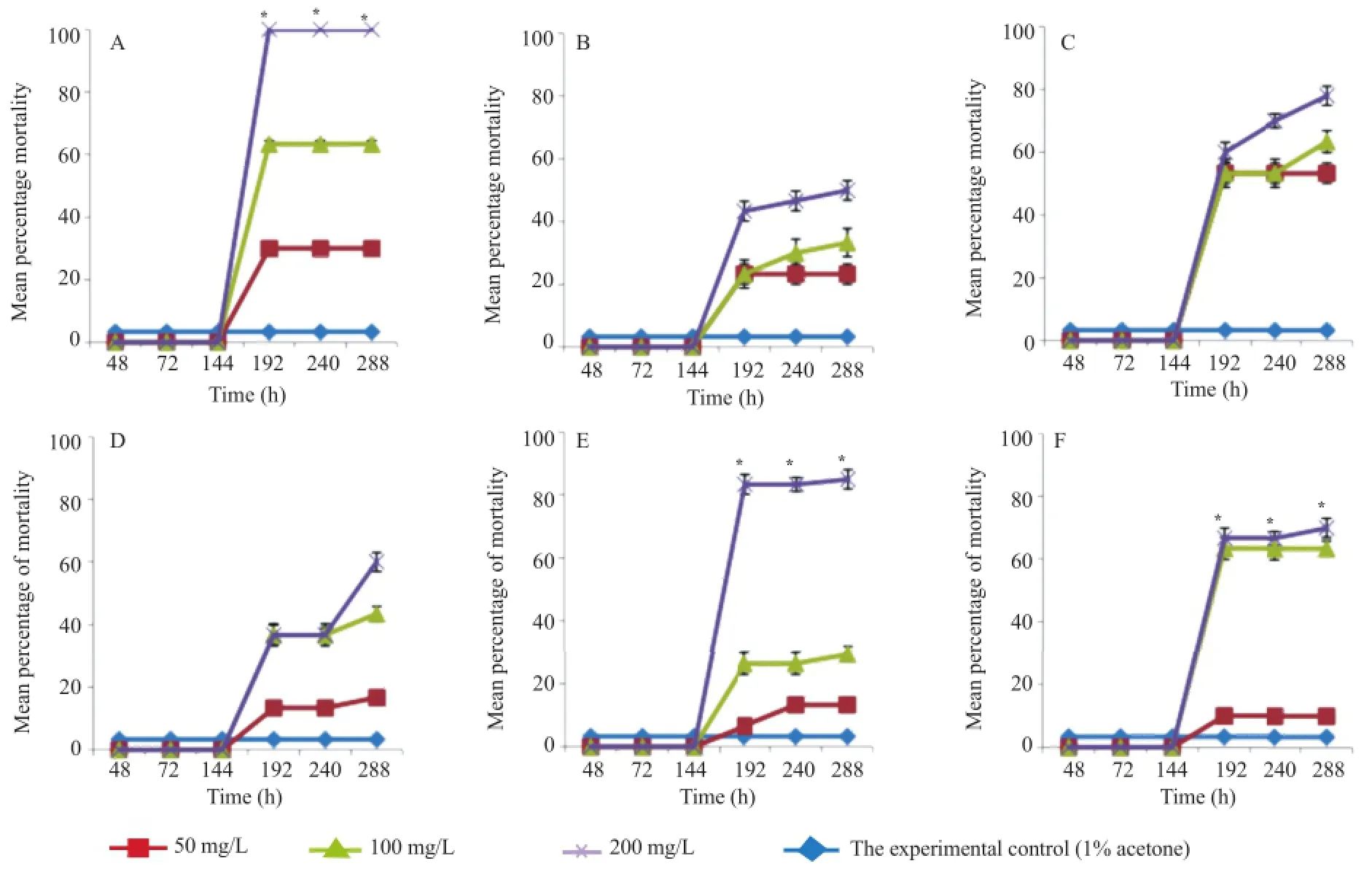
Figure 1.Mortality induced by different plant extracts on Ae.albopictus larvae.A:S.guineense root extract;B:S.guineense leaf extract;C:Z.heitzii fruit extract;D:Z.heitzii leaf extract;E:Z.heitzii bark extract;F:M.myristica fruit extract.Kruskal-Wallis non parametric test(P<0.05)was used for testing the larvicidal activity of each extract at various concentrations and times of exposure followed by a post hoc Dunnett C test.*:Significant difference(P<0.05)of means at 200 mg/L compared to the control from 192 h up to 288 h. Univariate General Linear Model test and Fisher test were used for the interaction between concentration and time.
LT50values were calculated at different concentrations. Among all the tested extracts,the fruit extract of Z.heitzii had the lowest LT50value of 145.68 h at the highest concentration(200 mg/L)followed by the root extract of S.guineense with a LT50of 192.00 h at the same concentration(Table 1).Generally,the results showed that the higher the concentration of the extract the lesser the exhibited LT50.Similar results were observed with the LC50.Longer time of exposure led to lesser LC50values. Regarding the LC50value,the fruit extract of Z.heitzii showed the lowest LC50value among all the tested extracts(Table 2). This extract exerted the best larvicidal activity among all the tested extracts with LC50values of 47.94,44.50 and 39.89 mg/L,at 192,240 and 288 h respectively.Second to this extract was the root extract of S.guineense.

Table 1LT50values calculated for the tested plant extracts at three different concentrations based on the log-probit regression analysis.

Table 2LC50values calculated for the tested plant extracts at three different time based on the log-probit regression analysis.
4.Discussion
The interest in developing pesticides of natural origin has increased during recent years because of the drawback of synthetic chemical pesticides,such as the impact on environment and the toxicity to non target organisms including humans and due to the development of resistance in targeted insect populations.Specifically,the possibility to exploit available low cost and renewable raw material,like wastes,for a possible individual use in urban area against mosquito vectors may lead to develop promising control strategies especially in developing countries[38].Several plant extracts have been reported to be biologically active against insect pests[39,40].Herein,the larvicidal activity of some Cameroonian plant extracts(roots and leaves of S.guineense,barks of Z.heitzii and leaves of M.myristica)against Ae.albopictus has been investigated.
The larvicidal efficacy of the tested extracts was dose dependent.The biological activity of the plant extracts is generally known to be due to the presence of various bioactive phytomolecules present in the plant,including alkaloids,terpenoids,and phenolics[41].However,the difference in toxicity expressed by the different plant species and parts may be due to the quantitative and qualitative variation in the chemical composition of the plant extracts.S.guineense induced full mortality(100%)of Ae.albopictus larvae within 8 days of exposure at 200 mg/L.A study conducted on the sheep ked parasite using the same extract reported about 47%mortality within 24 h of exposure[39].
The mortality depends on time of exposure,plant species and chemical composition.The variation in chemical composition could explain the differences in LC50and LT50values since the higher the concentration,the lesser the LT50,according to the extract and the part of the plant used.Likewise,in general,the higher the time of exposure the lesser the LC50.The fruit extract of Z.heitzii,exerted the best larvicidal activity among all the tested extracts with LC50values of 47.94,44.50 and 39.89 mg/L at 192 h(6th day),240 h(10th day)and 288 h(12th day)respectively,followed by the root extract of S.guineense.The variation of LC50and LT50values could be due to differences in the levels of toxicity of the specific insecticidal components in each plant extract.However,only the isolation and identification of the pure molecules exerting the larvicidal activity may allow evaluating more thoroughly these differences.
Various plant extracts have been tested as potential mosquito larvicides[42,43].The larvicidal and repellent effects of Albizzia amara and Ocimum basilicum against Aedes aegypti at different concentrations were assessed.The petroleum ether extract of Ageratum conyzoides leaves exhibited larvicidal activity with a LC50value of 425.60 and 267.90 mg/L after 24 and 48 h of exposure,respectively[44].Compared to the above results,the present study revealed low values of LC50between 192 h and 288 h of exposure.Similarly,another study reported the larvicidal effects of a neem cake methanol extract against Ae. albopictus larvae exposed to 50 and 100 mg/L for 20 days[20].Differentfractionsofincreasing polarity werealso successfully tested by the same authors following the method described by Nicoletti et al.[45]and showing a significant larvicidalactivity againstthe Ae.albopictus.Moreover,according to works of Bilal et al.[46]on the larvicidal activity of selected plant extracts against Ae.albopictus,all the extracts showed moderate larvicidal activity.The lowest LC50wasfound in Coriandrum sativum,Nigella sativa andSyzygium aromaticum at a dose of 363.7,377.5 and 403.4 mg/L,respectively,after 24 h exposure,while the amount of extracts used reduced to 263.9,300.8 and 342.2 mg/L,respectively,after 48 h.In terms of lethal time response again Coriandrum sativum,Nigella sativa and Syzygium aromaticum showed less time to produce 50%mortality(14.28,17.77 and 17.99 h). Yadav et al.[47]identified two plant species Vernonia cinerea and Prosopisjuliflora aspotentiallarvicide againstAe. albopictus.
The mode of action of most of the plant extracts on mosquito larvae is still unknown.As an example,a previous research documented that phytochemicals could interfere with the proper functioning of the mitochondria particularly at the proton transferring site[48].Some bioactive molecules of plant extracts have been found to primarily affect the mid-gut epithelial surface and secondarily the gastric caeca and the Malpighian tubules of the mosquito larvae[49].
Among the tested plant extracts,the 30%ethanol extracts of Z.heitzii(fruits in particular)and S.guineense(roots and leaves)demonstrated remarkable larvicidal activity against Ae.albopictus.These plants extracts are therefore promising as alternatives to synthetic insecticides in mosquito control programs,thus providing the basis to use the plant extracts against Ae. albopictus.
Future steps in the validation of these extracts as possible raw materials for the development of a new domestic insecticide will be the use of two to fourth instar larvae,the study of the physiological effects on the larvae,the determination of the chemical composition of the most effective extracts,the isolation of the most active fractions to possibly identify the molecules inducing the observed larvicidal effect and histopathological studies proving how the active substance act on the targeted hosts.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We would like to thank the Laboratory of Sustainable Management of the Agro-ecosystems of ENEA(Rome,Italy)for providing the facilities to carry out the biological assays.
References
[1]Gratz NG.Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004;18:215-27.
[2]Knudsen AB.Global distribution and continuing spread of Aedes albopictus.Parassitologia 1995;37:91-7.
[3]Benedict MQ,Levine RS,Hawley WA,Lounibos LP.Spread of the tiger:global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 2007;7:76-85.
[4]Bhatt S,Gething PW,Brady OJ,Messina JP,Farlow AW,Moyes CL,et al.The global distribution and burden of dengue. Nature 2013;496:504-7.
[5]Maciel-de-Freitas R,Avendanho FC,Santos R,Sylvestre G,Araújo SC,Lima JB,et al.Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak.PLoS One 2014;9(3):e92424.
[6]Weaver SC,Osorio JE,Livengood JA,Chen R,Stinchcomb DT. Chikungunya virus and prospects for a vaccine.Expert Rev Vaccines 2012;11:1087-101.
[7]Marcombe S,Farajollahi A,Healy SP,Clark GG,Fonseca DM. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved.PLoS One 2014;9(7): e101992.
[8]Forget G.Pesticides,necessary but dangerous poison.Ottawa: IDRC;1989.[Online]Available from:https://idl-bnc.idrc.ca/ dspace/bitstream/10625/24094/1/108896.pdf[Accessed on 25th June,2015]
[9]Carriger JF,Rand GM,Gardinali PR,Perry WB,Tompkins MS,Fernandez AM.Pesticides of potential ecological concern in sediment from South Florida canals:an ecological risk prioritization for aquatic arthropods.Soil Sediment Contam 2006;15:21-45.
[10]Bourtzis K,Dobson SL,Xi Z,Rasgon JL,Calvitti M,Moreira LA,et al.Harnessing mosquito-Wolbachia symbiosis for vector and disease control.Acta Trop 2014;132:S150-63.
[11]Rose RI.Pesticides and public health:integrated methods of mosquito management.Emerg Infect Dis 2001;7:17-23.
[12]Jaswanth A,Ramanathan P,Ruckmani K.Evaluation of mosquitocidal activity of Annona squamosa leaves against filarial vector mosquito,Culex quinquefasciatus Say.Indian J Exp Biol 2002;40:363-5.
[13]Park K.Park's textbook of preventive and social medicine.20th ed. Jabalpur:Banarsidas Bhanot Publishers;2009.
[14]Prabhu K,Murugan K,Nareshkumar A,Ramasubramanian N,Bragadeeswaran S.Larvicidal and repellent potential of Moringa oleifera against malarial vector,Anopheles stephensi Liston(Insecta:Diptera:Culicidae).Asian Pac J Trop Biomed 2011;1(2): 124-9.
[15]Allison LN,Dike SK,Opara FN,Ezike MN,Amadi AN.Evaluation of larvicidal efficacy and phytochemical potential of some selected indigenous plant against Anopheles gambiense and Culex quinquefasciatus.Adv Biosci Biotechnol 2013;4:1128-33.
[16]Arivoli S,Samuel T.Bioefficacy of Citrullus colocynthis(L.)Schrad(Cucurbitaceae)whole plant extracts against Anopheles stephensi,Aedes aegypti and Culex quinquefasciatus(Diptera: Culicidae).Int J Curr Res 2011;3:296-304.
[17]Liu XC,Liu QY,Zhou L,Liu QR,Liu ZL.Chemical composition of Zanthoxylum avicennae essential oil and its larvicidal activity on Aedes albopictus Skuse.Trop J Pharm Res 2014;13:399-404.
[18]Yadav R,Tyagi V,Tikar SN,Sharma AK,Mendki MJ,Jain AK,et al.Differential larval toxicity and oviposition altering activity of some indigenous plant extracts against dengue and chikungunya vector Aedes albopictus.J Arthropod Borne Dis 2014;8:174-85.
[19]Akono Ntonga P,Baldovini N,Mouray E,Mambu L,Belong P,Grellier P.Activity of Ocimum basilicum,Ocimum canum,and Cymbopogon citratus essential oils against Plasmodium falciparum and mature-stage larvae of Anopheles funestus.Parasite 2014;21: 33.
[20]Benelli G,Canale A,Conti B.Eco-friendly control strategies against the Asian tiger mosquito,Aedes albopictus(Diptera: Culicidae):repellency and toxic activity of plant essential oils and extracts.Pharmacologyonline 2014;1:44-51.
[21]Malele RS,Moshi MJ,Mwangi JW,Achola KJ,Munenge RW. Pharmacological properties of extracts from the stem bark of Syzygium guineense on the ileum and heart of laboratory rodents. Afr J Health Sci 1997;4:43-5.
[22]Ashebir M,Ashenafi M.Assessment of the antibacterial activity of some traditional medicinal plants on some food-borne pathogens. Ethiop J Health Dev 1999;13:211-6.
[23]Oketch-Rabah HA,Dossaji SF.Molluscicides of plant origin: molluscicidal activity of some Kenyan medicinal plants.South Afr J Sci 1998;94:299-301.
[24]Djoukeng JD,Abou-Mansour E,Tabacchi R,Tapondjou AL,Boud H,Lontsi D.Antibacterial triterpenes from Syzygium guineense(Myrtaceae).J Ethnopharmacol 2005;101:283-6.
[25]Ior LD,Otimenyin SO,Umar M.Anti-inflammatory and analgesic activities of the ethanolic extract of the leaf of Syzygium guineense in rats and mice.IOSR J Pharm 2012;2:33-6.
[26]Noudogbessi JP,Y´edomonhan P,Sohounhlou´e DC,Chalchat JC. Chemical composition of essential oil of Syzygium guineense(Willd.)DC.var.guineense(Myrtaceae)from Benin.Rec Nat Prod 2008;2:33-8.
[27]Pieme CA,Ngoupayo J,Nkoulou CH,Moukette BM,Nono BLN,Ama Moor VJ,et al.Syzyguim guineense extracts show antioxidant activities and beneficial activities on oxidative stress induced by ferric chloride in the liver homogenate.Antioxidants 2014;3:618-35.
[28]Tchi´egang C,Mbougueng PD.[Chemical composition of spices used in the preparation of Nah poh and Nkui of West Cameroon]. Tropicultura 2005;23:193-200.French.
[29]Mbaze LM,Poumale HM,Wansi JD,Lado JA,Khan SN,Iqbal MC,et al.Alpha-glucosidase inhibitory pentacyclic triterpenes from the stem bark of Fagara tessmannii(Rutaceae). Phytochemistry 2007;68:591-5.
[30]Ntchapda F,Maguirgue K,Adjia H,Etet PF,Dimo T.Hypolipidemic,antioxidant and anti-atherosclerogenic effects of aqueous extract of Zanthoxylum heitzii stem bark in diet-induced hypercholesterolemic rats.Asian Pac J Trop Med 2015;8:359-65.
[31]Moussavi N,Malterude KE,Mikolo B,Dawes D,Chandre F,Corbel V,et al.Identification of chemical constituents of Zanthoxylum heitzii stem bark and their insecticidal activity against the malaria mosquito Anopheles gambiae.Parasit Vectors 2015;8: 503.
[32]Mokondjimobe E,Joe MB,Barkha S,Dzeufiet PD,Chenal H,Otsudi'andjeka JB,et al.Fagaricine,a new immunorestorative phytomedicine from Zanthoxylum heitzii:preclinical and multicenter cohort clinical studies based on HIV-infected patients in six countries.Phytopharmacology 2012;2:26-45.
[33]Pauline N,Cabral BN,Anatole PC,Jocelyne AM,Bruno M,Jeanne NY.The in vitro antisickling and antioxidant effects of aqueous extracts Zanthoxyllum heitzii on sickle cell disorder.BMC Complement Altern Med 2013;13:162.
[34]Pieme CA,Santosh GK,Tekwu EM,Askun T,Aydeniz H,Ngogang JY,et al.Fruits and barks extracts of Zanthozyllum heitzii a spice from Cameroon induce mitochondrial dependent apoptosis and Go/G1 phase arrest in human leukemia HL-60 cells.Biol Res 2014;47:54.
[35]Mbaze LM,Lado JA,Wansi JD,Shiao TC,Chiozem DD,Mesaik MA,et al.Oxidative burst inhibitory and cytotoxic amides and lignans from the stem bark of Fagara heitzii(Rutaceae). Phytochemistry 2009;70:1442-7.
[36]Irvine FR.Woody plants of Ghana with special reference to their uses.London:Oxford University Press;1961,p.13-23.
[37]Calvitti M,Moretti R,Lampazzi E,Bellini R,Dobson SL. Characterization of a new Aedes albopictus(Diptera:Culicidae)-Wolbachia pipientis(Rickettsiales:Rickettsiaceae)symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens(Diptera:Culicidae).J Med Entomol 2010;47:179-87.
[38]Sukumar K,Perich MJ,Boobar LR.Botanical derivatives in mosquito control:a review.J Am Mosq Control Assoc 1991;7: 210-37.
[39]Gemeda N,Mokonnen W,Lemma H,Tadele A,Urga K,Addis G,et al.Insecticidal activity of some traditionally used Ethiopian medicinal plants against sheep ked Melophagus ovinus.J Parasitol Res 2014;2014.978537.
[40]Fayemiwo KA,Adeleke MA,Okoro OP,Awojide SH,Awoniyi IO. Larvicidal efficacies and chemical composition of essential oils of Pinus sylvestris and Syzygium aromaticum against mosquitoes. Asian Pac J Trop Biomed 2014;4:30-4.
[41]Vindhya K,Sampath Kumara KK,Neelambika HS,Leelavathi S. Preliminary phytochemical screening of Gardenia latifolia Ait.and Gardenia gummifera Linn.Res J Pharm Biol Chem Sci 2014;5: 527-32.
[42]Ghosh A,Chowdhury N,Chandra G.Plant extracts as potential mosquito larvicides.Indian J Med Res 2012;135:581-98.
[43]Murugan K,Murugan P,Noortheen A.Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector,Aedes aegypti(Insecta:Diptera:Culicidae). Bioresour Technol 2007;98:198-201.
[44]Sharma PD,Sharma OP.Natural products chemistry and biological properties of the Ageratum plant.Toxicol Environ Chem 1995;50: 213-32.
[45]Nicoletti M,Serafini M,Aliboni A,D'Andrea A,Mariani S.Toxic effects of neem cake extracts on Aedes albopictus(Skuse)larvae. Parasitol Res 2010;107:89-94.
[46]Bilal H,Akram W,Din S,Khan IA,Hassan SA,Arshad M. Larvicidal activity of selected plant extracts against Aedes albopictus Skuse(Diptera:Culicidae).Afr Entomol 2012;20(1): 8-12.
[47]Yadav R,Tikar SN,Sharma AK,Tyagi V,Sukumaran D,Jain AK,et al.Screening of some weeds for larvicidal activity against Aedes albopictus,a vector of dengue and chikungunya.J Vector Borne Dis 2015;52:88-94.
[48]Chakkaravarthy VM,Ambrose T,Vincent S,Arunachalam R,Paulraj MG,Ignacimuthu S,et al.Bioefficacy of Azadirachta indica(A.Juss)and Datura metel(Linn.)leaves extracts in controlling Culex quinquefasciatus(Diptera:Culicidae).J Entomol 2011;8:191-7.
[49]Rey D,Pautou MP,Meyran JC.Histopathological effects of tannic acid on the midgut epithelium of some aquatic dipteral larvae. J Invertebr Pathol 1999;73:173-81.
30 Jul 2015
Original article http://dx.doi.org/10.1016/j.apjtb.2016.09.004
Biapa Nya Prosper Cabral,Laboratory of Medicinal Plant Biochemistry,Food Science and Nutrition,Department of Biochemistry,Faculty of Science,University of Dschang,PO Box 67,Dschang,Cameroon.
 Asian Pacific Journal of Tropical Biomedicine2016年11期
Asian Pacific Journal of Tropical Biomedicine2016年11期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Human and animal sarcocystosis in Malaysia∶A review
- Therapeutic applications of collagenase(metalloproteases)∶A review
- Cytotoxic activity and phytochemical standardization of Lunasia amara Blanco wood extract
- Antiacanthamoebic properties of natural and marketed honey in Pakistan
- GC-MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica(Blanco)(Moraceae)leaves
- Anti-nitric oxide production,anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia
