淫羊藿次苷Ⅱ纳米脂质体的制备及包封率测定方法研究*
张 渊,姚晓东,苏 吉,何芋岐,周旭美,
(1.遵义医学院 药学院,贵州 遵义 563099;2.遵义医学院 院士工作站糖化学与糖生物学实验室,贵州 遵义 563099)
基础医学研究
淫羊藿次苷Ⅱ纳米脂质体的制备及包封率测定方法研究*
张渊1,姚晓东2,苏吉1,何芋岐1,周旭美1,2
(1.遵义医学院 药学院,贵州 遵义563099;2.遵义医学院 院士工作站糖化学与糖生物学实验室,贵州 遵义563099)
目的 制备淫羊藿次苷Ⅱ纳米脂质体并探索其包封率表征方法。方法 薄膜分散法制备淫羊藿次苷Ⅱ脂质体,Sephadex LH-20微柱-HPLC法测定包封率。分别加磷酸盐缓冲液(PBS,pH=7.4)和甲醇离心,将脂质体和游离的淫羊藿次苷Ⅱ分离。包封的药物浓度用HPLC法测定,色谱柱为TSKgel ODS C18,(250 mm×4.6 mm,5 μm),柱温30 ℃,流动相为乙腈: 水=55:45 (体积比),流速1 mL/min,检测波长270 nm。结果 制备的淫羊藿次苷Ⅱ脂质体包封率为: 95.6%,粒径: 86.7 nm,淫羊藿次苷Ⅱ脂质体胶体溶液经Sephadex LH-20微柱吸附后在相对离心力45g,PBS离心洗脱7次能够实现脂质体与游离药物的完全分离。结论 Sephadex LH-20微柱离心-HPLC法可用于测定淫羊藿次苷Ⅱ纳米脂质体的包封率,通过薄膜分散法可制备包封率高、粒径较小的淫羊藿次苷Ⅱ纳米脂质体。
淫羊藿次苷Ⅱ; 纳米脂质体; 包封率; 葡聚糖凝胶LH-20; 薄膜分散法
Icariside II (ICS),i.e.baohuoside I,is a flavonoid component extracted fromEpimediumkoreanumor metabolited from Icariin[1],with a wide range of pharmacological activities including anticancer,treating osteoporosis,and improving sexual function[2-4].In recent years,ICS was found to protect neurons[5]and anti-Alzheimer's disease significantly[6-7].Unfortunately,the clinical appilcation of ICS was limited for relatively low bioavailability resulted in its very poor solubility in aqueous solvent.The chemical structure of ICS was showed in Figure 1.
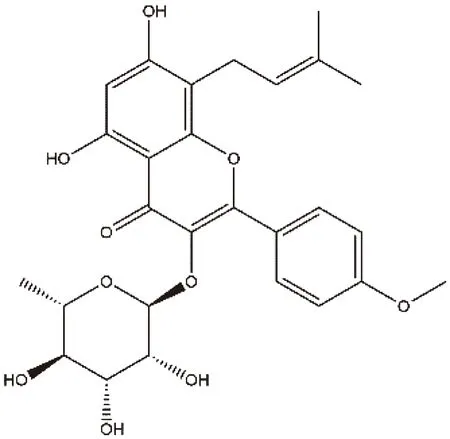
Fig 1 The chemical structure of icariside II (ICS)
Modern pharmaceutical preparation technology has become the main means to resolve the problem of bioavailability of poorly water-soluble drug.Phospholipid complexes,micelles,solid lipid nanoparticles[8],microspheres and nano-liposomes have caused widespread concern of researchers in related discipline.Nano-liposome has been playing an important role in drug delivery systems for its characteristics related to drug targeting[9-10],passing through BBB[11](blood brain barrier) and anti-tumor[12].Compared with common liposomes,nano-liposome has smaller particle size,better solubility and more stability.Nano-liposomes are particles that non-immunogenic,non-toxic,and structurally versatile.With the carriers of nano-liposomes,loaded drugs prolong retention time in blood and changed their distribution characteristics.To enhance its bioavailability,ICS-loaded nano-liposomes were prepared by film hydration method,and a method for its encapsulation efficiency was successfully established in this study.
1 Materials and methods
1.1Sample and reagentsIcariside II (>98% purity Batch No.: ZL2015051239) was purchased from Nanjing Zelang Ltd.Sephadex LH-20 was provided by Laboratory of Glycochemistry and Glycobiology,Academic Work station of Zunyi Medical University.Soya lecithin(Batch No.: 20140703) was purchased from Shanghai Tai Wei Ltd.Cholesterol was purchased from Sinopharm Chemical Reagent Ltd.Methanol,Chloroform (Chengdu Kelong Chemical Reagents Factory,AR),Acetonitrile (TEDIA,HPLC grade).
1.2EquipmentHigh performance liquid chromatography (Agilent 1260,USA),Injector (G1329B),Quaternary pump (G1311C),Diode array detector (G1315C).Column (TSKgel ODS C18250 mm×4.6 mm,5 μm),Rotary evaporator (RE2000A,Shanghai Yarong,Shanghai,China),EMD millipore Milli-QTMreference ultrapure water purification systems (USA),Ultrasonic cleaner (KQ500DV,Kunshan,Jiangsu,China),Transmission electron instrument (H-600 Hitachi,Japan),Medical Centrifuger (H1650,Hunan Xiangyi Laboratory Instrument Development Co.Ltd.),Centrifuger (AXTGL16M-II,Yancheng Anxing Experimental Instrument Co.Ltd.),Zetasizer Nano (S90,malvern,United Kingdom).
1.3Methods
1.3.1Nano-liposome preparationL-ICS were prepared by the modified thin-film hydration method.Briefly,weighted all samples into an eggplant-flask,added 500 μl methanol to dissolve ICS completely,then added 6 mL chloroform (ICS : Soya lecithin : cholesterol = 1:20:5,mass ratio).Solution was concentrated under reduced pressure to remove all chloroform.After that,3 ml phosphate buffer solution (PBS) was added and eluted with glass balls until the liposomes separated from eggplant-flask completely.Finally,L-ICS was obtained after sonicating for 30 minutes.
1.3.2Sephadex LH-20 micro-column packingA 5 ml syringe with the piston removed,were put a few cotton at the bottom.Flattened and injected Sephadex LH-20 on the cotton slowly,then added distilled water and centrifuged three times (pack height to the 2 ml scale).
1.3.3Methodological experiments
1.3.3.1HPLC analytical conditionsThe analysis was performed on a TSKgel ODS C18(250 mm×4.6 mm,5 μm) column,with a mobile phase consisted of acetonitrile : water = 55 : 45 (v/v) at a flow rate 1 ml/min,detection wavelength of 270 nm.Column temperature was set at 30 °C and the volume of sample injected to HPLC was 10 μl.
1.3.3.2Standard curve preparationA series concentrations of ICS methanol solution were injected to HPLC,then the standard curve were established by plotting the peak area versus the concentration of each standard sample.
1.3.3.3PrecisionICS methanol solutions with three concentrations were prepared .For intra-day variability test,repeated injection to HPLC was carried out 5 times within one day.While for inter-day variability test,the solutions were examined 5 days.
1.3.3.4Repeat experimentsA sample of ICS methanol solution was injected repeatedly to HPLC 6 times.
1.4Centrifug filter conditions of Sephadex LH-20 micro-column500 μl L-ICS was slowly injected to Sephadex LH-20 micro-column after its saturated with 50 μl blank liposome.5 min later,centrifuged 5 min at 45gand added 500 μl repeat 9 times again,then the next 7 times using methanol intead of PBS.Collected each eluent and added PBS to 5 ml as mother liquor.Aspirated 250 μl and added methanol to 1 000 μl,whirlpool mixed for 2 min,then centrifuged for 10 min at 9 700g.The supernatant was injected into HPLC.Plotting the concentrations represented as peak areas versus the centrifugal times.
1.5Free drug interference experiments2.0 mg ICS was added to 500 μl PBS and a suspension was obtained through ultrasonification.Then the similar procedure as that in 1.4 was carried out with centrifugal 16 times.The supernatant was injected into HPLC.Plotting the concentration versus the centrifugal times.
1.6Recovery experiments of free drugs100 μl,200 μl and 300 μl of ICS solution with concentration of 0.64 mg/ml were added to 500 μl free liposome (n=4).Then each concentration of three sample with the similar procedure as that in 1.4 was carried out,and the rest added methanol to 5ml directly .Finally,10 μl was injected to HPLC.Calculated recovery and RSD (Table 1).
Tab 1Recovery experiments of free drugs
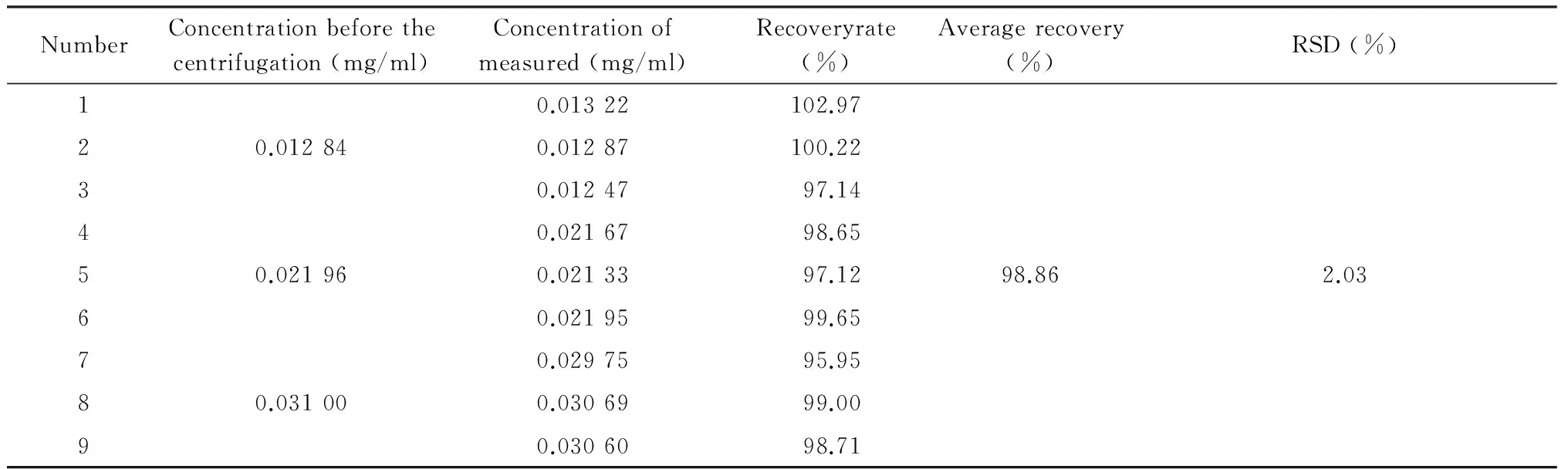
NumberConcentrationbeforethecentrifugation(mg/ml)Concentrationofmeasured(mg/ml)Recoveryrate(%)Averagerecovery(%)RSD(%)10.01322102.9720.012840.01287100.2230.0124797.1440.0216798.6550.021960.0213397.1298.862.0360.0219599.6570.0297595.9580.031000.0306999.0090.0306098.71
1.7Entrapment efficiency determinationCentrifuged 7 times at 45 g with PBS,collected all of eluent and added PBS to 5 ml,then sample of 250 μl was added by 750 μl methanol,whirlpool mixed 2 min,centrifuged 10 min at 9 700g.The supernatant was injected into HPLC.Entrapment efficiency (EE) was calculated used the following formula.
1.8Particle size determinationParticle size were determined by dynamic light scattring with a Malvern system.Samples were measured 5 times and the average partical size was calculated.Polydispersity index (PDI) was recorded for assessing the particle size distribution of L-IS.
1.9Transmission electron microscopyThe L-ICS was evaluated by transmission electron microscopy (TEM).A drop of L-ICS was instilled onto a carbon-coated copper grid,then stained with 1% phosphotungstic acid.The grid was dried in the air,then observed by TEM and photographed.
2 Results
The standard curve,Y= 27 447.51x- 88.43 (R2= 0.999 2) was shown in Figure 2.The ICS were linear over the range 0.001-0.350 mg/ml.R.S.D of inter-day and intra-day precision were 0.29% and 0.40%,respectively,while the repeat experiment was 0.34%.Recovery rates of free drug were 98.86%,its sufficient for the determination entrapment efficiency of L-ICS and indicated that no co-eluting with nano-liposomes.Centrifuging filter condition and free drug interference experiment were shown in Figure 3 and Figure 4.EE of three batches of L-ICS were 95.55%,93.02% and 98.25%,and their PDI was 0.255,0.278 and 0.284,respectively.Particle size distribution and TEM results were shown in Figure 5 and Figure 6.
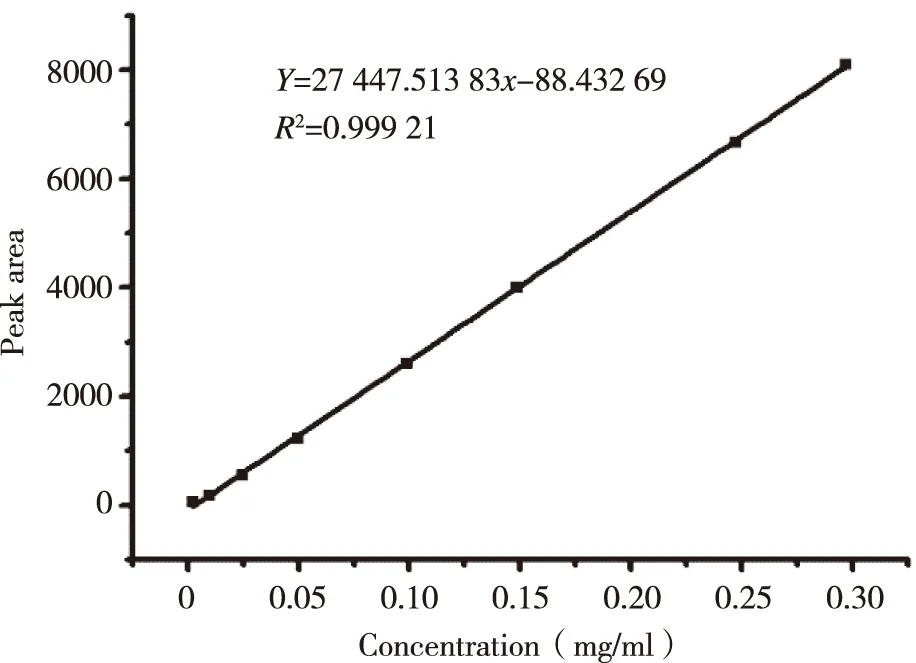
Fig 2 The standard curve of icariside II (ICS)
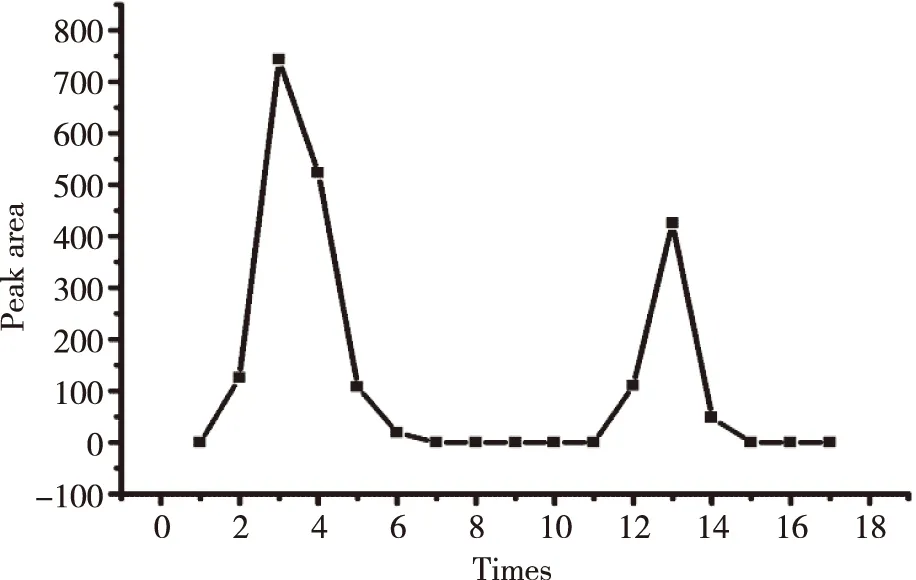
Fig 3 Plot of elution of icariside II nano-liposomes obtained from Sephadex LH-20 micro-column centrifugation separation method
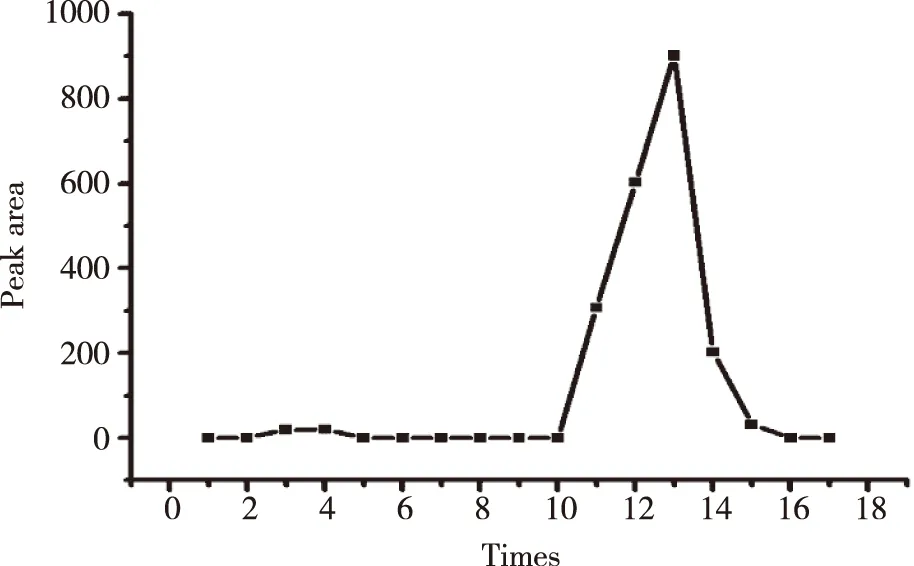
Fig 4 Plot of elution of icariside II free drug obtained from Sephadex LH-20 micro-column centrifugation separation method
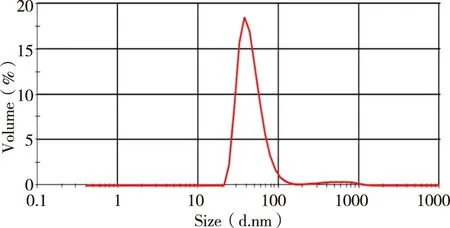
Fig 5 Icariside II nano-liposomes (L-ICS) particle size distribution
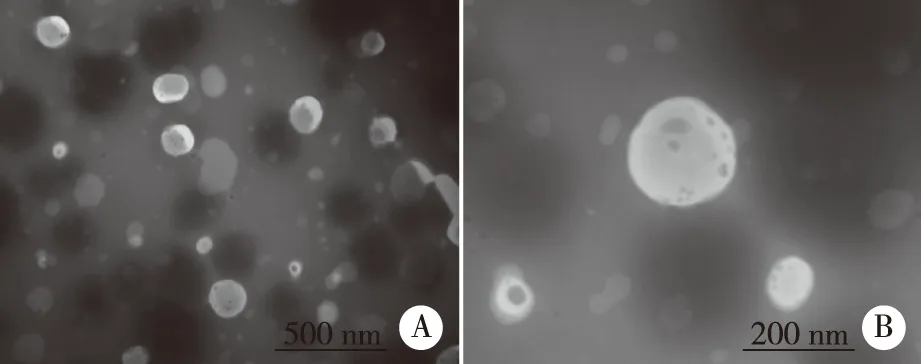
A: magnify ratio: 30 000×; B: magnify ratio: 100 000×.Fig 6 TEM of images icariside II nano-liposome (L-ICS)
3 Discussion
Encapsulation efficiency was an importance characterization parameter in evaluating preparation process and the quality of liposomes,which was usually determinated by the methods of dialysis,ultrafiltration centrifugation,ultrafiltration,sephadex column separation and so on.Based on different particle size between free drug and liposomes,ultrafiltration centrifugation was considered the fastest method,at present.The free drugs existing around liposomes in the form of particle because of its poor solubility in water.Generally,the particle size was larger than the nano-liposomes so that was difficult to be separated from the liposomes.Meanwhile,the particle had a great interference in dialysis,centrifugation and ultracentrifugation.Compared with the methods mentioned above,sephadex columns were used more widely.Sephadex G serials were more suitable for the separation of water-soluble drugs because of the network structure crosslinked dextran microparticles and 1-chloro-2,3-epoxypropane,while sephadex LH-20 also can tolerate an organic solvent with its hydroxypropylled structures[12-13].Drug properties,the kinds of sephadex,the size of nano-liposomes and centrifugal force all can have influence on determination of encapsulation efficiency through micro-column centrifugation.
In our experiments,we prepared L-ICS and established the micro-column centrifugation for determination of encapsulation efficiency successfully,then investigated the centrifugation times for separating nano-liposomes and the interference from free drugs at 45g.The results showed that L-ICS had been separated from free ICS,and sephadex LH-20 micro-column could be used in determination of the encapsulation efficiency of L-ICS.However,theinvivobehavior and targeting performance of L-ICS still need to be further investigated.That is,studies on distributioninvivoand pharmacokinetics of icariside II nano-liposomes should be carried out and L-ICS with tumor-targeting or brain-targeting effects should be prepared and exploited their anti-tumor and anti azheimer .
[1] Xu W,Zhang Y,Yang M,et al.LC-MS/MS method for the simultaneous determination of icariin and its major metabolites in rat plasma[J].Journal of Pharmaceutical and Biomedical Analysis,2007,45(4): 667-672.
[2] Zhang C,Yang L,Geng Y,et al.Icariside II,a natural mTOR inhibitor,disrupts aberrant energy homeostasis via suppressing mTORC1-4E-BP1 axis in sarcoma cells[J].Oncotarget,2016,7(19): 27819-27837.
[3] Lee K S,Lee H J,Ahn K S,et al.Cyclooxygenase-2 prostaglandin E 2 pathway mediates icariside II induced apoptosis in human PC-3 prostate cancer cells[J].Cancer Letters,2009,280(1): 93-100.
[4] Song J,Feng L,Zhong R L,et al.Icariside II inhibits the EMT of NSCLC cells in inflammatory microenvironment via down regulation of Akt/NF‐κB signaling pathway[J].Molecular Carcinogenesis,2016,DOI:10.1002/mc.22471
[5] Xiong B Q. Protective effect of Baohuoside on ischemic stroke and its mechanisms of action in rats[D].Zunyi:Medicay University,2014.
[6] Nie J,Luo Y,Huang X N, et al.Icariin inhibits beta-amyloid peptide segment 25-35 induced expression of beta-secretase in rat hippocampus[J].European Journal of Pharmacology,2010,626(3): 213-218.
[7] Yin C,Deng Y,Gao J,et al.Icariside II,a novel phosphodiesterase-5 inhibitor,attenuates streptozotocin-induced cognitive deficits in rats[J].Neuroscience,2016,328(22): 69-79.
[8] Wang A B, Li Q F,Zhou X M,Study on the optimized preparation and release in vitro of Icarrin- Loaded solid lipid nanoparticles[J].Journal of Zunyi Medical University,2014,37(6): 605-608.
[9] Jin X,Zhang Z H,Sun E, et al.Preparation of a nanoscale baohuoside I-phospholipid complex and determination of its absorption: in vivo and in vitro evaluations[J].International Journal of Nanomedicine,2011,7(6):4907-4916.
[10] Yan H M,Song J,Zhang Z H.et al.Optimization and anticancer activity in vitro and in vivo of baohuoside I incorporated into mixed micelles based on lecithin and Solutol HS 15[J].Drug Delivery,2015,1-8.
[11] Chen Z L,Huang M,Wang X R,et al.Transferrin-modified liposome promotes α-mangostin to penetrate the blood-brain barrier[J].Nanomedicine: Nanotechnology,Biology and Medicine,2016,12(2): 421-430.
[12] He Q,Tang J,Liu Y Y,The application of redox-responsive nanoparticles in tumor targeting drug delivery[J].Journal of Zunyi Medical University,2015,38(4): 321-331.
[13] Zhao F,Luan H S,Luo H F,et al.Determining the encapsulation efficiency of dexamethasone-loaded nanoparticles using sephadex gel column chromatography[J].Chin Pharm,2012,47(17): 1385-1390.
[收稿2016-06-15;修回2016-07-11]
(编辑:王静)
Preparion icariside Ⅱ nano-liposome and study of methods for determinating its entrapment efficiency
ZhangYuan1,YaoXiaodong2,SuJi1,HeYuqi1,ZhouXumei1,2
(1.Pharmacy School of Zunyi Medical University,Zunyi Guizhou 563099,China;2.Laboratory of Glycochemistry and Glycobiology,Academic Workstation of Zunyi Medical University,Zunyi Guizhou 563099,China)
Objective To prepare icariside II nano-liposome and determine its entrapment efficiency.Methods Icariside II nano-liposomes were prepared by film hydration method and its entrapment efficiency was determined by Sephadex LH-20 micro-column.The free drugs and nano-liposomes of icariside II were separated by centrifugated with PBS and methanol.Then,the concentration of icariside II entrapmented in the liposomes was determined by HPLC though column of TSKgel ODS C18(250 mm×4.6 mm,5 μm)with temperature of 30 ℃,mobile phase of acetonitrile-water (55:45,v/v) with a flow of 1.0 ml/min and detection wavelength of 270 nm.Results The nano-liposome of icariside II,with entrapment efficiency 95.6% and average size of 86.7 nm,were successfully prepared.The free drug of icariside II was separated by centrifuging with PBS for 7 times through Sephadex LH-20 micro-column.Conclusion The Sephadex LH-20 micro-column centrifugation and the HPLC method can be used for determination of entrapment efficiency of icariside II nano-liposomes with good dispersion and high entrapment efficiency.
icariside II; nano-liposome; entrapment efficiency; Sephadex LH-20; film hydration method
遵义市汇川区科技计划项目(NO:遵汇科合201513);贵州省遵义医学院院士工作站建设项目(NO:C-768)。
周旭美,女,教授,硕士生导师,研究方向:药物质量标准建立及新药研发,E-mail:546265853@qq.com。
R917
A
1000-2715(2016)04-0340-05

