阿瑞匹坦用于肺腺癌紫杉醇联合顺铂化疗引起恶心呕吐的临床研究
田 欣,吴丽娜,胡天玉,刘 洋,吴 荣,张振勇
阿瑞匹坦用于肺腺癌紫杉醇联合顺铂化疗引起恶心呕吐的临床研究
田欣,吴丽娜,胡天玉,刘洋,吴荣,张振勇*
目的观察阿瑞匹坦在肺腺癌紫杉醇联合顺铂方案化疗中的急性期及延迟期止吐疗效和安全性。方法50例肺腺癌患者,均接受紫杉醇联合顺铂的TP方案化疗,随机分为实验组及对照组,每组25例。对照组患者接受5-HT3受体拮抗剂托烷司琼、地塞米松预防止吐,实验组在对照组基础上联合应用阿瑞匹坦预防止吐。完成1个周期化疗后,评估两组患者在化疗后急性期及延迟期恶心呕吐情况及不良反应。结果50例患者均按期完成1个周期的TP方案化疗,实验组和对照组急性恶心、呕吐完全缓解率(CR)分别为44%和32%(χ2=0.764,P>0.05),有效率(RR)分别为80%和72%(χ2=0.439,P>0.05),差异无统计学意义。实验组和对照组延迟性恶心呕吐CR分别为52%和24%(χ2=4.160,P<0.05),RR分别为76%和44%(χ2=5.333,P<0.05),差异有统计学意义。两组患者不良反应主要包括头晕、乏力、呃逆、腹胀、便秘,均为轻度,差异无统计学意义。结论阿瑞匹坦联合托烷司琼、地塞米松方案预防肺腺癌紫杉醇联合顺铂化疗引起的急性期及延迟期恶心呕吐疗效确切,安全性高,值得临床进一步应用推广。
阿瑞匹坦;肺腺癌;紫杉醇;顺铂;化疗;恶心;呕吐
0 引言
肺癌是全世界发病率及死亡率极高的恶性肿瘤之一,肺腺癌是其中最常见的病理类型,临床上大部分肺腺癌患者需要接受紫杉醇联合顺铂的标准方案化疗[1-2]。该化疗方案尽管能使患者的总生存期及无病进展期明显获益,但随之产生的化疗相关性恶心呕吐也成为临床最常见的不良反应,有研究表明,化疗相关性恶心呕吐发生率高达70%~80%[3]。严重的恶心呕吐反应会影响患者生活质量,增加化疗期间的心理及经济负担,降低患者的治疗依从性,使患者恐惧及拒绝化疗,从而影响患者的治疗疗效及预后。目前,临床上5-HT3受体拮抗剂联合糖皮质激素是标准的止吐方案,但仍有部分应用高催吐化疗方案的患者止吐效果不明显,有研究表明,该止吐方案对于接近30%急性期的恶心呕吐和60%的延迟性恶心呕吐的控制水平仍较差[4-5],如何提高此类患者的止吐效果成为临床医生面对的挑战之一。阿瑞匹坦(Aprepitant)是首个被美国食品和药物监督管理局(FDA)批准的神经激肽-1受体拮抗剂,与5-HT3受体拮抗剂的作用机制不同,其可阻断神经激肽-1的作用,通过中枢机制抑制化疗引起的恶心呕吐。可在初次及重复治疗过程中,有效预防高致吐性抗肿瘤化疗药物导致的急性、延迟性恶心呕吐[6]。NCCN治疗指南推荐阿瑞匹坦联合5-HT3受体拮抗剂、糖皮质激素的三联止吐方案作为高催吐化疗方案的标准止吐治疗[7]。但目前国内关于阿瑞匹坦在肺腺癌紫杉醇联合顺铂化疗中止吐作用的报道很少,为此,我科对该类患者化疗期间采用含阿瑞匹坦的三联方案预防止吐,取得了较好的止吐效果,总结报道如下。
1 资料与方法
1.1临床资料2013年5月至2015年12月我科收治的50例肺腺癌患者,其中男20例,女30例,入组标准:①年龄≤70岁,KPS≥70分,预计生存期≥5个月;②均经病理学确诊,病理类型为肺腺癌;③初治患者,首次接受化疗,预计化疗疗程在2个周期以上;④均采用紫杉醇联合顺铂的TP方案化疗,紫杉醇135~175 mg/m2,d1,顺铂75 mg/m2,分1~3 d;⑤血常规、凝血、肝肾功能及心脏功能基本正常;⑥排除脑转移、肠梗阻等可能诱发恶心呕吐的并发症;⑦治疗前签署知情同意书。随机入组,对照组接受5-HT3受体拮抗剂及糖皮质激素的止吐方案,实验组在接受与对照组相同治疗的基础上联合应用阿瑞匹坦三联止吐方案,每组25例,两组患者一般资料比较差异无统计学意义,具有可比性。
1.2治疗方法对照组预防性止吐方案采用5-HT3受体拮抗剂托烷司琼5 mg+NS 100 mL 1次/d静滴d1~3,滴注时间>15 min;地塞米松5 mg 1次/d静推d1~3,均于化疗前30 min给药。实验组预防性止吐方案采用三药联合方案,即在对照组的基础上联合应用阿瑞匹坦口服给药,125 mg d1,80 mg d2~3,于化疗前1 h口服。
1.3疗效评价化疗24 h以内发生的恶心、呕吐为急性化疗相关性恶心呕吐(CINV);24 h以后发生的为延迟性CINV。恶心呕吐分度根据美国国家癌症研究所化疗药品不良反应评定标准(NCI-CTCAEV3.0)进行。恶心程度:0度:无恶心;Ⅰ度:食欲不振但无饮食习惯的改变;Ⅱ度:进食量减少但无明显的体重降低,脱水或营养不良输液补液<24 h;Ⅲ度:热量或体液量不足,需静脉补液、管饲或全静脉营养>24 h;Ⅳ度:出现危及生命的后果;Ⅴ度:死亡。呕吐程度:0度:24 h内无呕吐;Ⅰ度:24 h内发生呕吐<1次;Ⅱ度:24 h内发生呕吐2~5次,需静脉补液但<24 h;Ⅲ度:24 h内发生呕吐>6次,需静脉补液或全胃肠外营养>24 h;Ⅳ度:出现危及生命的后果;Ⅴ度:死亡。完全缓解(CR):恶心、呕吐均为0度;部分缓解(PR):有Ⅰ度恶心或呕吐;无效(F):恶心或呕吐达Ⅱ度及以上[8-9]。总有效率(RR)=(CR+PR)/总例数×100%。
1.4不良反应依据NCI-CTC为评价标准,观察和记录化疗期间以及化疗后出现的头晕、乏力、呃逆、腹胀、便秘等不良反应。
1.5统计学方法应用SPSS 17.0软件进行分析,计数资料比较行χ2检验,计量资料比较行t检验,等级资料比较行秩和检验。P<0.05为差异有统计学意义。
2 结果
2.1一般资料50例肺腺癌患者均有病理诊断依据,为初次接受化疗患者,所有患者均按期完成化疗,中途无退出。两组患者在年龄、性别、肿瘤分期、病理分化程度、是否吸烟等方面的基线特征基本类似,差异无统计学意义。见表1。
2.2急性恶心、呕吐控制情况比较实验组和对照组患者急性恶心、呕吐CR分别为44%和32%(χ2=0.764,P>0.05),RR分别为80%和72%(χ2=0.439,P>0.05),差异无统计学意义。见表2。
2.3延迟性恶心、呕吐控制情况比较实验组和对照组患者延迟性恶心、呕吐CR分别为52%和24%(χ2=4.160,P<0.05),RR分别为76%和44%(χ2=5.333,P<0.05),差异有统计学意义。见表3。
2.4不良反应两种止吐方案的最主要不良反应包括头晕、乏力、呃逆、腹胀、便秘,均为轻度,患者可耐受,未影响治疗。两组不良反应发生率比较差异无统计学意义(P>0.05)。见表4。
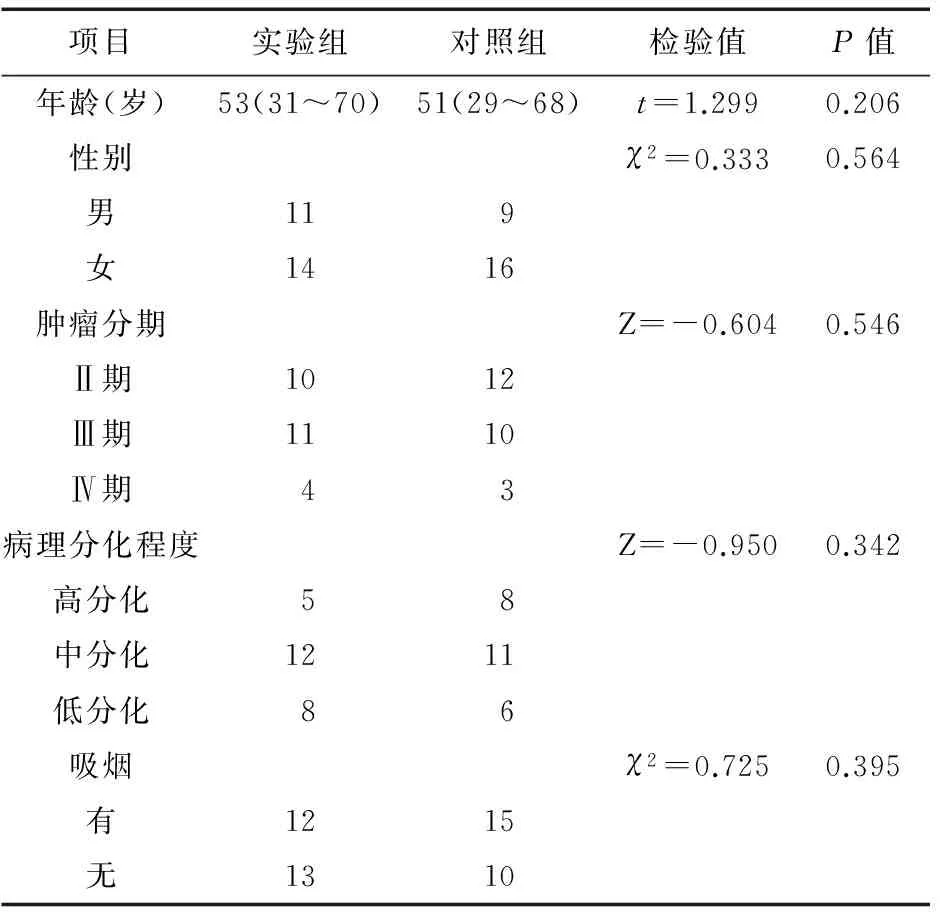
表1 两组患者基本特征分析(例)
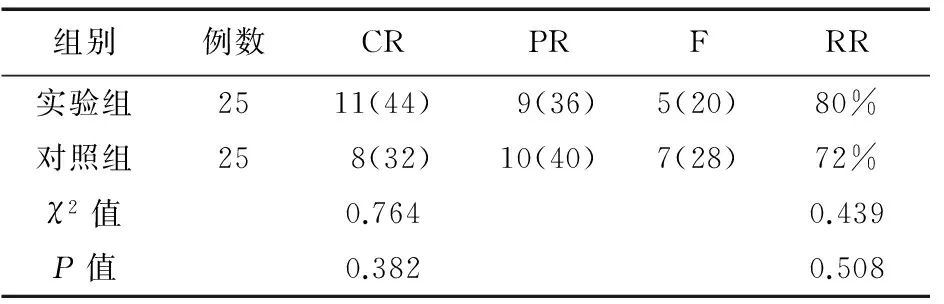
表2 两组患者急性恶心、呕吐控制情况(例,%)

表3 两组患者延迟性恶心、呕吐控制情况(例,%)

表4 两组患者不良反应分析(例,%)
3 讨论
随着环境污染的加剧及我国逐步迈入老龄化社会,肺癌的发病率逐年上升,其中腺癌是最常见的病理类型。大量研究表明,紫杉醇联合顺铂的TP方案化疗是中晚期肺腺癌的标准化疗方案,能改善患者生存期,提高治疗效果。而伴随该方案的最常见不良反应之一就是化疗导致的恶心、呕吐,在临床上给患者造成了不同程度的痛苦,严重降低患者生活质量,甚至导致脱水、电解质功能紊乱、营养不良,延误及中断治疗,影响疗效[10]。因此,如何预防及减轻此不良反应成为肿瘤科医师共同关注的课题。
阿瑞匹坦是首个被批准的神经激肽-1受体拮抗剂,不同于5-HT3受体拮抗剂,阿瑞匹坦的亲和力和选择性较高,可以穿过血脑屏障,拮抗P物质的呕吐效应,通过中枢机制抑制恶心呕吐[11-13]。大量研究已证实,5-HT3受体拮抗剂、地塞米松、口服阿瑞匹坦的三联方案能产生较佳的协同作用,可有效预防及缓解中重度致吐性化疗的急性期和延迟期的恶心、呕吐[14-17]。Nishimura等[18]进行了一项关于结直肠癌患者接受奥沙利铂化疗预防止吐的多中心、随机、对照Ⅲ期临床试验,结果表明,联合应用阿瑞匹坦+5-HT3受体拮抗剂+地塞米松的实验组,与仅应用5-HT3受体拮抗剂+地塞米松的对照组相比,急性及延迟性恶心、呕吐的控制率明显提高。Rapoport等[19]研究发现,在接受含蒽环类及环磷酰胺的AC方案或其他高催吐化疗方案的患者中,与昂丹司琼+地塞米松的预防止吐方案比较,联合应用阿瑞匹坦后,不同性别、年龄、地区的患者均得到了更好的止吐疗效。Kitazaki等[20]研究发现,在接受以顺铂或卡铂为基础方案化疗的肺癌患者中,含有阿瑞匹坦的三联止吐方案明显提高了急性及延迟性恶心呕吐的缓解率。国内的一项关于阿瑞匹坦在中国CINV患者中的随机、多中心、双盲、安慰剂对照的平行分组Ⅲ期临床研究表明,含阿瑞匹坦的三联方案在急性期呕吐发生率与对照组无明显差异,而迟发性呕吐发生率明显低于对照组[21]。
本研究的结果与国内外同类研究的结果保持了较高的一致性,其中实验组的急性恶心呕吐CR及RR高于对照组,但差异无统计学意义。而在延迟性恶心呕吐方面,实验组的CR及RR明显高于对照组,差异有统计学意义,表明阿瑞匹坦能提高急性及延迟性恶心呕吐控制效果,尤其在延迟性恶心呕吐方面显示了更佳的优势,提示含阿瑞匹坦的三联方案在临床恶性肿瘤化疗中具有较高的应用前景和价值。在不良反应方面,本研究实验组最常见的不良反应为头晕、乏力、呃逆、腹胀、便秘,但均为轻度,予对症处理后可缓解,不良反应的发生率与对照组相比,差异无统计学意义,没有患者因三联方案导致的不良反应中断治疗,证明阿瑞匹坦安全性好,不良反应轻微。
综上所述,阿瑞匹坦+5-HT3受体拮抗剂+地塞米松的三联止吐方案,安全有效,可以减轻由紫杉醇联合顺铂的TP方案化疗导致的恶心呕吐,提高急性期及延迟期恶心呕吐的缓解率,尤其对延迟期恶心呕吐效果更为明显,且患者总体耐受性好,使用方便,不良反应轻,可提高患者化疗期间的生活质量,使患者顺利完成化疗,保证化疗按期进行,适合接受高催吐化疗药物的恶性肿瘤患者。由于本研究病例数较少,需要临床继续积累样本深入评估。随着化疗药物及靶向药物的不断更新面世,我们可以期待阿瑞匹坦在多种恶性肿瘤化疗及靶向治疗领域发挥更为广阔的作用,同时,关于阿瑞匹坦能否在放疗导致的恶心呕吐以及癌痛患者由阿片类药物导致的恶心呕吐方面发挥作用有待于临床进一步观察。
[1]Tsao MS,Marguet S,Le Teuff G,et al.Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection[J].J Clin Oncol,2015,33(30):3439-3446.
[2]Fukuoka M,Wu YL,Thongprasert S,et al.Biomarker analyses and final overall survival results from a phase Ⅲ,randomized,open-label,first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS)[J].J Clin Oncol,2011,29(21):2866-7284.
[3]Shankar A,Roy S,Malik A,et al.Prevention of chemotherapy-induced nausea and vomiting in cancer patients[J].Asian Pac J Cancer Prev,2015,16(15):6207-6213.
[4]Tageja N,Groninger H.Chemotherapy-induced nausea and vomiting:an overview and comparison of three consensus guidelines[J].Postgrad Med J,2016,92(1083):34-40.
[5]Lu J,Ma L,Wang X,et al.Screening for prodromes of chemotherapy-induced vomiting and correlation between prodromes and chemotherapy-induced vomiting in lung cancer patients[J].Zhonghua Zhong Liu Za Zhi,2014,36(7):511-515.
[6]Schwartzberg LS,Rugo HS,Aapro MS,et al.New and emerging therapeutic options for the management of chemotherapy-induced nausea and vomiting[J].Clin Adv Hematol Oncol,2015,13(3 Suppl 3):3-13.
[7]Wang SY,Yang ZJ,Zhang Z,et al.Aprepitant in the prevention of vomiting induced by moderately and highly emetogenic chemotherapy[J].Asian Pac J Cancer Prev,2014,15(23):10045-10051.
[8]丁荣楣,王平,田奕,等.阿瑞匹坦辅助预防乳腺癌FAC方案化疗致恶心呕吐的临床观察[J].疑难病杂志,2015,14(1):45-48.
[9]陈丽昆,程颖,张红雨.阿瑞吡坦在中国肺癌患者中预防高剂量顺铂引起恶心和呕吐的疗效[J].中国新药与临床杂志,2015,34(10):757-763.
[10]Ishikawa A,Ohara G,Nakazawa K,et al.Chemotherapy-induced complications in patients with lung cancer:an evaluation by pharmacists[J].Mol Clin Oncol,2013,1(1):65-68.
[11]Davis MP.New therapies for ant-iemetic prophylaxis for chemotherapy[J].J Community Support Oncol,2016,14(1):11-20.
[12]Navari RM.The safety of ant-iemetic medications for the prevention of chemotherapy-induced nausea and vomiting[J].Expert Opin Drug Saf,2016,1(14):1-14.
[13]Ng TL,Clemons M,Hutton B,et al.Aprepitant versus dexamethasone to prevent delayed emesis after chemotherapy[J].J Clin Oncol,2014,32(20):2184-2185.
[14]Duggin K,Tickle K,Norman G,et al.Aprepitant reduces chemotherapy-induced vomiting in children and young adults with brain tumors[J].J Pediatr Oncol Nurs,2014,31(5):277-283.
[15]Kaushal P,Atri R,Soni A,et al.Comparative evaluation of triplet anti-emetic schedule versus double ant-iemetic schedule in chemotherapy-induced emesis in head and neck cancer patients[J].Ecancermedicalscience,2015,25(9):567.
[16]Hamada S,Hinotsu S,Kawai K,et al.Ant-iemetic efficacy and safety of a combination of palonosetron,aprepitant,and dexamethasone in patients with testicular germ cell tumor receiving 5-day cisplatin-based combination chemotherapy[J].Support Care Cancer,2014,22(8):2161-2166.
[17]农巧红,王树滨,彭小丹,等.阿瑞匹坦与托烷司琼联用或单用预防晚期肺癌顺铂化疗引起呕吐的比较研究[J].中国医药,2015,10(8):1123-1125.
[18]Nishimura J,Satoh T,Fukunaga M,et al.Combination anti-emetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial):a multicentre,randomised,controlled phase 3 trial[J].Eur J Cancer,2015,51(10):1274-1282.
[19]Rapoport BL.Efficacy of a triple anti-emetic regimen with aprepitant for the prevention of chemotherapy-induced nausea and vomiting:effects of gender,age,and region[J].Curr Med Res Opin,2014,30(9):1875-1881.
[20]Kitazaki T,Fukuda Y,Fukahori S,et al.Usefulness of anti-emetic therapy with aprepitant,palonosetron,and dexamethasone for lung cancer patients on cisplatin-based or carboplatin-based chemotherapy[J].Support Care Cancer,2015,23(1):185-190.
[21]张力.阿瑞匹坦在中国CINV患者中的应用研究[J].癌症进展,2011,9(6):610-612.
Clinical research on aprepitant for the prevention of nausea and vomiting due to paclitaxel and cisplatin chemotherapy in lung adenocarcinoma patients
TIAN Xin,WU Li-na,HU Tian-yu,LIU Yang,WU Rong,ZHANG Zhen-yong*
(The Second Deparment of Oncology,Shengjing Hospital of China Medical University,Shenyang 110004,China)
ObjectiveTo observe the acute and delayed antiemetic effects and safety of aprepitant for the prevention of paclitaxel and cisplatin chemotherapy-induced nausea and vomiting in lung adenocarcinoma carcinoma patients.MethodsFifty patients with lung adenocarcinoma carcinoma were randomly divided into the experimental group and control group,25 cases in each group.All the patients in the two groups received TP regimen chemotherapy (paclitaxel and cisplatin).The antiemetic regimen for control group was 5-HT3receptor antagonist tropisetron and dexamethasone;the regimen for experimental group consisted of aprepitant,tropisetron and dexamethasone.The acute and delayed antiemetic effects and adverse effects were evaluated after 1 cycle chemotherapy.ResultsAll 50 patients completed 1 cycle chemotherapy,the CR rates of acute antiemetic effect in experimental group and control group were 44% and 32%,there was no significant difference between the two groups (χ2=0.764,P>0.05);the RR rates of acute antiemetic effect in experimental group and control group were 80% and 72%,there was no difference between the two groups (χ2=0.439,P>0.05);the CR rates of delayed antiemetic effect in experimental group and control group were 52% and 24%,there were statistical difference between the two groups (χ2=4.160,P<0.05);the RR rates of delayed antiemetic effect in experimental group and control group were 76% and 44%,there was no difference between the two groups (χ2=5.333,P>0.05).The adverse effects mainly included dizziness,fatigue,hiccups,bloating and constipation,there was no statistical difference between the two groups.ConclusionAprepitant combined with tropisetron and dexamethasone has better antiemetic effects on the prevention of nausea and vomiting due to paclitaxel and cisplatin chemotherapy in lung adenocarcinoma patients,it is worth to be popularized.
Aprepitant;Lung adenocarcinoma carcinoma;Paclitaxel;Cisplatin;Chemotherapy;Nausea and vomiting
2016-02-26
中国医科大学附属盛京医院第二肿瘤病房,沈阳 110004
10.14053/j.cnki.ppcr.201609025

