地中海贫血患者心肌、肝脏铁沉积与血清铁蛋白相关性研究
邓小强,黄海波,尹晓林,周亚丽,管俊,覃明
地中海贫血患者心肌、肝脏铁沉积与血清铁蛋白相关性研究
邓小强1,黄海波2*,尹晓林3,周亚丽3,管俊2,覃明2
目的 定量评价地中海贫血患者心脏和肝脏铁过载,探讨心脏、肝脏铁沉积与血清铁蛋白(SF)相关性。材料与方法 应用3.0 T磁共振扫描基因确诊地中海贫血患者134例(包括中间型73例和重型61例),扫描包括:肝脏冠状面T2WI及横断面T1WI、T2WI、12回波梯度回波(T2*)序列;心脏标准横断面T2WI及两腔位、四腔位及短轴面电影和8回波梯度回波(T2*)成像。测量心肌、肝脏T2*值,所有受试者MRI扫描前1周完成SF检测。采用Spearman秩相关分析心肌、肝脏铁沉积和SF三者间相关性。结果 134例地中海贫血患者心肌T2*、肝脏T2*、SF中位数分别为23.35 (1.88~36.17) ms、1.33 (0.36~16.39) ms、1235.3 (105.1~14673.0) μg/L,心肌-肝脏T2*(rs=0.324,P=0.000)、心肌T2*-SF (rs=-0.491,P=0.000)、肝脏T2*-SF (rs=-0.697,P=0.000) 具有一定相关性。73例中间型地中海贫血患者心肌T2*、肝脏T2*、SF中位数分别为26.18 (7.09~36.17) ms、1.81 (0.37~16.39) ms、622.8 (105.1~10807.0) μg/L,心肌-肝脏T2*(rs=0.059,P=0.619)、心肌T2*-SF (rs=-0.166,P=0.161)无明显相关,但肝脏T2*-SF间中度负相关(rs=-0.583,P=0.000)。61例重型地中海贫血患者心肌T2*、肝脏T2*、SF中位数分别为18.80 (1.88~33.11) ms、0.72 (0.36~10.36) ms、3310.0 (313.0~14673.0) μg/L,心肌-肝脏T2*(rs=0.365,P=0.004)、心肌T2*-SF (rs=-0.359,P=0.004)、肝脏T2*-SF (rs=-0.707,P=0.000)具有轻中度相关性。结论 在一定范围内,地中海贫血患者心铁沉积与肝铁含量、SF具有较低或无相关性,其间相关性可能随病情加重而增加,而肝铁过载则与SF中度负相关。
地中海贫血;铁;铁蛋白类;血清学;磁共振成像
ACKNOWLEDGEMENTS This work was part of Guangxi Natural Science Foundation (NO. 2014GXNSFBA118187, 2015GXNSFAA139164).
地中海贫血(thalassemia,TM)为常染色体缺陷导致的一种或多种珠蛋白数量不足或缺乏,造成红细胞易被破坏的溶血性贫血。张之南等[1]将TM分为α型、β型和遗传性胎儿血红蛋白持续存在综合征(HPFH)。临床上β-重型及中间型(α和β) TM常因反复输血引起体内铁沉积而导致内分泌腺、肝脏、心脏等损害,患者死亡的最重要因素为心力衰竭[2],因此体内尤其心铁评估有重要意义。
标准水模、动物模型及临床研究[3-5]证实,MRI可在一定范围内准确评价水模或心铁、肝铁浓度,并指出心铁与肝铁、SF无相关或相关性低,且以SF或肝铁预测心铁沉积不可靠[6]。然而,不同程度与分型TM中心肌、肝脏铁沉积与SF相关性研究尚鲜有报道,笔者拟扫描134例TM患者,探讨不同程度与分型TM中心肌、肝脏铁沉积与SF相关性,以期为临床干预提供理论依据。
1 材料与方法
1.1资料
随机连续选取2014年6月至2015年7月解放军第303医院就诊138例中间型(TM intermediate)或重型(TM major)地中海贫血患者磁共振心肌、肝脏T2*扫描,扫描前7 d内行血清铁蛋白(SF)检测,剔除2例严重心率失常,1例不能配合,1例心肌测量未达要求外共134例纳入研究,中间型(αorβ)型TM 73例,男34例、女39例,年龄(31.3±11.3)岁,重型β-TM 61例,男33例、女28例,年龄(11.4±4.1)岁。纳入标准:基因诊断血红蛋白H病、中间型或重型β-TM;排除标准:资料不完整、严重心律失常及不能完成MR有效扫描者。实验获医院伦理委员会批准,志愿者或其家属知情并签署同意书。
1.2设备与方法
Philips Achieva 3.0 T TX MR扫描仪和16通道体部线圈,头先进仰卧位、呼气后屏气和(或)心舒中末期采集受试者肝门上一层肝实质、心室中部短轴面MR T2*图像,肝脏T2*成像前扫描冠状、横断面T2WI及横断面T1WI (参数略)以确定肝门位置,心肌T2*成像前扫描左室标准横断位-两腔心-四腔心BFFE电影序列(参数略)并以后两位置确定心室中部短轴面层面,MR T2*参数设置:TR 200 ms,FA 20°,肝脏12回波序列TEmin/max(ms) =0.6~1.3/7.8~16.0,心肌8回波序列TEmin/max(ms) =1.1/12.6,回波间隙为默认最小设置,余参数如表1。
1.3数据处理
SF数据来自实验室检测,肝脏、心脏T2*值由接受良好培训医师使用CMRtools和或结合Excel处理获得。心肌ROI位于室间隔,肝脏取4~5个ROI (30~50 mm2)且避开伪影及肉眼可见胆管、血管,ROI位于左右叶实质内,如CMRtools测量拟合度小于0.99时协同EXCEL处理或重新扫描,取三次平均值为结果。
1.4铁沉积分级、分度
铁沉积分级、分度标准根据文献[7-8]及结合本中心研究[5]制定。肝脏(单位:ms):0级(正常) T2*≥3.57,1级(轻度) 1.41≤T2*<3.57,2级(中度) 0.70≤T2*<1.41,3级(重度) 0.47≤T2*<0.7,4级(极重度) T2*<0.47;心肌(单位:ms):0级(正常) T2*≥10.0,1级(轻度) 7.0≤T2*<10.0,2级(中度) 5.0≤T2*<7.0,3级(重度) T2*<5.0;血清铁蛋白(单位:μg/L):0级(正常) SF男性≤300、女性≤200,1级(轻度) 0级<SF≤1500,2级(中度) 1500<SF≤2500,3级(重度) 2500<SF≤5000,4级(极重度) SF≥5000。
1.5统计学分析
2 结果
扫描成功率97.1% (134/138),中间型与重型β-TM组间性别无统计学意义(χ2=1.468,P=0.299),但重型组年龄显著小于中间型,其间差异有统计学意义(t=4.749,P=0.000)。
134例TM中铁沉积分度结果:(1)肝脏正常29例,轻度34例,中度31例,重度25例,极重度15例;(2)心肌正常114例,轻度7例,中度10例,重度3例;(3)血清铁蛋白正常8例,轻度62例,中度17例,重度35例,极重度12例。
中间型与重型β-TM患者肝脏和心肌铁沉积、SF分度及分布见表2,两组间差异有统计学意义(P<0.05)且重型TM体内铁沉积更明显。
134例心肌与肝脏T2*WI均满足定量要求,心肌T2*均由CMRtools完成测量,肝脏T2*计算由CMRtools完成70例(约52.2%),另64例需协同Excel测算(约47.8%)(图1,2),铁沉积越严重则同一回波时间的T2*WI相应心肌、肝脏信号越低,而拟合曲线越陡直、下降迅速。全部134例、中间型73例及重型61例TM中心肌、肝脏T2*和SF详细值如表3。134例TM心肌-肝脏T2*(rs=0.324,P=0.000)、心肌T2*-SF (rs=-0.491,P=0.000)、肝脏T2*-SF (rs=-0.697,P=0.000)具有轻或中度相关性;中间型73型TM心肌-肝脏T2*(rs=0.059,P=0.619)、心肌T2*-SF (rs=-0.166,P=0.161)无明显相关,但肝脏T2*-SF间中度负相关(rs=-0.583,P=0.000);重型61例TM心肌-肝脏T2*(rs=0.365,P=0.004)、心肌T2*-SF (rs=-0.359,P=0.004)、肝脏T2*-SF (rs=-0.707,P=0.000)呈轻或中度相关(图3,4)。
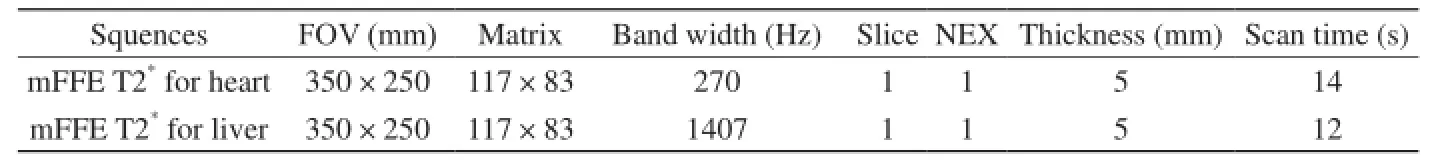
表1 梯度多回波序列参数设置Tab. 1 Parameters of MRI T2*protocols
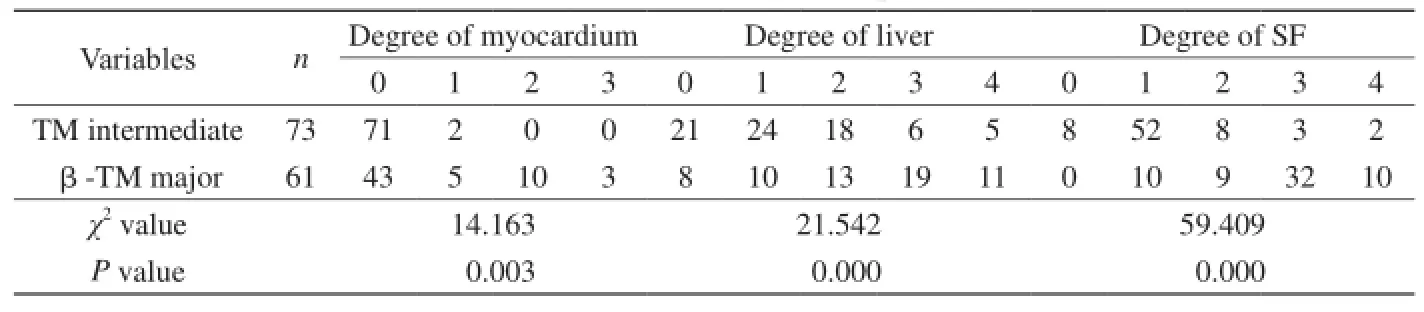
表2 中间型与重型β-地中海贫血体内铁沉积程度与分布表Tab. 2 Iron distribution and degree in patients with TM
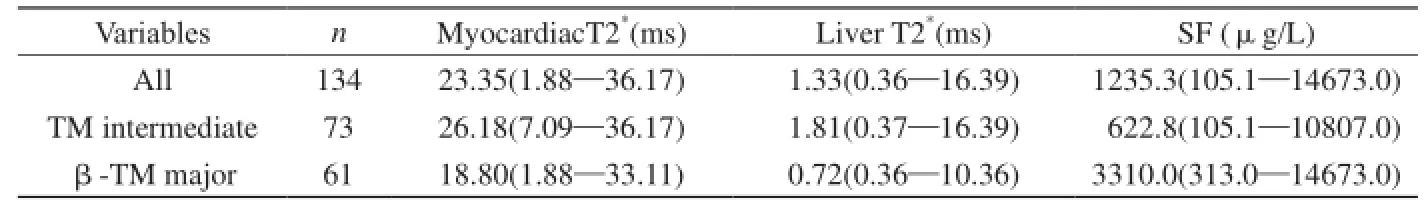
表3 地中海贫血患者心肌与肝脏T2*及血清铁蛋白详表Tab. 3 Myocardiac T2*, liver T2*and SF in patients with TM
3 讨论
输血依赖性患者红细胞被吞噬后,体内产生铁沉积将不可避免地影响内分泌腺体、肝脏以及心脏功能,其中心力衰竭为患者的致命因素,因此心脏与肝脏体内铁含量准确、早期监测对患者改善生活质量、延长生存周期有积极作用,不仅预防心肌、肝脏铁过载发生发展,而且还可逆转早期心肌病及大多数早中期肝纤维化。研究证实肝脏为体内储存铁的主要部位[9]且MR定量已经发展成为心脏和肝脏铁沉积诊断的惟一可信方法[10-11],有望替代有创性活检以减少穿刺创伤、出血、胆瘘等并发症,同时提高准确性、可重复性、受检者耐受性以及指导患者去铁治疗[12]。
磁共振定量体内铁原理[13]为应用自旋或梯度多回波序列采集信号,反映细胞内铁离子、含铁血黄素等顺磁性物质导致局部磁场不均匀、质子加速失相,采取一定函数模型计算不同回波时间与信号变化斜率而获得自旋-自旋弛豫值(T2或T2*),T2或T2*代表组织顺磁性物质浓度(即浓度越大、弛豫值越低),通过这个机制可重复地、无创性测量心脏、肝组织铁浓度并预测心脏损害程度。龙莉玲等[4]应用3.0 T自旋回波(8回波)序列扫描完全模拟人体的铁超负荷兔动物模型,证实在一定范围内,LIC与R2(即1/T2)高度相关(r=0.948,P<0.05),线性回归方程为LIC=96.426R2-0.92,R2=0.894。
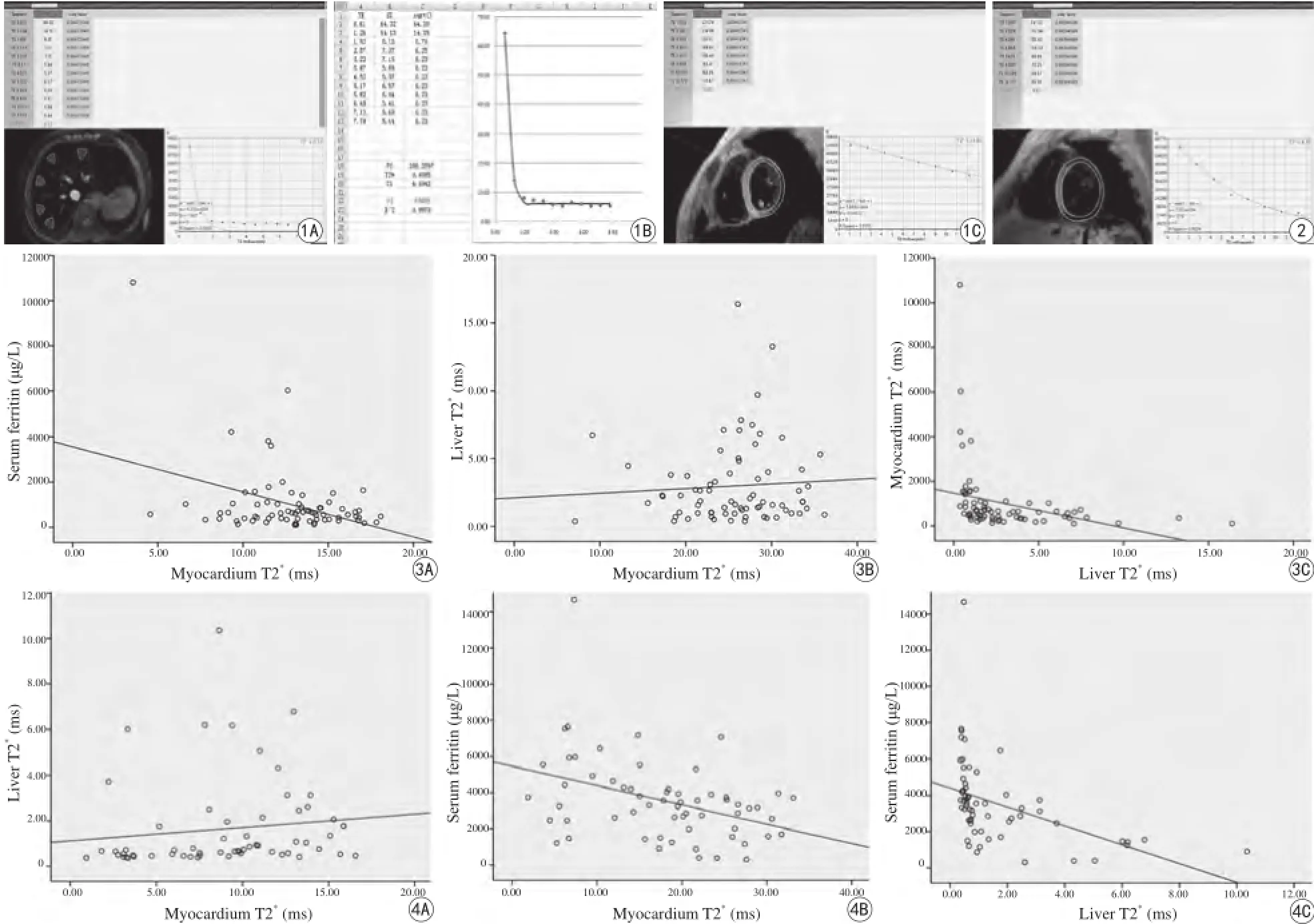
图1 A~C:女,24岁,中间型α-TM脾切除患者,Liver T2*=0.41 ms,Cardial T2*=24.80 ms,SF=6026.6 μg/L,显示心肌与肝铁和SF不平行,肝铁和SF水平相一致 图2 女,17岁,重型β-TM患者,Liver T2*=6.22 ms,Cardial T2*=6.67 ms,SF=1479.0 μg/L,提示心铁与肝铁、SF三间者均不一致 图3 A~C:中间型TM组心肌T2*、肝脏T2*、SF间散点图。A:Scatter plot of cardiac T2-SF for TM inter,rs=-0.059,P=0.619,R sq linear=0.144;B:Scatter plot of cardiac-hepatic T2*for TM inter,rs=-0.166,P=0.161,R sq linear=0.005;C:Scatter plot of hepatic T2*SF for TM inter,rs=-0.583,P=0.000,R sq linear=0.092 图4 A~C:重型β-TM组心肌T2*、肝脏T2*、SF间散点图。A:Scatter plot of cardiachepatic T2*for TM major,rs=0.365,P=0.004,R Sq linear=0.015;B:Scatter plot of cardiac T2*-SF for TM major,rs=0.359,P=0.004,R Sq linear=0.15;C:Scatter plot of hepatic T2*-SF for TM major,rs=-0.07,P=0.000,R Sq linear=0.203Fig. 1 A 24-year-old female patient with TM intermediate after surgery of spleen, of hepatic T2*(A, B), cardialT2*(C), SF were 0.41 ms, 24.80 ms, 6026.6 μg/L, respectively. It illustrates that no accordance between cardial iron overload with LIC, and SF as well, however, LIC matchs SF. Fig. 2 A 17-year-old female objective with β-TM major, liverT2*, cardial T2*, SF were 6.22 ms, 6.67 ms, 1479.0 μg/L, respectively. No agreements amongst cardial T2*, liver T2*. Fig. 3 A—C Scatter plots ofhepatic T2*(A), SF (B) against cardial T2*, and hepatic T2*(C) against SF with the linear ft (solid line), for TM intermediate. A: Scatter plot of cardiac T2-SF for TM inter, rs=-0.059, P=0.619, R sq linear=0.144. B: Scatter plot of cardiac-hepatic T2*for TM inter, rs=-0.166, P=0.161, R sq linear=0.005. C: Scatter plot of hepatic T2*SF for TM inter, rs=-0.583, P=0.000, R sq linear=0.092. Fig. 4 A—C Scatter plots of hepatic T2*(A), SF (B) against cardial T2*, and hepatic T2*(C) against SF with the linear ft (solid line), for TM major. A: Scatter plot of cardiac-hepatic T2*for TM major, rs=0.365, P=0.004, R Sq linear=0.015. B: Scatter plot of cardiac T2*-SF for TM major, rs=0.359, P=0.004, R Sq linear=0.15. C: Scatter plot of hepatic T2*-SF for TM major, rs=-0.07, P=0.000, R Sq linear=0.203.
本研究纳入满足要求分型与体内铁沉积不同的134例TM患者应用Philips 3.0 T MR扫描,结果显示,对所有病例,心肌T2*与肝脏T2*、SF均呈轻度相关且无明显规律,中间型TM组心肌T2*与肝脏T2*、SF未显示明确相关,其间相关性与相关程度在重型β-TM组有所增加。实验同时发现不论是否分型,肝脏T2*与SF均中度负相关,提示随病情加重三个变量间相关性具有增加趋势,而分型不同其相关性不完全一致,这些结果与以往国内外报道均不完全符合[14-16],笔者推测可能与学者研究病例数、病例选择、铁沉积程度、分型以及使用设备和实验设计等不相一致有关。吴学东等[14]应用1.5 T研究28例重型β-TM患者提示心肌T2*与肝脏T2*正相关(rs=0.378,P<0.05)、心肌T2*与SF负相关(rs=-0.479,P<0.05),肝脏T2*与SF无相关(rs=-0.163,P>0.05)。彭鹏等[6]以1.5 T扫描58~103例TM患者,结果认为心肌T2*与肝脏T2*正相关(rs=0.453,P<0.05)、心肌T2*与LIC低度负相关(rs=-0.402,P<0.05)且心肌T2*与SF未见线性相关(rs=-0.240,P>0.05)[15]。黄璐等[16]采用1.5 T磁共振对12只兔心肌铁超负荷模型扫描,结果显示心肌T2*、肝脏T2*、SF三变量间均无显著相关性(P>0.05)。由此可见,心肌铁过载、肝脏铁沉积和血清铁蛋白关系尚存在一定争议,这还有待于更科学、严谨、合理的实验设计进一步探讨,但多数学者已经取得以下初步共识即以SF或肝铁含量预测心铁过载、心功能不十分可靠。本研究结论支持这种观点且认为对于不同铁沉积程度与分型的TM病例,或许需要采用不同的临床干预措施与治疗方案。
实验过程还发现,体内铁沉积严重64例(约47.8%)需由CMRtools协同Excel完成肝脏T2*测算,说明3.0 T扫描仪对铁沉积检出敏感的同时还存在严重病例定量准确性难于保证的缺点(图1),这是铁定量超高场强临床应用需要特别注意的一点。为了解决该问题,笔者根据常规图像初步判断,正常和轻中度异常采用TEmin/max(ms)=1.3/16.8、严重病例则应用TEmin/max(ms)=0.6/7.8序列对肝铁过载不同程度患者扫描并观察CMRtools拟合曲线(当R2<0.99)协同EXCEL计算肝T2*值,结果本实验所有肝T2*值测算均满足定量要求,此为研究创新点之一。同时笔者另一项研究证实[3],重度铁沉积病例利用缩小首回波时间值的优化T2*序列可明显提高定量准确性和可信度。Wood等[17]实验证明,一般组织可测量的最小T2值大约为最短TE的5/7即更小首回波TE值可提高铁含量检测上限。
此外,需要说明的是,基于1.5 T的肝脏和心肌T2*参考值[7-8]为业内标准,且国内外至今尚无广泛认可的3.0 T铁沉积分级分度标准,因此本实验心铁诊断标准拟定大约为1.5 T的1/2、肝铁过载则参照本中心3.0 T标准水模扫描结果即PhC=7.008R2*+0.036[3],此亦为本研究将肝T2*代表铁含量而未转换为LIC的主要原因。
本研究创新点还包括,首次按基因将不同分型与分度基数较大的TM病例纳入研究,以及首先发现心肌铁沉积、肝脏铁过载、血清铁蛋白间相关性随病情加重而增加,且分型不同其间相关性不相一致。
综上所述,不同程度与分型TM中,心铁与肝铁、SF间相关性不完全一致,随病情加重其相关性有增加趋势,临床处理或许需要采用不同干预措施与方案,但总体而言,以肝铁或SF作为预测心铁异常和心功能状态不十分可靠,而以SF预估肝铁尚有一定价值。
本研究不足之处及展望:MR定量心肌、肝脏铁沉积具有无创性、无X线损害且可反复实施等诸多优点,但心律不齐、理解力低下、呼吸配合不良者尚难于完成扫描或心肌和肝脏T2*测量易受运动干扰,同时铁过载严重病例3.0 T扫描常出现CMRtools肝脏T2*计算失真尚需结合Excel给予纠正。此外,铁磁物置入、幽闭恐怖、微量泵等亦限制此技术全面应用,而受TEmin与磁敏感限制,3.0 T设备检测铁沉积上限不如1.5 T机型且易出现磁伪影。但相信在不久将来,随着软硬件开发与进步,这些问题可逐步得到解决,铁过载监测与疗效评估必将迎来新的高度并为临床干预提供可靠证据。
[References]
[1] Zhang ZN, Shen D. Diagnositc and effect-therapying criteria of hematopathy. 3th ed. Beijing: Sience Press, 2007: 29-34.张之南, 沈悌. 血液病诊断与疗效标准. 3版. 北京: 科学出版社, 2007: 29-34.
[2] Lekawanvijit S, Chattipakorn N. Iron overload thalassemic cardiomyopathy: iron status assessment and mechanisms of mechanical and electrical disturbance due to iron toxicity. Can J Cardiol, 2009, 25(4): 213-218.
[3] Huang HB, Zhou YL, Li ZZ, et al. Feasibility of multiple-echo GRE with parameters optimized protocol at 3.0 T MRI. Chin J Magn Reson Imaging, 2015, 6(7): 529-534.黄海波, 周亚丽, 李致忠, 等. 前瞻性3.0 T MRI梯度多回波序列参数优化可行性研究. 磁共振成像, 2015, 6(7): 529-534.
[4] Long LL, Peng P, Huang ZK, et al. Liver iron quantifcation by 3.0 T MRI: calibration on a rabbit model. Chin J Magn Reson Imaging, 2012, 3(6): 451-455.龙莉玲, 彭鹏, 黄仲奎, 等. 铁超负荷模型3.0 T MRI定量肝铁沉积可行性研究. 磁共振成像, 2012, 3(6): 451-455.
[5] Tanner MA, He TG, Westwood MA, et al. Multi-center validation of the transferability of the magnetic resonance T2*technique for the quantifcation of tissue iron. Haematologica, 2006, 91(10): 1388-1391.
[6] Peng P, Huang ZK, Long LL, et al. The relationship between heart and liver iron in thalassemia: a quantitative analysis using MRI. Chin J Radiol, 2012, 46(3): 244-247.彭鹏, 黄仲奎, 龙莉玲, 等. 地中海贫血患者心脏、肝脏铁沉积的MRI定量研究. 中华放射学杂志, 2012, 46(3): 244-247.
[7] Kerk P, Roughton M, Porter JB, et al. Cardiac T2*magnetic resonance for predication of cardiac complications in thalassemia magor. Circulation, 2009, 120(20): 1961-1968.
[8] Fischer R, Harmatz PR. Non-invasive assessment of tissue iron overload. Hematology, 2009, 215-221.
[9] Ding R, Jia JD. Progress in evaluation and treatment of hepatic iron overload. J Clin Hepatol, 2014, 30(9): 961-964.丁蕊, 贾继东. 肝脏铁过载的评估及治疗新进展. 临床肝胆病杂志, 2014, 30(9): 961-964.
[10] Huang L, Xia LM. Quantitative assessment of myocardial iron overload by functional magnetic resonance imaging. Huazhong University of Science and Technology, 2013.黄璐, 夏黎明. 功能磁共振成像对心肌铁超负荷的定量研究. 华中科技大学, 2013.
[11] Leung AW, Chu WC, Lam WW, et al. Magnetic resonance imaging assessment of cardiac and liver iron load in transfusion dependent patients. Pediatr Blood Cancer, 2009, 53(6): 1054-1059.
[12] Meloni A, Positano V, Ruffo GB, et al. Improvement of heart iron with preserved patterns of iron store by CMR-guided chelation therapy. Eur Heart J Cardiovasc Imaging, 2015,16(3): 325-334.
[13] Yang ZH, Feng F, Wang XY. A guide to technique of magnetic resonance imaging. Beijing: People' s Military Medical Press, 2014: 11.杨正汉, 冯逢, 王霄英. 磁共振成像技术指南. 北京: 人民军医出版社, 2014: 11.
[14] Wu XD, Jing YF, Pei FY, et al. Value of magnetic resonance imaging T2*tests in detecting heart and liver iron overload in patients with β-thalassemia major. J South Med Univ, 2013, 33(2): 249-252.吴学东, 井远方, 裴夫瑜, 等. 磁共振成像(T2*)检测重型地中海贫血患者心脏、肝脏铁负荷及其临床意义. 南方医科大学学报, 2013, 33(2): 249-252.
[15] Peng P, Long LL, Huang ZK, et al. Correlation study between MR quantitative cardiac iron accumulated and serum ferritin,liver iron concentration in patients with β-thalassemia major. Chin J Radiol, 2012, 46(11): 993-997.彭鹏, 龙莉玲, 黄仲奎, 等. β-重型地中海贫血MR定量心脏铁沉积与血清铁蛋白、肝铁浓度相关性研究. 中华放射学杂志, 2012, 46(11): 993-997.
[16] Huang L, Han R, Li ZW, et al. Quantitative assessment of iron load in myocardial overload rabbit model: preliminary study of MRI T2*map. Chin J Radiol, 2014, 48(3): 236-240.黄璐, 韩瑞, 李志伟, 等. MRI有效横向弛豫时间图对兔心肌铁超负荷模型铁负荷定量的初步研究. 中华放射学杂志, 2014, 48(3): 236-240.
[17] Wood JC, Ghugre N. Magnetic resonance Imaging assessment of excess iron in thalassemia, sickle cell disease and others iron overload diseases. Hemoglobin, 2008, 32(1-2): 85-96.
Correlation between myocardial iron deposit, liver iron concentration and serum ferritin in patients with thalassemia
DENG Xiao-qiang1, HUANG Hai-bo2*, YiN Xiao-lin3, ZHOU Ya-li3, GUAN Jun2, QIN Ming2
1Department of Radiology, Guangxi CAPE Hospital, Nanning 530003, China
2Department of Medical Imaging, 303rdHospital of PLA, Nanning 530021, China
3Department of Haematology, 303rdHospital of PLA, Nanning 530021, China
*Correspondence to: Huang HB, E-mail: jackie000528@163.com
Objective: To quantify the myocardial iron overload and liver iron concentration (LIC) in thalassemia (TM) patients and discuss the relationships amongst myocardial iron overload, LIC and serun ferritin (SF). Materials and Methods: Study protocol was approved by local ethics committee, informed consent was obtained. A total of 134 patients with TM (Intermediate, 73. Major, 61) diagnosed by gene were enrolled. A multiple fast-feld echo (mFFE) within a single breath-hold was performed using a 3.0 tesla MR unit to acquire 8 or 12 T2*weighted images in the heart or liver. T2*values of myocardium and liver were quantified based on mFFE-T2*protocol by a welltrained physician repectively, SF was obtained within 7 days before MRI. Spearman rank correlation was applied to analyse the relationships. Results: The median (range) of myocardial T2*, liver T2*and SF in 134 patients were 23.35 (1.88—36.17) ms, 1.33(0.36—16.39) ms, 1235.3 (105.1—14673.0) μg/L, respectively. There was weakly correlation between myocardial T2*and liver T2*(rs=0.324, P=0.000), as well as myocardial T2*and SF (rs=-0.491, P=0.000), moreover, liver T2*and SF were moderately linear related (rs=-0.697, P=0.000). Furthermore, the median (range) of myocardial T2*, liver T2*and SF in 73 TM inter mediate were 26.18 (7.09—36.17) ms, 1.81(0.37—16.39) ms, 622.8 (105.1—10807.0) μg/L, respecttively. There was no linear correlation between myocardial T2*and liver T2*(rs=0.059, P=0.619), likewise myocardial T2*and SF (rs=-0.166, P=0.161), however, liver T2*and SF was moderately related (rs=-0.583, P=0.000). The median (range) of myocardial T2*, liver T2*and SF in 61 TM major were 18.80 (1.88—33.11) ms, 0.72 (0.36—10.36) ms, 3310.0 (313.0—14673.0) μg/L, respectively. There was weak correlation between myocardial T2*and liver T2*(rs=0.365, P=0.004), and myocardial T2*and SF as well (rs=-0.359, P=0.004), in addition, liver T2*and SF was moderately related (rs=-0.707, P=0.000). Conclusions: Within a certain LIC limits, cardiac excess iron was weak or no linear correlation with LIC and SF, which might increase with condition worsed or its types, however, it showed a moderate negative relationship of LIC to SF.
Thalassemia; Iron; Ferritins; Serology; Magnetic resonance imaging
23 June 2016, Accepted 12 Aug 2016
广西壮族自治区自然科学基金(编号:2014GXNSFBA118187;2015 GXNSFAA139164)
1. 武警广西总队医院放射科,南宁530003
2. 解放军第303医院医学影像科,南宁 530021
3. 解放军第303医院血液科,南宁530021
黄海波,E-mail:Jackie000528@163. com
2016-06-23
接受日期:2016-08-12
R445.2;R730.264
A
10.12015/issn.1674-8034.2016.09.006
邓小强, 黄海波, 尹晓林, 等. 地中海贫血患者心肌、肝脏铁沉积与血清铁蛋白相关性研究. 磁共振成像, 2016, 7(9): 669-674.

