载脂蛋白Eε4基因对轻度认知功能障碍患者脑白质微细结构损害研究
李海涛,李桂花,哈斯也提·依不来音,朱国峰,马依日木·赛买提,阿依吐拉·卡迪尔,王 丹,杨新玲
载脂蛋白Eε4基因对轻度认知功能障碍患者脑白质微细结构损害研究
李海涛,李桂花,哈斯也提·依不来音,朱国峰,马依日木·赛买提,阿依吐拉·卡迪尔,王 丹,杨新玲
背景目前国内外对磁共振弥散张量成像(DTI)和轻度认知功能障碍(MCI)的相关性研究较多,但针对DTI临床评价MCI载脂蛋白Eε4(ApoEε4)基因携带者脑白质变化的研究还比较少见。目的利用功能影像学优势,研究ApoEε4基因对MCI患者脑白质微细结构损害,为临床早期治疗MCI患者提供理论依据。方法选取2012—2013年新疆医科大学第二附属医院、新疆医科大学附属中医院就诊的维吾尔族、汉族MCI患者26例,根据ApoE等位基因亚型测试结果,其中13例为ApoEε4基因携带者(A组),13例为非ApoEε4基因携带者(B组);另选取同期两院体检健康者15例为C组。对受试者进行DTI扫描,记录双侧颞叶、双侧海马、双侧顶叶、双侧枕叶、胼胝体压部、扣带回后部白质区域表观扩散系数(ADC)和部分各向异性(FA)值。结果3组双侧颞叶、双侧顶叶、双侧枕叶、扣带回后部ADC、FA值比较,差异均无统计学意义(P>0.05);3组双侧海马、胼胝体压部ADC、FA值比较,差异均有统计学意义(P<0.05);其中B组和C组双侧海马、胼胝体压部ADC值低于A组,C组双侧海马、胼胝体压部ADC值低于B组;B组双侧海马FA值高于A组,C组双侧海马、胼胝体压部FA值均高于A组和B组(P<0.05)。MCI ApoEε4基因携带者蒙特利尔认知评估量表(MoCA)评分为(20.1±3.2)分。相关性分析结果显示,MCI ApoEε4基因携带者双侧海马、胼胝体压部ADC值与MoCA评分呈正相关(P<0.05)。结论从脑白质微细结构损害的角度来说,MCI患者主要出现在双侧海马、胼胝体压部,且ApoEε4基因携带者较非携带者脑白质微细结构损害的程度变化更明显,且与MoCA评分存在相关性。
轻度认知障碍;弥散张量成像;载脂蛋白E4;海马;胼胝体
李海涛,李桂花,哈斯也提·依不来音,等.载脂蛋白Eε4基因对轻度认知功能障碍患者脑白质微细结构损害研究[J].中国全科医学,2016,19(26):3175-3179.[www.chinagp.net]
LI H T,LI G H,HASIYETI·Yibulaiyin,et al.Damage of apoEε4 gene on cerebral white matter microstructure of patients with mild cognitive impairment[J].Chinese General Practice,2016,19(26):3175-3179.
据不完全统计,国外65岁及以上者痴呆患病率为6.6%~15.8%[1]。轻度认知功能障碍(MCI)是阿尔茨海默病(AD)或其他类型痴呆的早期阶段,被认为是正常老年人在早期AD间的转换过渡阶段[2]。新疆维吾尔族、汉族老年人MCI标化患病率为10.58%[3],由于中、晚期AD的治疗效果不佳,所以早期诊断、早期干预迫在眉睫。
磁共振弥散张量成像(DTI)技术能反映大脑白质束超微结构完整性的情况,是目前唯一能在活体有效显示大脑白质纤维及其走行,并进行定量研究的方法,其两个重要指标为表观扩散系数(ADC)和部分各向异性(FA)[4]。TIAN等[5]报道载脂蛋白Eε4(ApoEε4)基因是MCI向AD转化中重要的风险因素;BRUEGGEN等[6]报道早期MCI患者脑白质微细结构纤维已经发生了不连续的损害改变,ApoEε4基因可能影响MCI患者的脑白质变化。目前国内外开展MCI和DTI的相关性研究很多,但具体到ApoEε4基因对早期MCI患者脑白质微细结构损害的相关报道比较少见。本研究利用DTI检测ADC及FA值反映MCI患者脑白质微细结构的变化特点,细化分析ApoEε4基因对MCI的影响,从而为评估新疆多民族MCI向AD转归的风险性提供临床指导作用。
1 资料与方法
1.1入选及排除标准入选标准:(1)符合美国精神病学会制定的精神障碍诊断和统计手册第4版(DSM-Ⅳ)中关于MCI的诊断标准[7]:①有记忆力减退的主观感觉;②日常生活能力量表、简易智能状态检查、总体衰退量表检测出现认知功能减退但尚不足以诊断痴呆;③社会及生活能力降低;④排除特发性因素引起的智能减退,且Hachinski缺血积分表≤4分;⑤病程>3个月。(2)蒙特利尔认知评估量表(MoCA)[8]评分为18~26分。排除标准:AD、帕金森合并痴呆、血管性痴呆(VD)及各种脑器质性疾病所致的痴呆。
1.2一般资料选取2012—2013年新疆医科大学第二附属医院、新疆医科大学附属中医院就诊的维吾尔族、汉族MCI患者26例,根据ApoE等位基因亚型测试结果,其中13例为ApoEε4基因携带者(A组),13例为非ApoEε4基因携带者(B组);另选取同期两院体检健康者15例为C组。3组受试者性别、年龄、民族、受教育年限比较,差异均无统计学意义(P>0.05,见表1)。

表1 3组患者一般资料比较
注:A组为载脂蛋白Eε4(ApoEε4)基因携带者,B组为非ApoEε4基因携带者,C组为体检健康者;a为χ2值
1.3ApoE等位基因亚型测试晨起或体检时空腹抽取外周静脉血4 ml,以2 000 r/min离心10 min,离心半径5 cm,弃上清液,-20 ℃保存。采用QIAGEN Blood Mini Kit试剂盒提取全血基因组DNA,然后利用HIXSON等[9]PCR及限制性内切酶ApoE基因亚型分型方法,通过红细胞裂解液分离静脉血样本中的白细胞,白细胞经预冷的磷酸盐缓冲液洗涤3次后,提取基因组DNA进行基因扩增,扩增引物为F4(5′-ACAGAATTCGCCCCGGCCTGGTACAC-3′)和F6(5′-TAAGCTTGGCACGGCTGTCCAAGGA-3′),扩增产物经HhaⅠ酶切(酶切位点为GCGC)后进行琼脂糖凝胶电泳,根据电泳后分离条带的位置及数量区分不同的ApoE等位基因亚型。
1.4DTI扫描DTI采用EPI序列,扫描参数:重复时间(TR)8 000 ms,回波时间(TE)83 ms,视野256 mm×256 mm,矩阵128×128,层厚2.0 mm,无间隔,总计获取32层,体素2.0 mm×2.0 mm×2.0 mm,b值分别为0、700 s/mm2,在32个各向同性方向上分别施加扩散敏感梯度,激励次数为1,总采集时间为546 s。扫描范围自枕骨大孔至颅顶部。将感兴趣区(ROI)放置在FA图上,分别置于双侧颞叶、双侧海马、双侧顶叶、双侧枕叶、胼胝体压部、扣带回后部白质区域。使用Neuro3D软件包计算出2D的彩色FA图,红色代表左右走行的纤维束,绿色代表前后走行的纤维束,蓝色代表上下走行的纤维束。检测由两名5年以上经验的影像科医师完成,患者需在安静配合状态下完成。

2 结果
2.13组不同部位ADC、FA值比较3组双侧颞叶、双侧顶叶、双侧枕叶、扣带回后部ADC、FA值比较,差异均无统计学意义(P>0.05);3组双侧海马、胼胝体压部ADC、FA值比较,差异均有统计学意义(P<0.05);其中B组和C组双侧海马、胼胝体压部ADC值低于A组,C组双侧海马、胼胝体压部ADC值低于B组;B组双侧海马FA值高于A组,C组双侧海马、胼胝体压部FA值均高于A组和B组,差异均有统计学意义(P<0.016,见表2)。MCIApoEε4基因携带者不同部位DTI纤维束图像见图1。
2.2MCIApoEε4基因携带者各部位ADC、FA值与MoCA评分的相关性MCIApoEε4基因携带者MoCA评分为(20.1±3.2)分。相关性分析结果显示,MCIApoEε4基因携带者双侧海马、胼胝体压部ADC值与MoCA评分呈正相关(P<0.05),双侧颞叶、双侧顶叶、双侧枕叶、扣带回后部ADC值以及双侧颞叶、双侧海马、双侧顶叶、双侧枕叶、胼胝体压部、扣带回后部FA值与MoCA评分无直线相关性(P>0.05,见表3、4)。
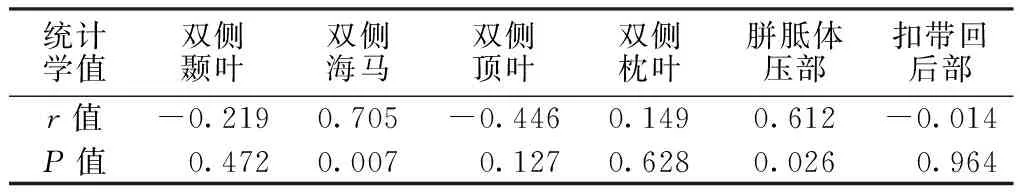
表3 MCI ApoEε4基因携带者各部位ADC值与MoCA评分的相关性

表4 MCI ApoEε4基因携带者各部位FA值与MoCA评分的相关性

表2 3组不同部位ADC、FA值比较〔M(QR)〕
注:与A组比较,aP<0.016;与B组比较,bP<0.016;ADC=表观扩散系数,FA=部分各向异性

注:A为小脑半球,B为小脑蚓部,C为大脑皮质,D为颞、顶叶,E为脑桥,F为海马
3 讨论
老年人MCI患病率处于较高水平,为12%~18%,每年10%~15%的MCI患者发展为AD,而健康人群每年转化为痴呆者仅为1%~2%[10]。因此早期发现及干预MCI,对于延缓甚至阻止痴呆的发生或进展具有深远意义,可以使患者在较长一段时期内保持基本的认知功能,对于维持和改善患者生活质量有积极作用。
AD最为常见的遗传危险因素是ApoEε4等位基因,其增加了人群罹患AD的风险,携带ApoEε4等位基因的健康老年人与未携带ApoEε4等位基因的健康老年人相比,在认知能力、脑部结构和脑功能上存在一定的差异性[11]。REDEL等[12]报道认为ApoEε4等位基因在患者出现认知功能障碍或在出现临床症状的前10年,就已经开始调节大脑功能状态。ALICHNIEWICZ等[10]研究认为MCI患者携带ApoEε4基因型较正常对照组显著增多。在MCI患者中ApoEε4等位基因的出现率介于正常老年人与AD之间,因此认为ApoEε4等位基因是MCI的危险因素[13]。ALEXOPOULOS等[14]认为ApoEε4基因可选择性地影响情节记忆,其对情节记忆的影响相对于其他认知领域强,即RISACHER等[15]研究发现,携带ApoEε4等位基因是遗忘型MCI的危险因素。VANA等[16]研究表明认知功能正常的老年人携带至少一个ApoEε4等位基因较易转变为遗忘型MCI。
DTI技术能反映大脑白质超微结构完整性的情况,因此在痴呆的研究中具有良好的发展前景。虽然有研究认为MRI对于准确发现脑白质的变化不是十分肯定,但目前多数研究对DTI研究VD、AD、MCI得出的结论仍持十分肯定的态度[17-18]。DTI两个重要的指标分别是ADC和FA。ADC主要反映水分子弥散运动的快慢而不指示运动的方向性,如神经元丢失、神经退变及髓鞘脱失等病变均可导致水分子运动空间加大,各向异性减低,弥散加快。FA主要反映组织各向异性弥散程度变化,FA值越大说明神经纤维排列越紧密,微细结构越好,走向较为一致,纤维相对完整。SELNES等[19]报道DTI显示MCI患者脑区的ADC增加是早期AD的典型表现,特别是海马的ADC增加比海马萎缩更为敏感,更容易发展为AD。在AD患者中,研究认为脑后部区域如扣带回后部、颞叶白质、海马旁回灰质、胼胝体压部等部位,DTI异常似乎更为集中[20]。AD患者DTI变化似乎与MCI相似,而多种损害的MCI患者转化成AD的比例比单一损害的MCI更高。DTI的应用对AD、MCI的全面研究有很大帮助,通常发现MCI患者ADC值增加,FA值减低和伴影像学指标改变不如AD明显[21]。因此三维立体弥散张量纤维束示踪成像图及定量的数据指标给临床提供了很有价值的信息。
本研究结果显示,3组双侧海马和胼胝体压部ADC和FA值比较存在差异,其中ApoEε4基因携带者双侧海马、胼胝体压部ADC高于非ApoEε4基因携带者和正常对照者; ApoEε4基因携带者双侧海马FA值低于非ApoEε4基因携带者和正常对照者,胼胝体压部FA值低于正常对照者;说明脑白质微细结构变化较快的损害部位主要集中在双侧海马及胼胝体压部,与THILLAINADESAN等[22]报道的涉及的部位比较广泛不一致。另外本研究结果提示:双侧海马、胼胝体压部的ADC值与MoCA评分呈正相关。说明双侧海马、胼胝体压部的脑白质纤维束的微细结构损害越严重,认知功能障碍越严重。
综上所述,MCI ApoEε4基因携带者较非携带者脑白质微细结构损害的变化程度更明显,且与MoCA评分相关。
作者贡献:李海涛负责试验设计与实施、资料收集整理、撰写论文、成文并对文章负责;李桂花、哈斯也提·依不来音、朱国峰、马依日木·赛买提、阿依吐拉·卡迪尔、王丹进行试验实施、评估、资料收集;杨新玲进行质量控制及审校。
本文无利益冲突。
[2]DA X,TOLEDO J B,ZEE J,et al.Integration and relative value of biomarkers for prediction of MCI to AD progression:spatial patterns of brain atrophy,cognitive scores,APOE genotype and CSF biomarkers[J].Neuroimage Clin,2013(4):164-173.
[3]周晓辉,朱晓琼,库木斯·巴雅合买提,等.新疆维吾尔族和汉族老年人轻度认知功能障碍的现况调查[J].中华老年医学杂志,2009,28(10):865-869.
[4]O′BRYANT S E,JOHNSON L,BALLDIN V,et al.Characterization of Mexican Americans with mild cognitive impairment and Alzheimer′s disease[J].J Alzheimers Dis,2013,33(2):373-379.
[5]TIAN J,SHI J,BAILEY K,et al.Association between apolipoprotein E e4 allele and arteriosclerosis,cerebral amyloid angiopathy,and cerebral white matter damage in Alzheimer′s disease[J].J Neurol Neurosurg Psychiatry,2004,75(5):696-699.
[6]BRUEGGEN K,GROTHE M J,DYRBA M,et al.The European DTI study on dementia- a multicenter DTI and MRI study on Alzheimer′s disease and mild cognitive impairment[J].Neuroimage,2016.pii:S1053-8119(16)30011-8.
[7]American Psychiatric Association.Diagnostic and statistical manual of mental disorders[M].4th ed.Washington:American Psychiatric Association,1994.
[8]HORTON D K,HYNAN L S,LACRITZ L H,et al.An abbreviated Montreal Cognitive Assessment(MoCA)for dementia screening[J].Clin Neuropsychol,2015,29(4):413-425.
[9]HIXSON J E,VERNIER D T.Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hha Ⅰ[J].J Lipid Res,1990,31(3):545-548.
[10]ALICHNIEWICZ K K,BRUNNER F,KLÜNEMANN H H,et al.Structural and functional neural correlates of visuospatial information processing in normal aging and amnestic mild cognitive impairment[J].Neurobiol Aging,2012,33(12):2782-2797.
[11]FRANK G,HENNIG-FAST K,KLÜNEMANN H H,et al.Differential impact of ApoE ε4 on cortical activation during famous face recognition in cognitively intact individuals and patients with amnestic mild cognitive impairment[J].Alzheimer Dis Assoc Disord,2011,25(3):250-261.
[12]REDEL P,BUBLAK P,SORG C,et al.Deficits of spatial and task-related attentional selection in mild cognitive impairment and Alzheimer′s disease[J].Neurobiol Aging,2012,33(1):195.e27-42.
[13]AMLIEN I K,FJELL A M,WALHOVD K B,et al.Mild cognitive impairment:cerebrospinal fluid tau biomarker pathologic levels and longitudinal changes in white matter integrity[J].Radiology,2013,266(1):295-303.
[14]ALEXOPOULOS P,GUO L H,JIANG M,et al.Amyloid cascade and tau pathology cerebrospinal fluid markers in mild cognitive impairment with regards to Alzheimer′s disease cerebral metabolic signature[J].J Alzheimers Dis,2013,36(2):401-408.
[15]RISACHER S L,KIM S,SHEN L,et al.The role of apolipoprotein E(APOE)genotype in early mild cognitive impairment(E-MCI)[J].Front Aging Neurosci,2013,5:11.
[16]VANA L,KANAAN N M,UGWU I C,et al.Progression of tau pathology in cholinergic Basal forebrain neurons in mild cognitive impairment and Alzheimer′s disease[J].Am J Pathol,2011,179(5):2533-2550.
[17]PARNETTI L,CHIASSERINI D,EUSEBI P,et al.Performance of aβ1-40,aβ1-42,total tau,and phosphorylated tau as predictors of dementia in a cohort of patients with mild cognitive impairment[J].J Alzheimers Dis,2012,29(1):229-238.
[18]CLAXTON A,BAKER L D,WILKINSON C W,et al.Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer′s disease[J].J Alzheimers Dis,2013,35(4):789-797.
[19]SELNES P,AARSLAND D,BJØRNERUD A,et al.Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment[J].J Alzheimers Dis,2013,33(3):723-736.
[20]LIU J,YIN C,XIA S,et al.White matter changes in patients with amnestic mild cognitive impairment detected by diffusion tensor imaging[J].PLoS One,2013,8(3):e59440.
[22]THILLAINADESAN S,WEN W,ZHUANG L,et al.Changes in mild cognitive impairment and its subtypes as seen on diffusion tensor imaging[J].Int Psychogeriatr,2012,24(9):1483-1493.
(本文编辑:贾萌萌)
Damage of ApoEε4 Gene on Cerebral White Matter Microstructure of Patients with Mild Cognitive Impairment
LIHai-tao,LIGui-hua,HASIYETI·Yibulaiyin,ZHUGuo-feng,MAYIRIMU·Saimaiti,AYITULA·Kadier,WANGDan,YANGXin-ling.DepartmentofNeurology,theSecondAffiliatedHospitalofXinjiangMedicalUniversity,Urumqi830002,China
Correspondingauthor:YANGXin-ling,DepartmentofNeurology,theSecondAffiliatedHospitalofXinjiangMedicalUniversity,Urumqi830002,China;E-mail:poplar862@sohu.com
BackgroundAt present,there are many researches about the correlation between the magnetic resonance diffusion tensor imaging(DTI)and mild cognitive impairment(MCI)both at home and abroad,but studies on the changes of cerebral white matter by clinical evaluation of DTI on MCI ApoEε4 gene carriers are still relatively rare.ObjectiveTo study the damage of ApoEε4 gene on cerebral white matter microstructure of MCI patients by taking advantages of functional imaging and to provide a theoretical basis for early clinical treatment of MCI patients.Methods26 Uyghur and Han patients with MCI,who received treatment in the Second Affiliated Hospital of Xinjiang Medical University and Affiliated Chinese Medicine Hospital of Xinjiang Medical University from 2012 to 2013,were selected.According to the test results of ApoE allele subtypes,13 were ApoEε4 gene carriers(A group),13 cases of non-ApoEε4 gene carriers(B group);and another 15 who were healthy after physical examination at the same period in these two hospitals were enrolled as C group.DTI scanning of the subjects were performed to record the apparent diffusion coefficient(ADC)and fractional anisotropy(FA)values of the bilateral,temporal lobe,hippocampus,parietal lobe,occipital lobe,splenium of corpus callosum,and posterior cingulate gyrus.ResultsThere were no significant differences among the three groups in ADC and FA values of bilateral temporal lobe,parietal lobe,occipital lobe,and posterior cingulated gyrus(P>0.05);the comparison of ADC and FA values of bilateral hippocampus and splenium of corpus callosum among the three groups were significantly different(P<0.05);the ADC values of bilateral hippocampus and splenium of corpus callosum in B and C group were lower than those in A group,ADC value of bilateral hippocampus and splenium of corpus callosum in C group was lower than that in B group;FA value of bilateral hippocampus in B group was higher than that in A group,FA value of bilateral hippocampus and splenium of corpus callosum in C group was higher than that in A and B group(P<0.05).MoCA score of MCI ApoEε4 gene carriers was(20.1±3.2).Correlation analysis showed that ADC values of bilateral hippocampus and splenium of corpus callosum of MCI ApoEε4 gene carriers were positively correlated with MoCA score(P<0.05).ConclusionFrom the perspective of the damage of white matter microstructure,it mainly occurs in bilateral hippocampus and splenium of corpus callosum of MCI patients.Moreover,the changing extent of the damage in white matter microstructure of ApoEε4 gene carriers is more obvious than that of non-ApoEε4 gene carriers,and has correlation with MoCA score.
Mild cognitive impairment;Diffusion tensor imaging;Apolipoprotein E4;Hippocampus;Corpus callosum
新疆维吾尔自治区自然科学技术基金面上项目(2014211C088)
830002新疆乌鲁木齐市,新疆医科大学第二附属医院神经内科(李海涛,哈斯也提·依不来音,朱国峰,马依日木·赛买提,阿依吐拉·卡迪尔,王丹,杨新玲);新疆医科大学附属中医院脑病二科(李桂花)
杨新玲,830002新疆乌鲁木齐市,新疆医科大学第二附属医院神经内科;E-mail:poplar862@sohu.com
R 741
A
10.3969/j.issn.1007-9572.2016.26.010
2016-06-21;
2016-07-29)

