18F-FDG PET/CT显像在胰腺淋巴瘤与胰腺癌鉴别诊断中的价值
任胜男 张建 袁渊 胡胜平 程超 董爱生 左长京
18F-FDG PET/CT显像在胰腺淋巴瘤与胰腺癌鉴别诊断中的价值
任胜男张建袁渊胡胜平程超董爱生左长京
目的评估18F-FDG PET/CT显像在胰腺淋巴瘤(PL)与胰腺癌(PC)鉴别诊断中的价值。方法回顾性分析经病理证实的16例PL患者的18F-FDG PET/CT资料,并与同期随机抽取的32例经病理证实的PC患者进行比较。分析患者的年龄,胰腺病变的位置、直径及最大标准化摄取值(SUVmax),胰管有无扩张,远端胰腺有无萎缩,血清CA19-9有无升高,胰腺外组织侵犯的情况。结果16例PL患者中男性8例,女性8例,平均年龄(46±17)岁,11.1%(1/9)患者血CA19-9升高;32例PC患者中男性15例,女性17例,平均年龄(61±12)岁,81.3%(26/32)患者血CA19-9升高。PL组与PC组的性别分布差异无统计学意义,但PL组的平均年龄较PC组小,血CA19-9升高例数较PC组少,差异均有统计学意义(P值均<0.05)。16例PL中12例为胰腺弥漫大B细胞淋巴瘤,2例为B淋巴母细胞性淋巴瘤/白血病,1例滤泡性淋巴瘤,1例间变大T细胞性淋巴瘤。两组胰腺病灶部位的差异无统计学意义,但PL组胰腺病灶直径显著大于PC组[(6.6±3.3)cm比(4.3±1.8)cm,P=0.038],胰管扩张、远端胰腺组织萎缩例数显著少于PC组(3/16比17/32,1/16比13/32),胰腺病灶SUVmax显著高于PC组(12.0±5.5比8.6±3.8),差异均有统计学意义(P值均<0.05)。SUVmax诊断临界值为9.95,约登指数为0.406,诊断PL的敏感性为68.8%,特异性为71.9%。PL组胰腺外骨、肾脏及脾脏侵犯率显著高于PC组(56.3%比6.25%,43.8%比3.1%,50.0%比6.3%),肝转移率显著低于PC组(12.5%比75.0%),差异均有统计学意义(P值均<0.01)。两组胰腺外其他部位侵犯率的差异无统计学意义。结论相对年轻的患者发现胰腺肿块体积较大,伴有明显脱氧葡萄糖(FDG)摄取,且无明显胰管扩张或远端胰腺组织萎缩,累及骨、肾脏及脾脏而无肝转移者应考虑胰腺淋巴瘤。
淋巴瘤;胰腺肿瘤;体层摄影术,发射型计算机;体层摄影术,X线计算机;脱氧葡萄糖;诊断,鉴别
Fund program:National Natural Science Foundation of China (81471714)
胰腺淋巴瘤(pancreatic lymphoma, PL)可以分为原发性和继发性。原发PL很罕见,在所有胰腺恶性肿瘤中占比不到0.5%,淋巴瘤侵犯胰腺所致的继发性PL较常见[1]。这两种类型PL的临床表现均无特异性,表现为腹痛、体重减轻、黄疸、乏力和发热。在影像学上两种PL均可表现为类似胰腺癌(pancreatic cancer, PC)的肿块[2],但是两者治疗和预后明显不同,因此治疗前对于PL与PC的鉴别诊断非常重要[3]。最近几年18F-FDG PET/CT广泛应用于肿瘤诊断分期和疗效评价,在胰腺肿瘤及淋巴瘤的诊治中发挥了重要作用[4-5]。本研究旨在探讨18F-FDG PET/CT全身显像在PL与PC鉴别诊断中的临床价值。
材料与方法
一、一般资料
回顾性分析2010年8月至2015年3月间在上海长海医院行18F-FDG PET/CT检查,并经病理诊断为PL的16例患者图像,随机抽取同期经病理证实为PC的32例患者作为对照组。入组标准:(1)18F-FDG PET/CT检查发现胰腺病变伴其他组织器官异常摄取;(2)经手术、穿刺活检取得病理或影像随访证实;(3)18F-FDG PET/CT检查与穿刺活检时间相隔不超过2周;(4)18F-FDG PET/CT检查及穿刺活检前均未行放疗、化疗等抗肿瘤治疗。16例PL患者中9例检查血清肿瘤指标CA19-9;32例PC患者均检查血CA19-9。
二、仪器和检查方法
检查前患者禁食6 h以上,空腹血糖<11.1 mmol/L。静脉注射3.70~5.55 MBq/kg体重18F-FDG后患者休息45~60 min,然后行PET/CT常规扫描。采用西门子Biograph trupoint 64层52环HD PET/CT进行图像采集。全身CT扫描使用140 mA电流,120 kV电压,扫描时间为18.67~21.93 s,层厚为3 mm。全身PET扫描覆盖6~7个床位,每个床位扫描时间为2.5 min,扫描范围自颅底至股骨中段。PET和CT图像融合以及后处理在多模式工作台上执行,建立横断位、冠状位、矢状位图像和三维重建图像。
三、图像分析
PL及PC组患者影像资料随机分配给两名有经验的核医学科医师盲法阅片,分别记录胰腺病灶位置(头、颈、体和尾部)及大小;胰腺病灶最大标准化摄取值SUVmax;有无胰管扩张和远端胰腺实质萎缩;其他组织侵犯情况。
四、统计学处理
结 果
一、临床特征及实验室检查
16例PL患者中男性8例,女性8例,年龄19~72岁,平均(46±17)岁;32例PC患者中男性15例,女性17例,年龄29~81,平均(61±12)岁。PL组与PC组的性别分布差异无统计学意义(P>0.05),但PL组的平均年龄较PC组小,差异有统计学意义(t=-3.259,P=0.004)。检测血CA19-9的9例PL患者中仅1例升高(11.1%),32例PC患者中26例升高(81.3%),两组间差异有统计学意义(χ2= 12.407,P=0.001)。
16例PL的病理类型: 12例为胰腺弥漫大B细胞淋巴瘤,2例为B淋巴母细胞性淋巴瘤/白血病,1例滤泡性淋巴瘤,1例间变大T细胞性淋巴瘤。
二、胰腺淋巴瘤和胰腺癌的18F-FDG PET/CT表现
1.胰腺病灶形态学改变:PL位于胰头、颈、体、尾部分别为11、3、9、8例次;PC分别为10、8、14、11例次,两者间差异无统计学意义(P>0.05)。PL组胰腺病灶直径为0.7~11.0 cm,平均(6.6±3.3)cm;PC组胰腺病灶直径为1.8~8.9 cm,平均(4.3±1.8)cm,两组间差异有统计学意义(t=2.221,P=0.038)。PL组3例胰管扩张,1例出现远端胰腺组织的萎缩;PC组17例胰管扩张,13例远端胰腺萎缩,两组间差异均有统计学意义(χ2=5.186,P=0.023;χ2=4.550,P=0.033)。
2.胰腺病灶FDG摄取情况:PL组与PC组胰腺病变均可见FDG异常摄取,PL组的SUVmax范围为4.2~23.9,中位值为12.0±5.5;PC组SUVmax范围为4.0~19.7,中位值为8.6±3.8,两组间差异有统计学意义(P=0.025)。通过ROC曲线计算曲线下面积,确定常规扫描中鉴别PL和PC的SUVmax诊断临界值为9.95,约登指数最大(0.406),鉴别诊断的敏感性为68.8%,特异性为71.9%。
PL组及PC组中均有2例出现弥漫性摄取,其余均为局灶性摄取,两组摄取方式的差异无统计学意义(P>0.05)。
3.胰腺外病变:PL组胰腺外异常FDG摄取部位分别为:淋巴结高摄取13例次(81.3%);多骨侵犯9例次(56.3%);脾脏侵犯8例次(50.0%);肾脏及腹膜侵犯各7例次(43.8%);肾上腺、胃肠道侵犯各5例次(31.3%);附件侵犯3例次(18.8%);肺、胸膜、心包膜、肝脏、乳腺、子宫及肌肉侵犯各2例次(12.5%);脑、鼻咽部、胆囊及前列腺侵犯各1例次(6.3%)。PC组胰腺外异常FDG摄取部位分别为:肝脏侵犯24例次(75.0%);淋巴结高摄取20例次(62.5%);肺侵犯7例次(21.9%);腹膜侵犯5例次(12.5%);肾上腺、胃肠道侵犯各3例次(9.4%);脾脏及多骨侵犯各2例次(6.3%);肾脏、胆囊及附件侵犯各1例次(3.1%)。PL多侵犯骨、肾脏及脾脏(图1),PC多侵犯肝脏(图2),两组间差异有统计学意义(χ2=12.398,P=0.001;χ2=9.919,P=0.002;χ2=9.868,P=0.002;χ2=14.360,P=0.001)。两组间胰腺外其他侵犯部位的差异均无统计学意义(P值均>0.05)。
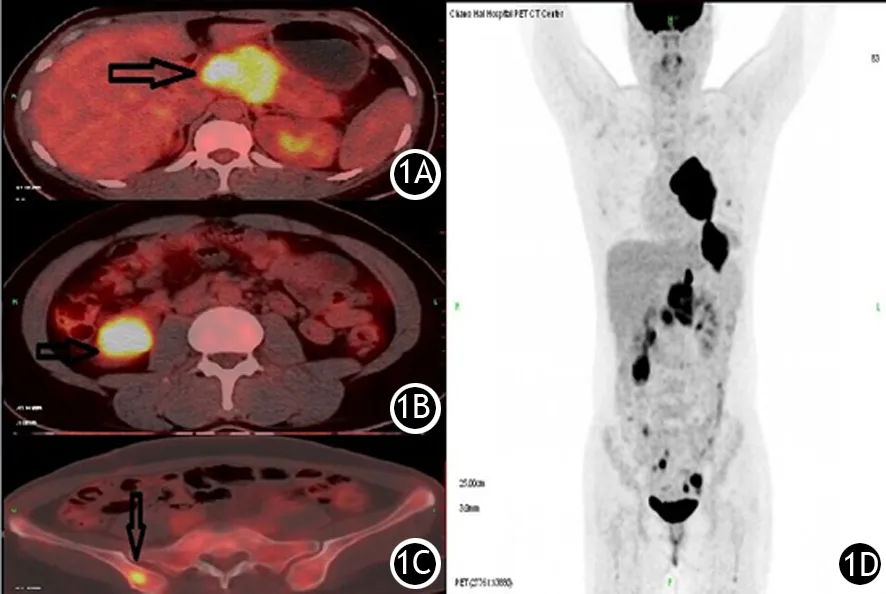
图1 胰腺淋巴瘤患者(女,29岁)18F-FDG PET/CT显像图。1A.胰颈部高代谢肿块;1B.右肾下极高FDG摄取病灶;1C.右髂骨高FDG摄取病灶;1D.PET全身MIP图
讨 论
PL是非上皮源性的胰腺恶性肿瘤,其主要病理学类型是B细胞非霍奇金淋巴瘤(NHL),弥漫大B细胞性淋巴瘤(DLBCL)是其中最常见的类型,其中超过50%的患者可出现淋巴结外侵犯,累及胰腺的比例只有0.6%~2.1%[6]。PC约90%为起源于腺管上皮的导管腺癌,胰腺导管腺癌在胰腺实性恶性肿瘤中约占85%~95%[7]。PL在影像学上可表现为类似PC的肿块,化疗是主要的治疗方法,预后明显不同于PC,因此通过影像学特征鉴别诊断PL与PC有着至关重要的作用。PL在CT上通常表现为向周围侵犯的胰腺肿块,合并后腹膜淋巴结肿大,但这些表现并不能准确地区分于常见的PC。作为全身扫描的影像学检查方法18F-FDG PET可同时发现淋巴结内外病变部位,这是包括增强CT在内的常规检查方法所不能发现的。本研究通过分析PL在18F-FDG PET上的形态学特征及FDG的摄取方式,从而获得与PC鉴别诊断的影像学特征。
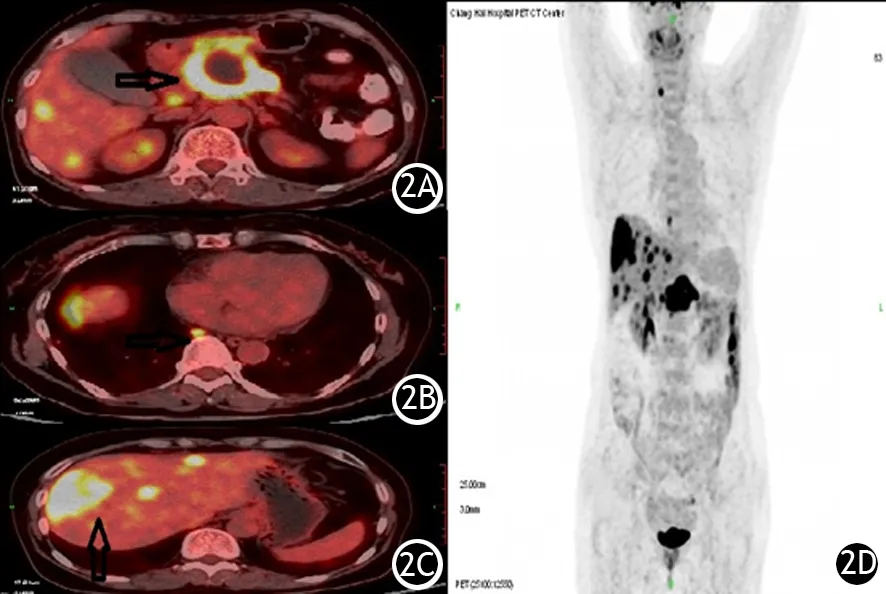
图2 胰腺癌患者(男,58岁)18F-FDG PET/CT显像图。2A.胰颈部高代谢肿块;2B.横膈上淋巴结高FDG摄取病灶;2C.肝脏多发高FDG摄取病灶;2D.PET全身MIP图
本研究结果显示,年龄分布在两组中有明显差异,PL发生于相对年轻人群,与王光宪[7]及Scialpi等[8]研究结果相似。本研究还发现PL胰腺病灶的直径通常大于PC的直径,其他文献[9-11]也报道了相似的结果。可能的原因是PC没有包膜,易侵犯周围血管神经结构,引起胰胆管阻塞,相对容易引起临床症状而被发现。胰管扩张、远端胰腺组织萎缩及CA19-9升高更常见于PC,这与PC多起源于胰腺导管上皮细胞相关,肿瘤容易累及胰腺导管,而PL起源于非导管上皮,质地较软,累及胰管较轻[6]。
18F-FDG PET显像不仅可以提供形态学相关信息,还可以通过FDG摄取反映肿瘤糖酵解代谢率。Abe等[12]和Tummala等[13]研究报道PL与PC均有明显的FDG摄取,这与肿瘤细胞葡萄糖转运蛋白水平升高相关。本研究评估了常规显像中SUVmax在PL与PC诊断中的价值,发现常规扫描PL组SUVmax值比PC组高,这可能与PL肿块体积较大、坏死率低、肿瘤细胞密集相关。SUVmax9.95作为最佳诊断界值时,敏感性和特异性分别为68.8%和71.9%。SUV值与肿瘤的增殖能力、癌细胞分化程度[14]及侵袭性相关[15],不同病理类型的淋巴瘤及胰腺癌SUV摄取有明显差异。但本研究有1例PL的SUVmax值仅为4.2,而PC中发现SUVmax值高达19.7,因此在临床工作中SUVmax值不能作为唯一依据来鉴别诊断PL与PC。
Dong等[16]研究发现PL中胰腺外组织侵犯最常见部位为淋巴结(89%),其次为骨(78%),而未发现肝脏受侵犯。Blastik等[17]对426例PC尸检患者研究发现,PC转移最常见的部位是肝脏(61.3%),其次分别为淋巴结(57.0%)、腹膜(23.7%)、肺及胸膜(22.1%),肾上腺、骨、肾脏及脾脏相对少见。本研究发现PL与PC在胰腺外组织器官侵犯分布上也有差异,骨、肾脏及脾脏侵犯更容易出现在PL中,而肝脏为PC组中最常见转移部位。究其原因可能与PL、PC不同的生物学特性相关,PC容易侵犯门静脉从而引起肝内转移,而PL很少侵犯门静脉。
综上所述,相对于PC患者,PL更常见于相对年轻人群,通常不伴有CA19-9升高,胰腺病灶的体积较大,胰管扩张和远端胰腺萎缩均不常见,FDG摄取明显增高,胰腺外组织器官(骨、肾脏及脾脏)的侵犯较PC常见。
[1]Nayer H, Weir EG, Sheth S, et al. Primary pancreatic lymphomas: a cytopathologic analysis of a rare malignancy[J]. Cancer, 2004,102(5):315-321. DOI: 10.1002/cncr.20488.
[2]Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects[J]. AJR Am J Roentgenol, 2000,174(3):671-675. DOI: 10.2214/ajr.174.3.1740671.
[3]Haji AG, Sharma S, Majeed KA, et al. Primary pancreatic lymphoma: Report of three cases with review of literature[J]. Indian J Med Paediatr Oncol, 2009,30(1):20-23. DOI: 10.4103/0971-5851.56331.
[4]张建, 贾宁阳, 余仲飞, 等. FDG PET与增强CT异机融合图像在胰腺病变良恶性鉴别诊断及胰腺癌分期中的价值[J].中华胰腺病杂志,2014,14(6):374-379.DOI:10.3760/cma.j.issn.1674-1935.2014.06.004.
[5]梁颖, 吴宁, 方艳, 等. 18F-FDG PET/CT显像SUVmax、MTV和TLG判断弥漫性大B细胞淋巴瘤的预后价值[J].中华核医学与分子影像杂志,2015,35(2):97-101.DOI:10.3760/cma.j.issn.2095-2848.2015.02.005.
[6]Fujinaga Y, Lall C, Patel A, et al. MR features of primary and secondary malignant lymphoma of the pancreas: a pictorial review[J]. Insights Imaging, 2013,4(3):321-329. DOI: 10.1007/s13244-013-0242-z.
[7]王光宪, 郭大静, 杨华, 等. 胰腺继发性淋巴瘤的CT诊断[J].临床放射学杂志,2011,30(6):899-901.
[8]Scialpi M, Reginelli A, D′Andrea A, et al. Pancreatic tumors imaging: An update[J]. Int J Surg, 2016,28 Suppl 1:S142-S155. DOI: 10.1016/j.ijsu.2015.12.053.
[9]Leite NP, Kased N, Hanna RF, et al. Cross-sectional imaging of extranodal involvement in abdominopelvic lymphoproliferative malignancies[J]. Radiographics, 2007,27(6):1613-1634. DOI: 10.1148/rg.276065170.
[10]Sata N, Kurogochi A, Endo K, et al. Follicular lymphoma of the pancreas: a case report and proposed new strategies for diagnosis and surgery of benign or low-grade malignant lesions of the head of the pancreas[J]. JOP, 2007,8(1):44-49.
[11]王永超, 李强. 原发性胰腺淋巴瘤的临床分析及与胰腺癌的鉴别[J].中国肿瘤临床,2014,41(2):113-116.DOI:10.3969/j.issn.1000-8179.20131732.
[12]Abe Y, Tamura K, Sakata I, et al. Unique intense uptake demonstrated by (18)F-FDG positron emission tomography/computed tomography in primary pancreatic lymphoma: A case report[J]. Oncol Lett, 2010,1(4):605-607. DOI: 10.3892/ol_00000107.
[13]Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: An overview[J]. J Gastrointest Oncol, 2011,2(3):168-174. DOI: 10.3978/j.issn.2078-6891.2011.036.
[14]李汉华, 王慧玲, 区应亮, 等. PET/CT及肿瘤病理特征与胰头癌手术预后的关系[J].广东医学,2015,36(6):896-898.
[15]胡娜, 吴永港, 肖立志, 等. 18F-FDGPET/CT代谢活性参数及其在淋巴瘤中的应用[J].国际放射医学核医学杂志,2015,39(4):342-347.DOI:10.3760/cma.j.issn.1673-4114.2015.04.015.
[16]Dong A, Cui Y, Gao L, et al. Patterns of FDG uptake in pancreatic non-Hodgkin′s lymphoma lesions[J]. Abdom Imaging, 2014,39(1):175-186. DOI: 10.1007/s00261-013-0041-5.
[17]Blastik M, Plavecz E, Zalatnai A. Pancreatic carcinomas in a 60-year, institute-based autopsy material with special emphasis of metastatic pattern[J]. Pancreas, 2011,40(3):478-480. DOI: 10.1097/MPA.0b013e318205e332.
(本文编辑:屠振兴)
Diagnostic value of18F-FDG PET/CT in differentiating pancreatic lymphoma and pancreatic carcinoma
RenShengnan,ZhangJian,YuanYuan,HuShengping,ChengChao,DongAisheng,ZuoChangjing.
DepartmentofNuclearMedicine,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433,China
Correspondingauthor:ZuoChangjing,Email:changjing.zuo@qq.com
ObjectiveTo evaluate the differential diagnostic value of18F-FDG PET/CT between pancreatic lymphoma(PL) and pancreatic carcinoma(PC). MethodsThe18F-FDG PET-CT data of 16 patients who were pathological diagnosed with PL were retrospectively reviewed and compared with those of 32 consecutive pancreatic cancer patients who were pathologically diagnosed and randomly enrolled. The age, location, diameter and the maximum standard uptake values (SUVmax) of pancreatic lesions, pancreatic ductal dilatation, distal pancreatic atrophy, serum CA19-9 level and extrapancreatic organs involvement were analyzed. ResultsThe 16 patients with PL included 8 men and 8 women, the mean age was (46±17)year,and 11.1%(1/9) patients had elevated CA19-9. The 32 patients with PC included 15 men and 17 women, the mean age was (61±12)year, and 81.3% patients had elevated CA19-9. There were no significant differences on gender between the two groups, while the mean age of PL patients was younger than that of PC, elevated CA19-9 was less common than that in PC, and the differences were statistically significant (allP<0.05). There were 12 cases of diffusive large B cell lymphoma, 2 cases of B lymphoblastic lymphoma /leukaemia, 1 case of follicular lymphoma and 1 case of dysplastic large T cell lymphoma in 16 PL patients. There was no significant difference on the site of pancreatic lesions between the two groups , but long diameter of PL lesions was larger than that of PC [(6.6±3.3)vs(4.3±1.8)cm,P=0.038]. Dilated pancreatic duct and distal parenchyma atrophy in PL were less than those in PC (3/16vs17/32, 1/16vs13/32), and SUVmaxof PL lesions was significantly higher than that of PC (12.0±5.5vs8.6±3.8), indicating that the differences were statistically significant (allP<0.05). The cut-off value of SUVmax was 9.95, and Youden′s index was 0.406 with the sensitivity and specificity of 68.8% and 71.9% for differentiating PL from PC. The incidence of extrapancreatic lesions including bone marrow and kidney and spleen infiltration was significantly more frequent in patients with PL than that in patients with PC(56.3%vs6.25%,43.8%vs3.1%,50.0%vs6.3%), while the incidence of liver metastases was significantly lower than that in PC (12.5%vs5.0%), indicating that the differences were statistically significant (allP<0.01). There were no significant differences on the incidence of other extrapancreatic lesions. ConclusionsPL should be considered in relatively younger patients and manifested as a bulky mass with significant FDG uptake and extrapancreatic involvement of bone, kidney and spleen but without distinct pancreatic ductal dilation or distal parenchymal atrophy or liver metastasis.
Lymphoma;Pancreatic neoplasms;Tomography, emission-computed;Tomography, X-ray computed;Deoxyglucose;Diagnosis, differential
10.3760/cma.j.issn.1674-1935.2016.04.007
200433上海,第二军医大学长海医院核医学科(任胜男、张建、胡胜平、程超、董爱生、左长京),放射科(袁渊)
左长京,Email:changjing.zuo@qq.com
国家自然科学基金(81471714)
2016-02-24)

