pEgr1-Trail重组质粒联合电离辐射对MCF-7细胞杀伤效应的实验研究
曲 莉,赵大力,谢忠伟,刘 斌,李小冬,王 丹,刘 扬,齐亚莉
(1.北华大学公共卫生学院,吉林吉林 132011;2.吉林(市)出入境检验检疫局,吉林吉林 132013;3.吉林大学公共卫生学院,吉林长春 130021)
pEgr1-Trail重组质粒联合电离辐射对MCF-7细胞杀伤效应的实验研究
曲莉1,赵大力2,谢忠伟2,刘斌1,李小冬1,王丹1,刘扬3,齐亚莉1
(1.北华大学公共卫生学院,吉林吉林132011;2.吉林(市)出入境检验检疫局,吉林吉林132013;3.吉林大学公共卫生学院,吉林长春130021)
目的研究pEgr1-Trail重组质粒联合电离辐射对MCF-7细胞的杀伤效应,为增强肿瘤放疗效果提供实验证据.方法应用Annexin V-EGFP/PI双染法检测细胞凋亡;应用酶标仪检测细胞增殖;应用流式细胞仪检测细胞周期.结果不同处理组作用MCF-7细胞后24 h、不同剂量X射线照射后24 h后,各处理组MCF-7细胞的生长抑制率呈上升态势,生长抑制由弱到强的顺序为:2.0Gy,pEgr1-Trail+0.5Gy,5.0Gy,pEgr1-Trail+1.0Gy,pEgr1-Trail+2.0Gy,Egr1-Trail+5.0Gy.各处理组联合2.0Gy X射线照射后6 h,细胞凋亡率开始上升,48 h达高峰,其中pEgr1-Trail+2.0Gy组与其他各处理组比较差异具有统计学意义,且细胞凋亡最为显著(P<0.05).在细胞周期方面,2.0Gy X射线照后6 h各处理组S期细胞百分数呈现上升趋势,24 h达高峰;照后24 h,G2+M期细胞百分数开始上升,且各处理组比较差异均具有统计学意义(P<0.05);照后48 h,G2+M期细胞百分数达到最高,依旧是pEgr1-Trail+2.0Gy组上升最显著.结论pEgr1-Trail重组质粒联合电离辐射作用于肿瘤细胞表现出协同杀伤效应,促凋亡作用强于单独X射照射组或pEgr1-Trail基因干预组.
pEgr1-Trail重组质粒;电离辐射;MCF-7细胞;杀伤效应
【引用格式】曲莉,赵大力,谢忠伟,等.pEgr1-Trail重组质粒联合电离辐射对MCF-7细胞杀伤效应的实验研究[J].北华大学学报(自然科学版),2016,17(3):344-348.
肿瘤治疗主要以手术、放疗、化疗以及基因治疗为主.目前,多种方法联合使用已成为肿瘤治疗的主要手段[1].肿瘤的基因-放射治疗既能提高肿瘤的辐射敏感性,又能减少正常组织的放射损伤,因而成为肿瘤治疗的新思路[2].本研究利用Egr-1启动子能够诱导Trail基因表达增强以及Trail基因对肿瘤的促凋亡作用,将放射治疗和基因治疗结合,探讨 pEgr1-Trail重组质粒联合电离辐射对MCF-7细胞杀伤效应的影响.
1 材料与方法
1.1细胞系和主要试剂
pEgr1-Trail重组质粒由吉林大学公共卫生学院放射卫生教研室刘扬博士惠赠,MCF-7细胞为本研究室保存.用含10%FBS的高糖DMEM培养基在37℃,5%CO2培养箱中培养,当MCF-7细胞融合度达到70%左右,使用0.25%的胰酶消化传代培养. Annexin V/PI凋亡检测试剂盒(南京凯基);碘化丙啶(美国Sigma);DMEM培养基(美国Gibco).
1.2照射条件
医用X射线深部治疗机(丹东市康佳医用机厂),其电压为180 kV,电流为20mA,滤板为0.25 mm Cu和1.0mm Al,剂量率为0.41Gy/min.
1.3细胞增殖的检测
使用酶标仪检测各处理组细胞增殖情况.将MCF-7细胞接种于96孔板,每孔大约接种6×104个细胞,设6个复孔,培养12 h后,使用不同浓度pEgr1-Trail重组质粒转染MCF-7细胞,24 h后给予2.0Gy X照射,并于照后6,12,24和48 h每孔加入10μLCCK-8试剂,培养2 h后,使用酶标仪(A490)检测各孔吸光度值.
1.4细胞凋亡的检测
Annexin V-EGFP/PI双染法检测细胞凋亡.将MCF-7细胞接种于24孔培养板,每孔5.0×105个细胞,设6个复孔,不同浓度pEgr1-Trail重组质粒转染MCF-7细胞,培养24 h,再经2.0 Gy X线照射后,分别于6,12,24和48 h收集细胞,并给予相应的处理:1)1 500 r/min离心3 min;2)0.01 mol/L PBS洗涤2次;3)800μL缓冲液重悬细胞沉淀;4)加入Annexin V-EGFP 6μL+PI 6μL;5)混匀后室温避光保存15min.分别收集1.0×104个细胞,2 h内使用流式细胞仪检测不同处理组细胞凋亡情况,应用CellQuest系统软件进行数据处理,用凋亡细胞百分率描述分析结果.
1.5细胞周期的检测
使用24孔培养板接种MCF-7细胞,细胞密度为每孔5.0×105个,设6个复孔.不同浓度pEgr1-Trail重组质粒转染MCF-7细胞,在培养箱中培养24 h,再经2.0Gy X射线照射,分别于照射后6,12,24和48 h收集细胞,离心、清洗细胞步骤同细胞凋亡的检测.最后,分别加入RNAse A 100μL和PI 200μL,混匀后室温避光保存20min,分别收集1.0 ×104个细胞,2 h内流式细胞仪检测各期细胞百分率,并使用ModFit软件分析数据.
2 结 果
2.1pEgr1-Trail重组质粒对MCF-7细胞生长的影响
以不同浓度的pEgr1-Trail重组质粒转染MCF-7细胞,分别于转染12,24和48 h后使用酶标仪检测其吸光度值.结果表明:同一时点,随着pEgr1-Trail重组质粒浓度的增加,细胞生长被抑制,并呈现出剂量反应关系.即剂量10 mol重组质粒转染MCF-7细胞培养48 h后,检测到MCF-7细胞的生长受到抑制.随着重组质粒浓度的升高与0mol组比较,差异具有统计学意义(P<0.05或P<0.01);随着时间的延长,细胞生长亦受到抑制.见表1.
表1 MCF-7细胞转染不同浓度pEgr1-Trail重组质粒后吸光度值变化Tab.1 Absorbance value changes of MCF-7 cells after being infected by different concentrations of pEgr1-Trail recombinant plasm id (n=6,±s)
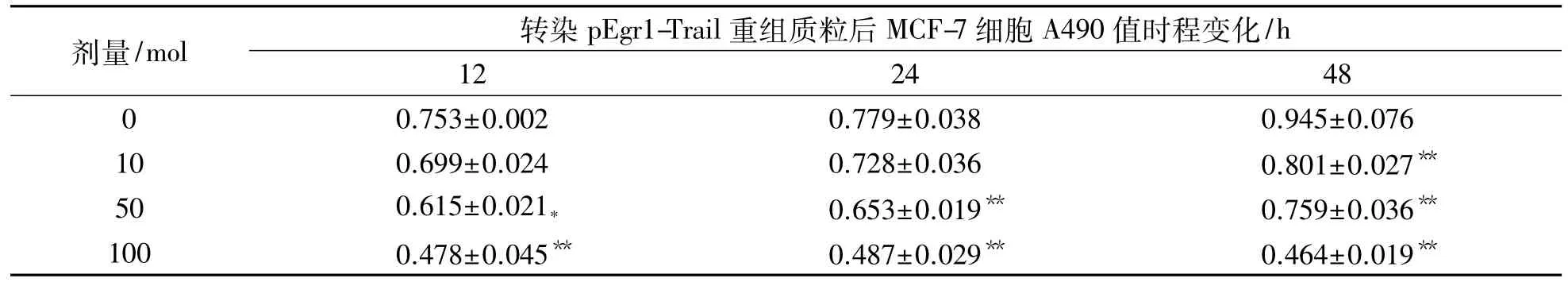
表1 MCF-7细胞转染不同浓度pEgr1-Trail重组质粒后吸光度值变化Tab.1 Absorbance value changes of MCF-7 cells after being infected by different concentrations of pEgr1-Trail recombinant plasm id (n=6,±s)
注:与0mol组比较,*:P<0.05,**:P<0.01
剂量/mol 转染pEgr1-Trail重组质粒后MCF-7细胞A490值时程变化/h 12 24 48 0 0.753±0.002 0.779±0.038 0.945±0.076 10 0.699±0.024 0.728±0.036 0.801±0.027**50 0.615±0.021* 0.653±0.019** 0.759±0.036**100 0.478±0.045** 0.487±0.029** 0.464±0.019**
2.2不同照射剂量各组吸光度值变化
以10mol重组质粒转染MCF-7细胞,培养24 h后分别给予不同剂量X射线照射,再培养24 h,采用酶标仪检测 A490值.结果提示:各处理组MCF-7细胞生长受到抑制,并呈现剂量反应关系. pEgr1-Trail组从1.0Gy开始与对照组和空质粒组比较差异均具有统计学意义(P<0.01或P<0.05).见表2.
表2 不同照射剂量MCF-7细胞各处理组吸光度值变化Tab.2 Absorbance value changes of MCF-7 cells after exposing under different dosages of irradiation (n=6,±s)
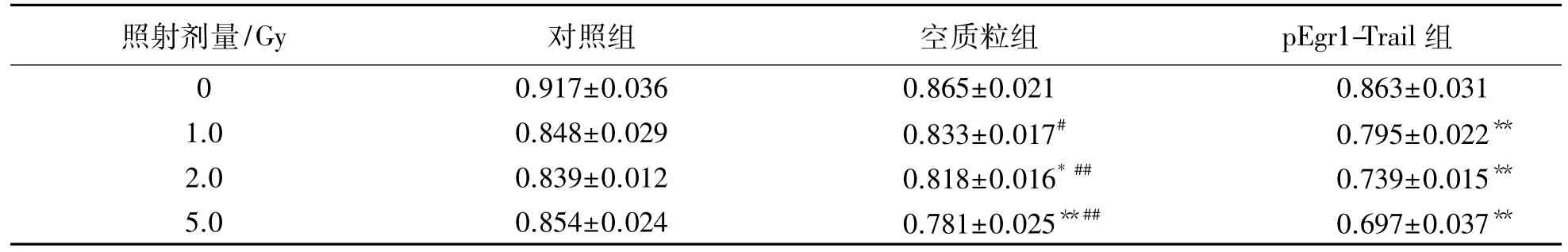
表2 不同照射剂量MCF-7细胞各处理组吸光度值变化Tab.2 Absorbance value changes of MCF-7 cells after exposing under different dosages of irradiation (n=6,±s)
注:与对照组比较,*:P<0.05,**:P<0.01;与pEgr1-Trail组比较,#:P<0.05,##:P<0.01
照射剂量/Gy 对照组 空质粒组 pEgr1-Trail组0 0.917±0.036 0.865±0.021 0.863±0.031 1.0 0.848±0.029 0.833±0.017# 0.795±0.022**2.0 0.839±0.012 0.818±0.016*## 0.739±0.015**5.0 0.854±0.024 0.781±0.025**## 0.697±0.037**
2.3重组质粒联合2.0 Gy X射线照射对MCF-7细胞生长抑制的时程效应
以10mol重组质粒转染MCF-7细胞,培养24 h后给予2.0Gy X射线照射,分别应用CCK-8试剂盒,于照后6,12,24和48 h使用酶标仪检测其吸光度值.结果表明:2.0Gy X线照射后,重组质粒转染的各处理组细胞生长均受到抑制,具有时程量效关系.与其他各组比较,pEgr1-Trail+2.0 Gy组细胞生长抑制程度最为明显,且差异具有统计学意义(P<0.05或P<0.01).见表3.
表3 2.0Gy X线照射后各不同处理组MCF-7细胞吸光度值时程变化Tab.3 Absorbance-time value changes of MCF-7 cells after 2.0Gy X-ray irradiation in different treatment groups (n=6,±s)
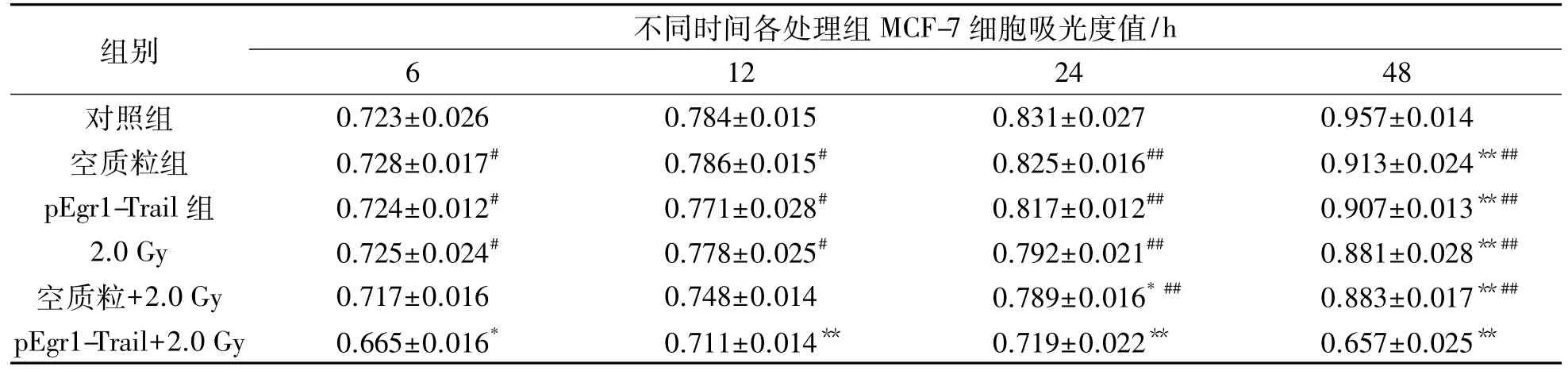
表3 2.0Gy X线照射后各不同处理组MCF-7细胞吸光度值时程变化Tab.3 Absorbance-time value changes of MCF-7 cells after 2.0Gy X-ray irradiation in different treatment groups (n=6,±s)
注:与对照组比较,*:P<0.05,**:P<0.01;与pEgr1-Trail+2.0Gy组比较,#:P<0.05,##:P<0.01
组别 不同时间各处理组MCF-7细胞吸光度值/h 6 12 24 48对照组 0.723±0.026 0.784±0.015 0.831±0.027 0.957±0.014空质粒组 0.728±0.017# 0.786±0.015# 0.825±0.016## 0.913±0.024**##pEgr1-Trail组 0.724±0.012# 0.771±0.028# 0.817±0.012## 0.907±0.013**##2.0Gy 0.725±0.024# 0.778±0.025# 0.792±0.021## 0.881±0.028**##空质粒+2.0Gy 0.717±0.016 0.748±0.014 0.789±0.016*## 0.883±0.017**##pEgr1-Trail+2.0Gy 0.665±0.016* 0.711±0.014** 0.719±0.022** 0.657±0.025**
2.4重组质粒联合2.0 Gy X射线照射对MCF-7细胞凋亡的作用
以10mol重组质粒转染MCF-7细胞,经培养箱培养24 h后,给予2.0Gy X射线照射,分别于照射后6,12,24和48 h收集细胞,经Annexin V-EGFP和PI双荧光染色,流式细胞仪分析细胞凋亡情况.结果表明:照射后6 h各处理组细胞开始出现凋亡,48 h达到峰值,其中以pEgr1-Trail+2.0Gy组细胞凋亡最明显,与其他各处理组比较差异具有统计学意义(P<0. 05或P<0.01).见表4.
表4 2.0Gy X线照射后各不同处理组MCF-7细胞凋亡变化Tab.4 Apoptosis changes of MCF-7 cells after 2.0Gy X-ray irradiation in different treatment groups (n=6,±s)
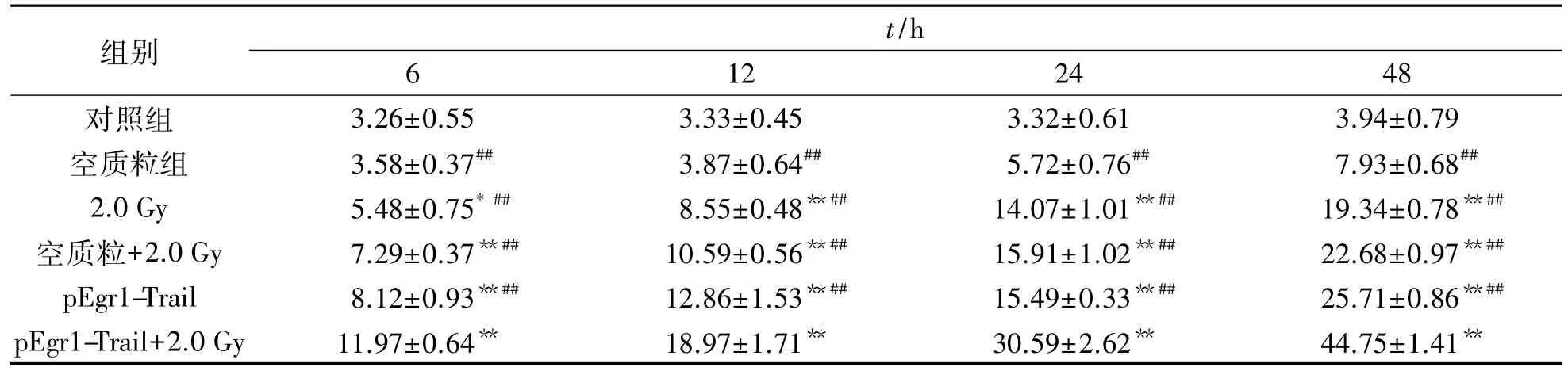
表4 2.0Gy X线照射后各不同处理组MCF-7细胞凋亡变化Tab.4 Apoptosis changes of MCF-7 cells after 2.0Gy X-ray irradiation in different treatment groups (n=6,±s)
注:与对照组比较,*:P<0.05,**:P<0.01;与pEgr1-Trail+2.0Gy组比较,##:P<0.01
组别t/h 6 12 24 48对照组 3.26±0.55 3.33±0.45 3.32±0.61 3.94±0.79空质粒组 3.58±0.37## 3.87±0.64## 5.72±0.76## 7.93±0.68##2.0Gy 5.48±0.75*## 8.55±0.48**## 14.07±1.01**## 19.34±0.78**##空质粒+2.0Gy 7.29±0.37**## 10.59±0.56**## 15.91±1.02**## 22.68±0.97**##pEgr1-Trail 8.12±0.93**## 12.86±1.53**## 15.49±0.33**## 25.71±0.86**##pEgr1-Trail+2.0 Gy 11.97±0.64** 18.97±1.71** 30.59±2.62** 44.75±1.41**
2.5重组质粒联合2.0 Gy X射线照射对MCF-7细胞周期进程的作用
以10mol重组质粒转染MCF-7细胞,在培养箱中培养24 h后,再经2.0Gy X射线照射处理,并于照射后6,12,24和48 h收集细胞,经PI单荧光染色,采用流式细胞仪分析细胞周期的变化.结果表明:照射后6 h各处理组细胞S期百分数开始增加,12 h后达到峰值水平(P<0.01),24 h后G2+M期细胞百分数开始增加,除空质粒组外,其余各组与对照组比较差异均具有统计学意义(P<0.01),48 h后G2+M期细胞百分数达到最高,以pEgr1-Trail+2.0Gy组增加最明显,且差异具有统计学意义(P<0.01).见表5.
表5 2.0Gy X线照射后各不同处理组MCF-7细胞周期变化Tab.5 Cell cycle changes of MCF-7 cells after 2.0Gy X-ray irradiation in different treatment groups (n=6,±s)
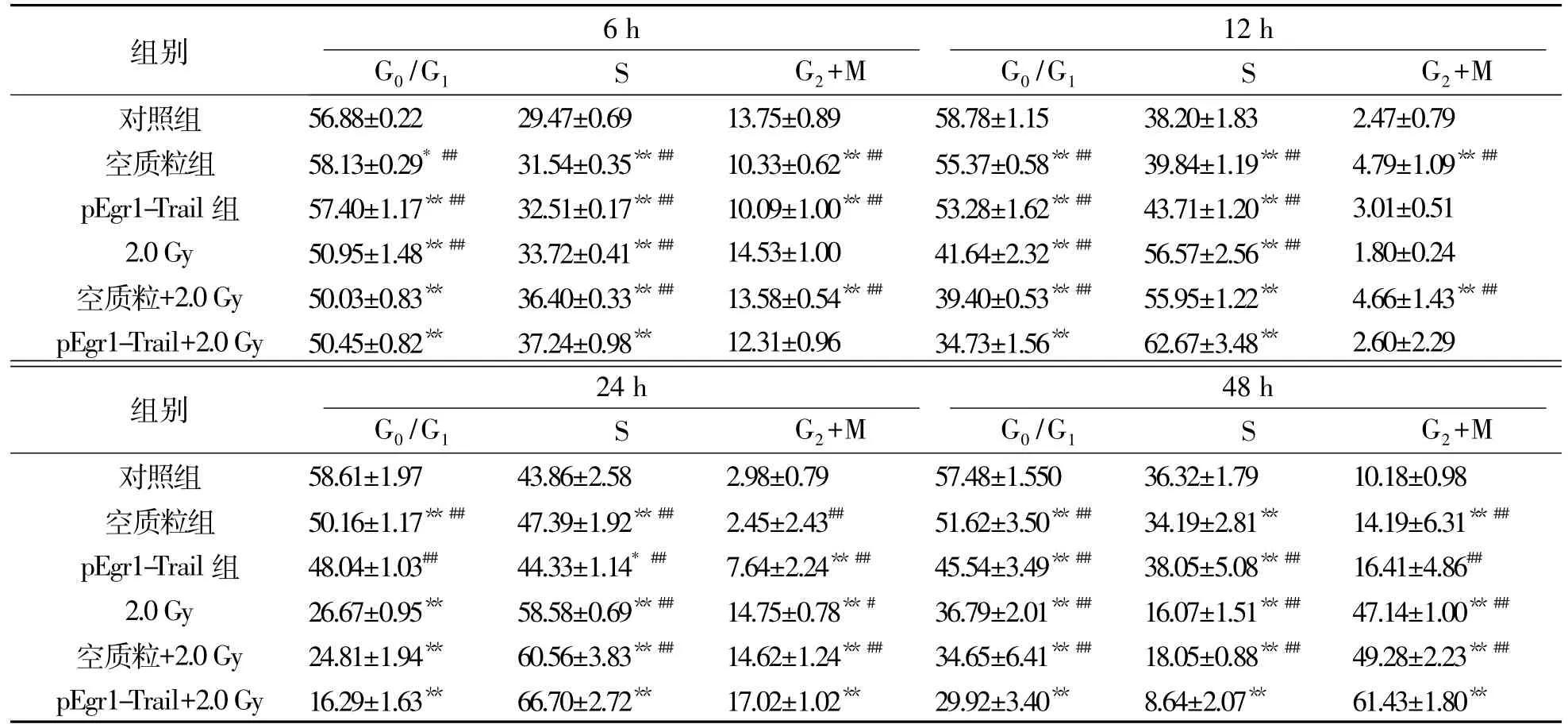
表5 2.0Gy X线照射后各不同处理组MCF-7细胞周期变化Tab.5 Cell cycle changes of MCF-7 cells after 2.0Gy X-ray irradiation in different treatment groups (n=6,±s)
注:与对照组比较,*:P<0.05,**:P<0.01;与pEgr1-Trail+2.0 Gy组比较,##:P<0.01
组别 6 h 12 h G0/G1 S G2+M G0/G1 S G2+M对照组 56.88±0.22 29.47±0.69 13.75±0.89 58.78±1.15 38.20±1.83 2.47±0.79空质粒组 58.13±0.29*## 31.54±0.35**## 10.33±0.62**## 55.37±0.58**## 39.84±1.19**## 4.79±1.09**##pEgr1-Trail组 57.40±1.17**## 32.51±0.17**## 10.09±1.00**## 53.28±1.62**## 43.71±1.20**## 3.01±0.51 2.0Gy 50.95±1.48**## 33.72±0.41**## 14.53±1.00 41.64±2.32**## 56.57±2.56**## 1.80±0.24空质粒+2.0Gy 50.03±0.83** 36.40±0.33**## 13.58±0.54**## 39.40±0.53**## 55.95±1.22** 4.66±1.43**##pEgr1-Trail+2.0Gy 50.45±0.82** 37.24±0.98** 12.31±0.96 34.73±1.56** 62.67±3.48** 2.60±2.29组别 24 h 48 h G0/G1 S G2+M G0/G1 S G2+M对照组 58.61±1.97 43.86±2.58 2.98±0.79 57.48±1.550 36.32±1.79 10.18±0.98空质粒组 50.16±1.17**## 47.39±1.92**## 2.45±2.43## 51.62±3.50**## 34.19±2.81** 14.19±6.31**##pEgr1-Trail组 48.04±1.03## 44.33±1.14*## 7.64±2.24**## 45.54±3.49**## 38.05±5.08**## 16.41±4.86##2.0Gy 26.67±0.95** 58.58±0.69**## 14.75±0.78**# 36.79±2.01**## 16.07±1.51**## 47.14±1.00**##空质粒+2.0Gy 24.81±1.94** 60.56±3.83**## 14.62±1.24**## 34.65±6.41**## 18.05±0.88**## 49.28±2.23**##pEgr1-Trail+2.0Gy 16.29±1.63** 66.70±2.72** 17.02±1.02** 29.92±3.40** 8.64±2.07** 61.43±1.80**
3 讨 论
3.1重组质粒联合电离辐射对MCF-7细胞杀伤的协同作用
10mol重组质粒转染MCF-7细胞后,在培养箱培养24 h后,分别给予不同剂量X射线照射,各处理组MCF-7细胞随着照射剂量的增加,其生长抑制率呈现上升趋势,以pEgr1-Trail组最明显.另外,pEgr1-Trail联合2.0Gy X射线照射对MCF-7细胞生长抑制的时程效应,也说明重组质粒联合电离辐射具有杀伤肿瘤细胞的协同作用.本实验结果与相关文献报道一致[3].
3.2重组质粒联合电离辐射对MCF-7细胞凋亡的影响
本实验用pEgr1-Trail联合2.0Gy X射线照射,观察MCF-7细胞凋亡情况.实验结果提示:MCF-7细胞经照射后6 h,各处理组细胞凋亡率开始增加,48 h达高峰,pEgr1-Trail+2.0Gy组与其他各处理组比较差异最显著.实验结果提示:电离辐射联合重组质粒组的促凋亡作用强于单独X线照射组,此结果与相关报道[4-6]一致;Egr-1可调控Trail基因,促进肿瘤细胞凋亡,同时与电离辐射联合作用可发挥协同杀伤效应.
3.3重组质粒联合电离辐射对MCF-7细胞周期各时相的影响
流式细胞仪分析了不同处理组MCF-7细胞周期各时相的情况.经2.0Gy X射线照射6 h后,各处理组细胞S期百分数开始增高,12 h达到峰值;24 h后G2+M期细胞百分数开始上升,48 h达高峰,其中以pEgr1-Trail+2.0 Gy组变化最明显.以上结果表明:各处理组MCF-7细胞周期以S和G2+M期阻滞为主,G0/G1期阻滞不显著;与前面的实验结果相似,依然是重组质粒联合2.0Gy X射线照射组导致S和G2+M期阻滞最显著,这可能与MCF-7细胞内p53的状态有关,突变的p53不能引起细胞G0/G1期阻滞[7-8].
Trail作为肿瘤坏死因子超家族成员之一,是肿瘤坏死因子相关凋亡诱导配体,在调节细胞死亡、免疫反应和炎症等方面发挥着重要作用.上述实验结果证实了Trail基因的肿瘤杀伤效应,以及联合放疗而产生的协同杀伤作用.利用Egr-1作为启动子,并调控Trail基因表达,构建辐射诱导表达载体,可提高肿瘤基因-放射治疗的效果,已成为肿瘤治疗的新方法和新思路.
[1]A l-Khami A A,Mehrotra S,Nishimura M I.Adoptive immunotherapyof cancer:Genetransfer of Tcell specificity[J].Self Nonself,2011,2(2):80-84.
[2]Desilet N,Campbell T N,Choy F Y.p53-based anticancer therapies:An empty promise?[J].Curr Issues Mol Biol,2010,12(3):143-146.
[3]Lim L Y,Vidnovic N,Ellisen L W.Mutant p53 mediates survival of breast cancer cells[J].Curr Issues Mol Biol,2009,12(3):143-146.
[4]He X,Liu J,Yang C,et al.5/35 fiber-modified conditionally replicative adenovirus armed with p53 shows increased tumor-suppressing capacity to breast cancer cells[J].Hum Gene Ther,2011,22(3):283-292.
[5]Zhang Y,Shi Y,Li X,et al.Inhibition of the p53-MDM2 interaction by adenovirus delivery of ribosomal protein L23 stabilizes p53 and induces cell cycle arrest and apoptosis in gastric cancer[J].J Gene Med,2010,12 (2):147-156.
[6]Li Y,Li L J,Zhang ST,et al.In vitro and clinical studies of gene therapy with recombinant human adenovirus-p53 injection for oral leukoplakia[J].Clin Cancer Res,2009,15(21):6724-6731.
[7]Idogawa M,Sasaki Y,SuzukiI H,et al.A single recombinant adenovirus expressing p53 and p21-targeting artificial microRNAs efficientlyinduces apoptosis in human cancer cells[J].Clin Cancer Res,2009,15(11): 3725-3732.
[8]Song X,Varker H,Elchelbaum M,et al.Treatment of lung cancer patients and concomitant use of drugs interacting with cytochrome P450 isoenzymes[J].Lung Cancer,2011,74(1):103-111.
【责任编辑:陈丽华】
Experimental Research on Killing Effects of pEgr1-Trail Recombinant Plasmid Combined with Ionizing Radiation on MCF-7 Cell
Qu Li1,Zhao Dali2,Xie Zhongwei2,Liu Bin1,Li Xiaodong1,Wang Dan1,Liu Yang3,Qi Yali1
( 1.Public Health College of Beihua University,Jilin 132011,China; 2.Jilin Province ( City) Entry-Exit Inspection and Quarantine Bureau,Jilin 132013,China; 3.School of Public Health,Jilin University,Changchun 130021,China)
Objective To study the killing effects of pEgr1-Trail recombinant plasmid combined with ionizing radiation on MCF-7 cell,and to provide experimental evidence for improving curative effects of radiotherapy for tumor. Method Annexin V-EGFP /PI was used to detect cell apoptosis,cell proliferation was detected by ELIASA,and cell cycle was determined by flow cytometer.Results After being irradiated for 24 h by differentdoses of X-ray,the growth inhibition rates of MCF-7 cell in different treatment groups all increased with the order of 2.0 Gy,pEgr1 Trail+0.5 Gy,5.0 Gy,pEgr1-Trail+1.0 Gy,pEgr1-Trail+2.0 Gy,pEgr1-Trail+5.0 Gy.Compared with other groups,PEgr1-Trail group had significant difference ( P<0.05) . In the combination of 2.0 Gy X-ray irradiation for 6 h,apoptosis rates of each group started to rise,and reached peak at 48 h.Compared with other treatment group,cell apoptosis of pEgr1-Trail +2.0 Gy group was the most obvious ( P<0.05) .As for cell cycle, compared with the control group,cell percentage in S phase of each treatment group began to rise after being irradiated by 2.0 Gy X-ray for 6 h,and got to peak at 12 h ( P<0.05) .After 24 h,cell percentages in G2+M phase began to rise,and the differences among different treatment groups were all significant ( P<0.05) .After 48 h,cell percentages in G2+M phase were the highest,and the percentage was aslo the most obvious in pEgr1-Trail+2.0 Gy group.Conclusion PEgr1-Trail recombinant plasmid combined with ionizing radiation exhibit synergistic effect on killing tumour cells,their synergistic effect on promoting apoptosis is better than the single use of X-ray irradiation or gene interference.
recombinant plasmid;ionizing radiation;MCF-7 cells;killing effects
R730
A
1009-4822(2016)03-0344-05
10.11713/j.issn.1009-4822.2016.03.013
2015-10-12
吉林省教育厅科学技术研究项目(2014192).
曲莉(1976-),女,硕士,讲师,主要从事肿瘤毒理学研究,E-mail:418997433@qq.com;通信作者:齐亚莉(1973-),女,博士,副教授,硕士生导师,主要从事肿瘤的放射基因治疗和流行病学研究,E-mail:374494617@qq.com.

