ConA重复给药建立ACLF免疫状态动态转变的小鼠模型*
张楠楠 杨淑殷 陈柳莹 尹 珊 王十锦 刘三海 王蓓蓓,3 汪 铮 李 海#
上海交通大学医学院附属仁济医院消化科 上海市消化疾病研究所1(200001) 首都医科大学附属北京地坛医院传染病研究所2 新发突发传染病研究北京市重点实验室3
·论著·
ConA重复给药建立ACLF免疫状态动态转变的小鼠模型*
张楠楠1杨淑殷1陈柳莹1尹珊1王十锦1刘三海2王蓓蓓2,3汪铮1李海1#
上海交通大学医学院附属仁济医院消化科上海市消化疾病研究所1(200001) 首都医科大学附属北京地坛医院传染病研究所2新发突发传染病研究北京市重点实验室3
背景:慢加急性肝衰竭(ACLF)是我国常见的肝衰竭类型,目前尚缺乏能有效模拟ACLF免疫状态动态转变的动物模型。目的:通过刀豆球蛋白A(ConA)重复给药,建立模拟ACLF免疫状态动态转变的动物模型。方法:小鼠随机分为对照组和ConA重复给药组,ConA重复给药组小鼠给予球后内眦静脉丛注射ConA 15 mg/kg,每隔48 h一次,共5次,对照组给予等体积0.9%NaCl溶液。CBA法检测外周血IL-6、IL-10、IL-12、TNF-α、IFN-γ、MCP-1水平,并测定IL-10/TNF-α比值;流式细胞术检测外周血中单核细胞HLA-DR表达、CD4+T细胞数量及其比例以及PD-1表达。结果:随着给药次数增加,ConA重复给药组小鼠外周血细胞因子从促炎细胞因子为主转变成抗炎细胞因子为主。与对照组相比,ConA重复给药组外周血中单核细胞HLA-DR表达下降(P<0.05);CD4+T细胞数量和比例下降(P<0.05),PD-1表达上调(P<0.05)。结论:本研究通过ConA重复刺激成功建立了模拟ACLF免疫状态从全身炎症反应综合征(SIRS)到代偿性抗炎反应综合征(CARS)动态转变的动物模型。
关键词慢加急性肝衰竭;伴刀豆球蛋白A;免疫状态;细胞因子类;模型,动物
Establishment of Model of Dynamic Change of Immune Status of ACLF Induced by ConA Repeated Administration in MiceZHANGNannan1,YANGShuyin1,CHENLiuying1,YINShan1,WANGShijin1,LIUSanhai2,WANGBeibei2,3,WANGZheng1,LIHai1.1DivisionofGastroenterologyandHepatology,RenJiHospital,SchoolofMedicine,ShanghaiJiaoTongUniversity;ShanghaiInstituteofDigestiveDisease,Shanghai(200001);2InstituteofInfectiousDiseases,BeijingDitanHospital,CapitalMedicalUniversity,Beijing;3BeijingKeyLaboratoryofEmergingInfectiousDiseases,Beijing
Correspondence to: LI Hai, Email: haili_17@126.com
慢加急性肝衰竭(ACLF)是临床常见的肝衰竭类型,晚期常合并多脏器功能衰竭,死亡率可高达30%~50%[1]。东方ACLF的病因以乙型肝炎感染为主,西方则以酒精性肝炎为基础。ACLF的急性诱因包括感染、过度饮酒、外科手术、肝脏毒性药物等[2-4]。尽管东西方ACLF的病因和急性诱因不一,但引起机体免疫应答和免疫演变的过程大致相同,主要表现为急性肝损伤诱因导致的机体早期免疫亢进即全身炎症反应综合征(SIRS),逐渐演变为以抗炎细胞因子白细胞介素(IL)-10为主的代偿性抗炎反应综合征(CARS)[4-8]。
2014年世界胃肠病组织工作小组提出了ACLF的“优先和次优先”研究主题,其中发展有效的动物实验模型以深入研究ACLF病理生理机制和研制可能的治疗药物作为三大优先主题之一,因此建立模拟ACLF动态免疫演变的动物模型是深入阐明该疾病的关键切入点。本研究通过给予小鼠刀豆球蛋白A(ConA)重复刺激,旨在建立反复急性肝损伤诱导的ACLF免疫状态动态演变的动物模型。
材料与方法
一、实验动物、主要试剂和仪器
1. 实验动物:SPF级6~8周龄C57BL/6雄性小鼠20只,体质量20~23 g,购于中国医学科学院医学实验动物研究所。给予小鼠清洁饮食,昼夜规律光照。
2. 主要试剂和仪器:ConA购于美国Sigma公司;低温多用途离心机购于德国Thermo公司;小鼠CBA试剂盒、抗小鼠CD45-FITC抗体、抗小鼠NK1.1-PE、抗小鼠CD3-PerCP抗体、抗小鼠CD4-APC抗体、抗小鼠CD11b-FITC抗体、抗小鼠Gr-1 PE抗体、抗小鼠MHC-Ⅱ-PerCP抗体、抗小鼠CD48-APC、抗小鼠PD-1-FITC抗体、绝对细胞计数管、流式细胞仪(BD FACS Calibur)购于美国BD公司。
二、实验方法
1. 模型制备:小鼠随机分为对照组和ConA重复给药组,每组各10只。ConA重复给药组小鼠给予球后内眦静脉丛注射ConA 15 mg/kg,每隔48 h一次,共5次,建立ConA重复给药模型。对照组给予等体积0.9% NaCl溶液。
2. 细胞因子的检测:每次给予ConA或0.9% NaCl溶液后3 h眼球取血,2 000 r/min离心 20 min,分离血清。根据CBA试剂盒说明书,通过流式细胞仪检测IL-6、IL-10、IL-12、TNF-α、IFN-γ、单核细胞趋化蛋白(MCP)-1水平。
3. 单核细胞HLA-DR表达、CD4+T细胞数量和程序性死亡受体(PD)-1表达的检测:ConA末次刺激后48 h取小鼠外周血,制成单细胞悬液,应用流式细胞仪检测单核细胞HLA-DR表达、CD4+T细胞数量和PD-1表达。
三、统计学分析

结果
一、促炎/抑炎细胞因子的动态变化过程
第1次ConA刺激后,促炎细胞因子TNF-α(P<0.000 1)水平、IL-12(P=0.015 2)、IFN-γ(P=0.001 8)、MCP-1(P<0.000 1)、IL-6(P=0.000 2)较刺激前显著升高,抗炎细胞因子IL-10水平显著升高(P=0.002 3);第5次ConA刺激后,TNF-α、IL-12、IFN-γ、MCP-1、IL-6水平均由峰值明显降低,而IL-10继续维持高水平(图1A)。给予ConA刺激后,IL-10/TNF-α比值持续升高(图1B)。说明随着ConA刺激次数增加,小鼠的主导细胞因子由第1次ConA刺激后以促炎细胞因子为主导的SIRS转变为第5次ConA刺激后以抑炎细胞因子IL-10为主导的CARS阶段。
二、HLA-DR表达
ConA第5次给药后,外周血单核细胞HLA-DR表达较对照组明显下降(平均荧光强度:3.56对11.76),差异有统计学意义(P<0.000 1)(图2、图3)。说明ConA重复刺激后机体处于先天性免疫系统受损状态。
三、外周血CD4+T细胞数量和PD-1表达
与对照组相比,ConA第5次刺激后,小鼠外周血CD4+T细胞数量明显下降[(1 417±1 386)/mL对(1 986±323)/mL,P=0.350 5],CD4+T细胞比例亦明显下降(33.69%对59.97%,P<0.000 1)(图3)。说 明ConA重复刺激后小鼠适应性免疫系统受损。
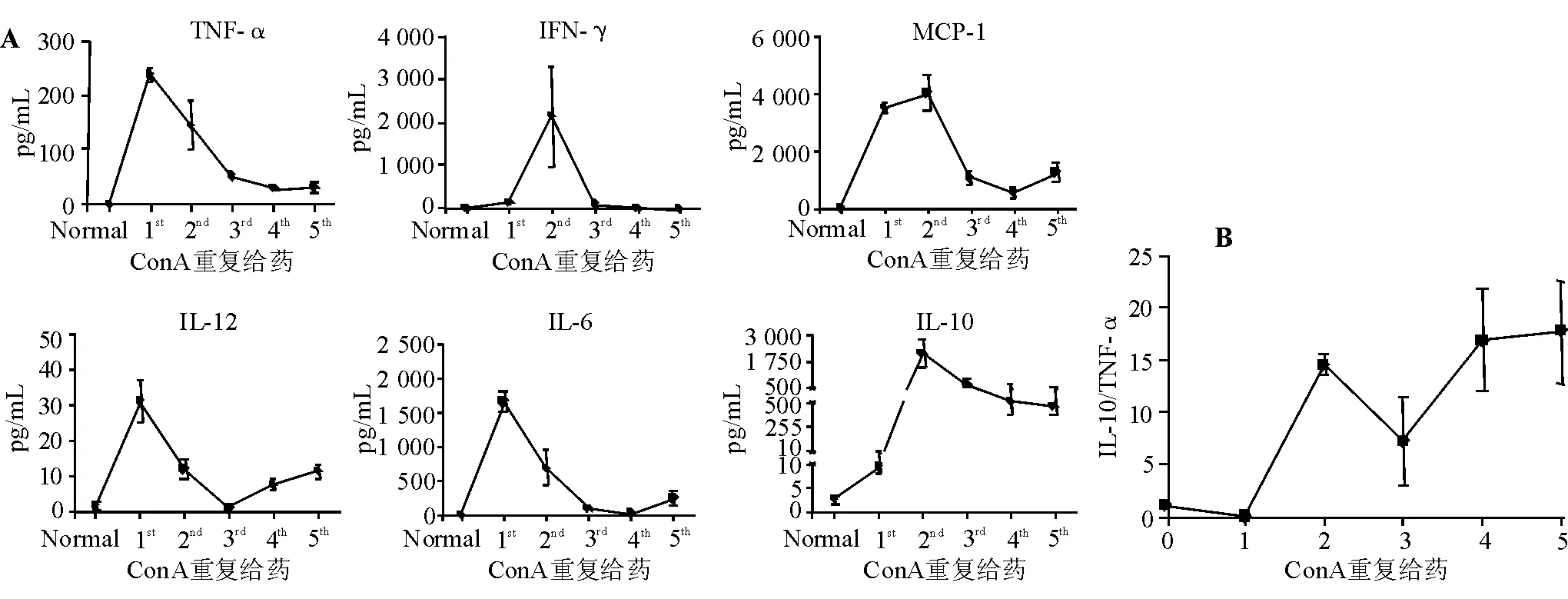
A:外周血细胞因子水平;B:IL-10/TNF-α比值
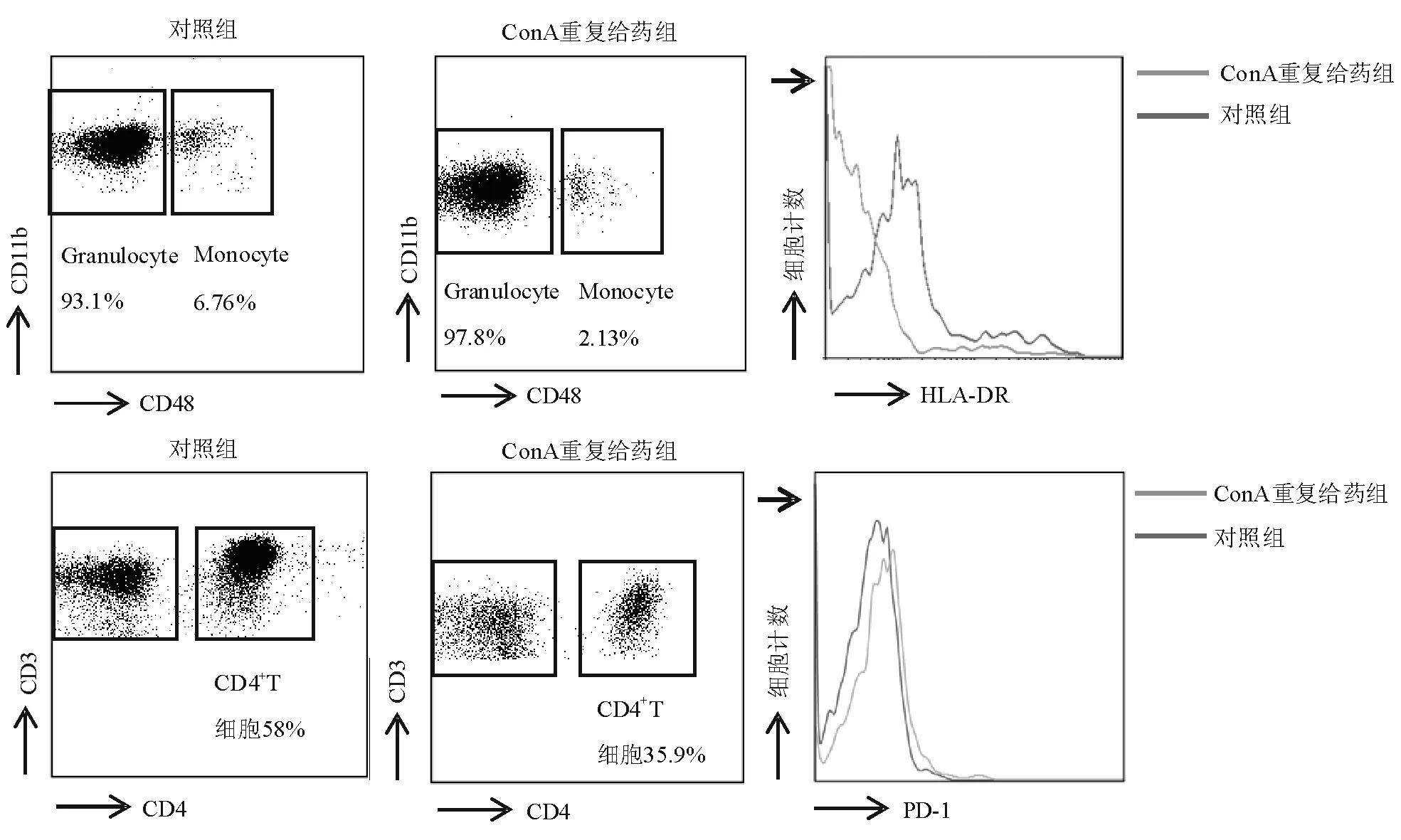
图2 流式细胞术检测外周血HLA-DR和PD-1表达
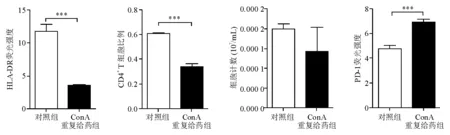
图3 外周血HLA-DR表达、CD4+T细胞比例、数量和PD-1表达
与对照组相比,ConA重复给药组小鼠的外周血CD4+T细胞表面PD-1表达明显上调(平均荧光强度:6.90对4.79,P<0.000 1)(图2、图3),表明具有正向免疫调节功能的CD4+T细胞受损。
讨论
已有研究表明免疫状态的相互转变在ACLF发病和转归中起有重要作用[4-8]。ACLF的免疫状态可分为SIRS和随后的CARS[8]。外周血细胞因子是反映机体免疫状态的重要指标,SIRS主要表现为外周血促炎细胞因子(TNF-α等)明显升高且占主导地位的细胞因子风暴;CARS主要表现为促炎细胞因子明显降低而抗炎细胞因子(IL-10等)分泌增加并占主导地位[9-11]。
本研究中,ConA首次给药后,小鼠外周血促炎细胞因子TNF-α、IFN-γ、MCP-1、IL-6急剧升高,随着给药次数增加,促炎细胞因子水平逐渐下降,末次给药时基本处于正常范围;但IL-12水平变化不明显。抗炎细胞因子IL-10在首次给药轻微升高,第2次给药后继续升高并达到峰值,随这给药次数增加,IL-10仍维持较高水平。细胞因子的变化说明首次给药后小鼠处于免疫亢进状态(SIRS),即促炎细胞因子急剧升高,而抗炎细胞因子处于较低水平;第3次ConA刺激后,促炎细胞因子水平逐渐下降而抗炎细胞因子维持较高水平,小鼠呈现免疫耐受状态(CARS)。
ConA是一种植物来源的凝集素,对肝脏细胞具有特异性毒性作用[12-13]。ConA单次给药能引起肝脏大面积坏死,外周血促炎细胞因子(TNF-α、IFN-γ)显著升高,机体处于免疫亢进状态(SIRS)[14-15],同时单核细胞HLA-DR表达升高[16-17]。
CARS主要表现为促炎细胞因子(TNF-α等)明显降低而抗炎细胞因子(IL-10等)分泌增加并占主导地位。据文献报道,ACLF患者的免疫状态以CARS状态为主,持续CARS状态是导致ACLF患者死亡的主要原因[4,18-19]。ACLF的免疫抑制主要表现为先天性和适应性免疫系统功能抑制[8,20-24],因此本研究选择HLA-DR反映先天性免疫系统在免疫抑制时单核细胞呈递抗原的能力,同时选择CD4+T细胞反映免疫抑制时适应性免疫系统功能的变化。文献[25-27]报道,PD-1(CD279)是一种重要的免疫抑制分子,免疫抑制时CD4+T细胞通过PD-1介导其凋亡,诱导外周免疫耐受。本研究结果表明ConA 5次刺激小鼠后,HLA-DR表达明显降低,外周血CD4+T细胞数量和比例均明显降低,PD-1表达明显升高。说明ConA 5次刺激后机体免疫系统处于抑制状态,即发生了先天性和适应性免疫系统的功能障碍。
综上所述,本研究通过连续ConA重复刺激成功建立了ACLF免疫状态动态演变的动物模型,对探索ACLF的病理生理过程、筛选有效的治疗药物以及研究新的有效治疗方法等具有重要意义。
参考文献
1 Moreau R, Jalan R, Gines P, et al; CANONIC Study Investigators of the EASL-CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis[J]. Gastroenterology, 2013, 144 (7): 1426-1437.
2 Duseja A, Chawla YK, Dhiman RK, et al. Non-hepatic insults are common acute precipitants in patients with acute on chronic liver failure (ACLF) [J]. Dig Dis Sci, 2010, 55 (11): 3188-3192.
3 Katoonizadeh A, Laleman W, Verslype C, et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study[J]. Gut, 2010, 59 (11): 1561-1569.
4 Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) [J]. Hepatol Int, 2009, 3 (1): 269-282.
5 Laleman W, Verbeke L, Meersseman P, et al. Acute-on-chronic liver failure: current concepts on definition, pathogenesis, clinical manifestations and potential therapeutic interventions[J]. Expert Rev Gastroenterol Hepatol, 2011, 5 (4): 523-537.
6 Jalan R. Acute-on-chronic liver failure: from concept to a new syndrome[J]. Curr Opin Crit Care, 2011, 17 (2): 152.
7 Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis[J]. Curr Opin Crit Care, 2011, 17 (2): 165-169.
8 Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure[J]. J Hepatol, 2012, 57 (6): 1336-1348.
9 Rosenbloom AJ, Pinsky MR, Bryant JL, et al. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction[J]. JAMA, 1995, 274 (1): 58-65.
10Osuchowski MF, Welch K, Siddiqui J, et al. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality[J]. J Immunol, 2006, 177 (3): 1967-1974.
11Osuchowski MF, Craciun F, Weixelbaumer KM, et al. Sepsis chronically in MARS: systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis[J]. J Immunol, 2012, 189 (9): 4648-4656.
12Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A[J]. J Clin Invest, 1992, 90 (1): 196-203.
13Mizuhara H, O’Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6[J]. J Exp Med, 1994, 179 (5): 1529-1537.
14Küsters S, Gantner F, Künstle G, et al. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A[J]. Gastroenterology, 1996, 111 (2): 462-471.
15Sass G, Heinlein S, Agli A, et al. Cytokine expression in three mouse models of experimental hepatitis[J]. Cytokine, 2002, 19 (3): 115-120.
16Yang Q, Liu Y, Shi Y, et al. The role of intracellular high-mobility group box 1 in the early activation of Kupffer cells and the development of Con A-induced acute liver failure[J]. Immunobiology, 2013, 218 (10): 1284-1292.
17Antoniades CG, Berry PA, Wendon JA, et al. The importance of immune dysfunction in determining outcome in acute liver failure[J]. J Hepatol, 2008, 49 (5): 845-861.
18Mookerjee RP, Stadlbauer V, Lidder S, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome[J]. Hepatology, 2007, 46 (3): 831-840.
19Berry PA, Antoniades CG, Carey I, et al. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis[J]. Intensive Care Med, 2011, 37 (3): 453-460.
20Stadlbauer V, Mookerjee RP, Hodges S, et al. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis[J]. J Hepatol, 2008, 48 (6): 945-951.
21Antoniades CG, Wendon J, Vergani D. Paralysed monocytes in acute on chronic liver disease[J]. J Hepatol, 2005, 42 (2): 163-165.
22Wasmuth HE, Kunz D, Yagmur E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis[J]. J Hepatol, 2005, 42 (2): 195-201.
23Dong X, Gong Y, Zeng H, et al. Imbalance between circulating CD4+ regulatory T and conventional T lymphocytes in patients with HBV-related acute-on-chronic liver failure[J]. Liver Int, 2013, 33 (10): 1517-1526.
24Albillos A, Hera Ad Ade L, Reyes E, et al. Tumour necrosis factor-alpha expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin[J]. J Hepatol, 2004, 40 (4): 624-631.
25Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance[J]. Trends Immunol, 2001, 22 (5): 265-268.
26Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion[J]. Nat Med, 2002, 8 (8): 793-800.
27Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation[J]. J Exp Med, 2000, 192 (7): 1027-1034.
(2015-12-14收稿;2016-01-26修回)
DOI:10.3969/j.issn.1008-7125.2016.06.002
Background: Acute-on-chronic liver failure (ACLF) is a commonly seen liver failure in China, and lacking an animal model that can effectively simulate the dynamic change of immune status of ACLF. Aims: To establish an animal model that can simulate dynamic change of immune status of ACLF by repeated administration of concanavalin A (ConA). Methods: Mice were randomly divided into normal control group and ConA repeated administration group. Mice in ConA repeated administration group were injected with ConA 15 mg/kg through retrobulbar angular vein every 48 hours for 5 times, and mice in control group were injected with same volume of 0.9% NaCl solution. Serum levels of IL-6, IL-10, IL-12, TNF-α, IFN-γ, MCP-1 in peripheral blood were assessed by CBA assay, and the ratio of IL-10/TNF-α was calculated. The expression of HLA-DR, number and proportion of CD4+T cells and the expression of PD-1 of monocytes in peripheral blood were detected by flow cytometry. Results: Peripheral blood cytokines changed from predominated proinflammatory cytokines into predominated anti-inflammatory cytokines with the increasing in time of administration in ConA repeated administration group. Compared with control group, HLA-DR expression of monocytes in peripheral blood was significantly decreased (P<0.05), number and proportion of CD4+T cells were significantly decreased (P<0.05), and PD-1 expression was significantly increased (P<0.05) in ConA repeated administration group. Conclusions: An animal model of ACLF immune status from systemic inflammatory response syndrome (SIRS) to compensatory anti-inflammatory response syndrome (CARS) induced by repeated ConA stimulation is successfully established.
Key wordsAcute-On-Chronic Liver Failure;Concanavalin A;Immune Status;Cytokines;Models, Animal
*本课题为国家自然基金项目(30971333、81170421、81470869)
#本文通信作者,Email: haili_17@126.com

