对乙酰氨基酚诱导的急性肝损伤和肝衰竭模型中线粒体基因组转录改变
明雅南 李春敏 张静怡 刘晓琳 茅益民
·论著·
对乙酰氨基酚诱导的急性肝损伤和肝衰竭模型中线粒体基因组转录改变
明雅南李春敏张静怡刘晓琳茅益民
200001上海交通大学医学院附属仁济医院消化内科,上海市消化疾病研究所
【摘要】目的通过探索对乙酰氨基酚(APAP)诱导的急性肝损伤和急性肝衰竭模型中线粒体基因组转录水平的改变与疾病进展之间的关联,为AILI预后的预测提供新的生物标记。方法将90只小鼠随机分为3组:对照组、APAP导致的DILI组(AILI,300 mg/kg)和APAP导致的急性肝衰竭组(AILF,750 mg/kg)。禁食16 h后,腹腔注射体积相近的0.9%氯化钠溶液和不同剂量APAP,在0、1、3、6、12 h等不同时间点时,每组随机选取6只小鼠处死,留取小鼠的血浆和肝脏组织。检测每只小鼠ALT、AST、ROS变化水平; 提取肝脏总RNA,采用RT-PCR技术检测线粒体基因组基因的转录水平。结果与对照组相比,腹腔注射APAP后,两组均可见转氨酶明显升高,AILI组在6-12 h时ALT达到峰值(5 000~10 000 U/L);AILF组12 h时ALT水平超过10 000 U/L,明显高于AILI组(P<0.05)。APAP处理后,两组小鼠均可见典型的3区微泡性脂肪变,AILF组可见典型的急性肝衰竭的大块性坏死。与对照组相比,AILI和AILF组肝脏中ROS从1 h开始,各时间点均可见显著升高,且1 h时,AILF组ROS的生成量约为AILI组的2.5倍(P<0.05)。AILI组COX1在6 h时显著升高(P<0.05),而对照组和AILF组均未见显著升高。与对照组相比,3 h时,AILI组和AILF组可见CYTB、COX2和ATP8显著降低(P<0.05);6 h时,AILI组和AILF组COX1、ND1、ND5和ATP8转录水平显著降低(P<0.05);12 h时,AILI组和AILF组中NADH各亚基均可见显著降低(P<0.05)。AILI组与AILF组相比, 6 h时,AILF组中 ATP6的转录水平显著低于AILI组和对照组(P<0.05);12 h时,AILF组的肝脏中CYTB、COX2、ATP8以及NADH的2、3、5和6亚基的转录水平显著低于AILI组(P<0.05)。结论线粒体基因组除COX1在AILI组明显上调,其他存在明显改变的基因均出现降低趋势,且AILF组变化更明显。线粒体基因组转录水平改变对于预测AILI预后有潜在价值。
【关键词】对乙酰氨基酚;肝损伤;肝衰竭;线粒体;转录
药物的肝脏毒性是新药研发失败及上市后被撤回最主要的原因之一,尽管药物导致肝损伤的机制复杂,但线粒体功能损伤是其的重要环节[1,2]。线粒体基因组(mtDNA)位于线粒体内膜上的环状DNA,是可编码13种合成线粒体呼吸链和氧化磷酸化功能蛋白必备的RNA、rRNA和tRNA,但由于缺少组蛋白保护,而且相对于细胞核DNA对复制和转录过程中出现各种错误后完善的修复机制,线粒体DNA的修复机制并不完善,导致其更易受到氧化应激产物(ROS)的损伤[3-5],影响线粒体氧化磷酸化功能,进而影响能量生成。有研究提示,血浆中线粒体生物标记物明显升高与预后不良明显相关,线粒体结构和功能严重受损者,存活率更低[6]。
对乙酰氨基酚(APAP)是最常见的固有型药物性肝损伤(DILI)的代表药物,APAP过量应用已成为西方国家急性肝衰的重要原因之一[7]。P450酶代谢产生的毒性产物N-乙酰对苯醌亚胺(NAPQI)造成线粒体结构和功能的损伤,是其主要的发病机制。肝内过多的NAPQI能够耗竭肝内解毒NAPQI的谷胱甘肽(GSH),并损害线粒体复合体Ⅱ和Ⅲ的酶活性[8-9],最终导致线粒体功能受损,产生大量的ROS。本研究通过构建APAP导致的DILI动物模型(AILI)和急性肝衰竭动物模型(AILF),采用实时PCR技术对两种具有不同预后的动物模型在不同时间点mtDNA的转录水平进行分析,探索mtDNA在DILI的发生发展过程中的变化特点,以发现可以预测疾病预后的潜在生物标记。
资料和方法
一、实验动物与试剂仪器
90只C57BL/6小鼠购于上海斯莱克动物实验中心;APAP、戊巴比妥钠购于sigma公司(美国);全自动生化分析仪(SIEMEN SADVIA 1800,美国);全自动脱水机(ASP300)、石蜡包埋机(EG1150C)、脱蜡机(Auto tainer XL)等均来自于德国Leica公司;Bio-Rad CFX96荧光定量PCR仪(美国);Trizol、Prime Script TM反转录试剂盒、SYBR@Premix Ex TaqTM II购于 Takara公司(日本);PCR引物购于上海生工有限公司。
90只小鼠,随机分为对照组、AILI组和AILF组,每组30只;腹腔分别注射体积相近的0.9%氯化钠溶液、APAP-300 mg/kg、APAP-750 mg/kg。处理后的动物在0、1、3、6、12 h,每组随机抽出6只小鼠,麻醉,取血,留取肝脏组织。提取肝脏总RNA,检测AILI组和AILF组小鼠肝脏组织ROS 的生成量和mtDNA转录水平,观察其随时间变化的特点。
二、血浆转氨酶检测
戊巴比妥钠麻醉小鼠,抗凝管眼球取血,取血后,3000×g,4℃离心,取上清液,稀释10倍,检测小鼠的ALT和AST。
三、ROS检测
新鲜的肝脏100 mg,用组织清洗液清洗后,用匀浆器制成肝脏匀浆,按照试剂盒说明检测肝脏匀浆中ROS的产生量,并采用BCA的方法检测肝脏匀浆的蛋白浓度,校正肝脏内ROS的产量。
四、实时PCR检测线粒体RNA
TRIZOL试剂盒提取组织总RNA。NanoDrop ND-1000测定RNA的浓度、质量和纯度,A260/A280>1.8作为RNA合格的标准,根据 PrimeScript反转录试剂盒合成cDNA,然后按照SYBR@Premix Ex Taq II说明扩增线粒体基因,mRNA水平以18S作为内参对照,结果以相对含量表示不同基因的转录水平。
五、统计学分析
统计分析应用SPSS 19.0软件,作图应用GraphPad Prime 6.01软件。计量资料用均数±标准差表示,组间比较采用t检验,P<0.05为差异有统计学意义。
结果
一、动物模型建立
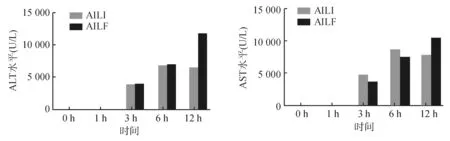
与对照组相比,腹腔注射APAP后,AILI组和AILF组均可见转氨酶明显升高,AILI组在6~12 h时ALT达到高峰(5 000~10 000 U/L);AILF组12 h时ALT水平超过10 000 U/L,明显高于AILI组(P<0.05)。见图1。12 h时,AILF组肝脏组织学可见典型的急性肝衰竭表现-大块性肝坏死,病变周围细胞也表现为明显的弥漫性的肝细胞变性;AILI组损伤范围局限,损伤周围的肝细胞形态结构正常。上述组织学特点提示,APAP诱导的AILI和AILF造模成功。
二、ROS的表达变化
与对照组相比,APAP处理后1 h,AILI组和AILF组可见肝内ROS生成均明显增多,且AILF组约为AILI组2.5倍(P<0.05),两组肝内ROS均随时间延长,含量越高(P<0.05)。见表1。
三、线粒体基因组转录水平变化
各组mtDNA检测结果显示AILI组COX1在6 h时显著升高(P<0.05),而对照组和AILF组均未见显著升高。与对照组相比,3 h时,AILI组和AILF组可见CYTB、COX2和ATP8显著降低(P<0.05);6 h时,AILI组和AILF组COX1、ND1、ND5和ATP8转录水平显著降低(P<0.05);12 h时,AILI组和AILF组中NADH各亚基均可见显著降低(P<0.05)。AILI组与AILF组相比, 6 h时,AILI组COX1显著高于AILF组(P<0.05),AILF组中ATP6的转录水平显著低于AILI组和对照组(P<0.05);12 h时,AILF组的肝脏中CYTB、COX2、ATP8以及NADH的2、3、5和6亚基的转录水平显著低于AILI组(P<0.05)。见表2,表3。
注:AILI组和AILF组与对照组比较*P<0.05;AILI组和AILF组比较§P<0.05。

组别0h1h3h6h12h对照组927.02±183.471013.68±23.37993.68±141.48936.68±128.03977.35±42.11AILI组1060.35±162.721739.15±655.62*3519.14±806.51*4069.00±1046.64*6371.32±1910.92*AILF组993.68±262.374518.59±913.71*§4061.37±422.22*5015.99±654.29*5555.80±977.36*
讨论
AILI发生过程中,线粒体氧化应激并生成ROS[10],损伤线粒体蛋白和DNA,并导致线粒体膜通透性转变孔(mitochondrial membrane permeability transition,MPT)开放,MPT的发生最终导致线粒体膜电位改变、ATP生成中断,胞内离子稳态平衡被打破,肝细胞肿胀坏死。因此,线粒体结构和功能损伤是DILI早期重要的发病机制[11,12]。mtDNA是线粒体内重要的组成成分,能够编码表达13种呼吸链复合体的必需蛋白,其数量减少和功能改变可能直接影响肝细胞代谢状态及存活[13]。

表2 不同组别各时间点NADH亚基中线粒体编码基因的转录水平
注:*:与对照组比较,P<0.05;§:与AILI组比较,P<0.05
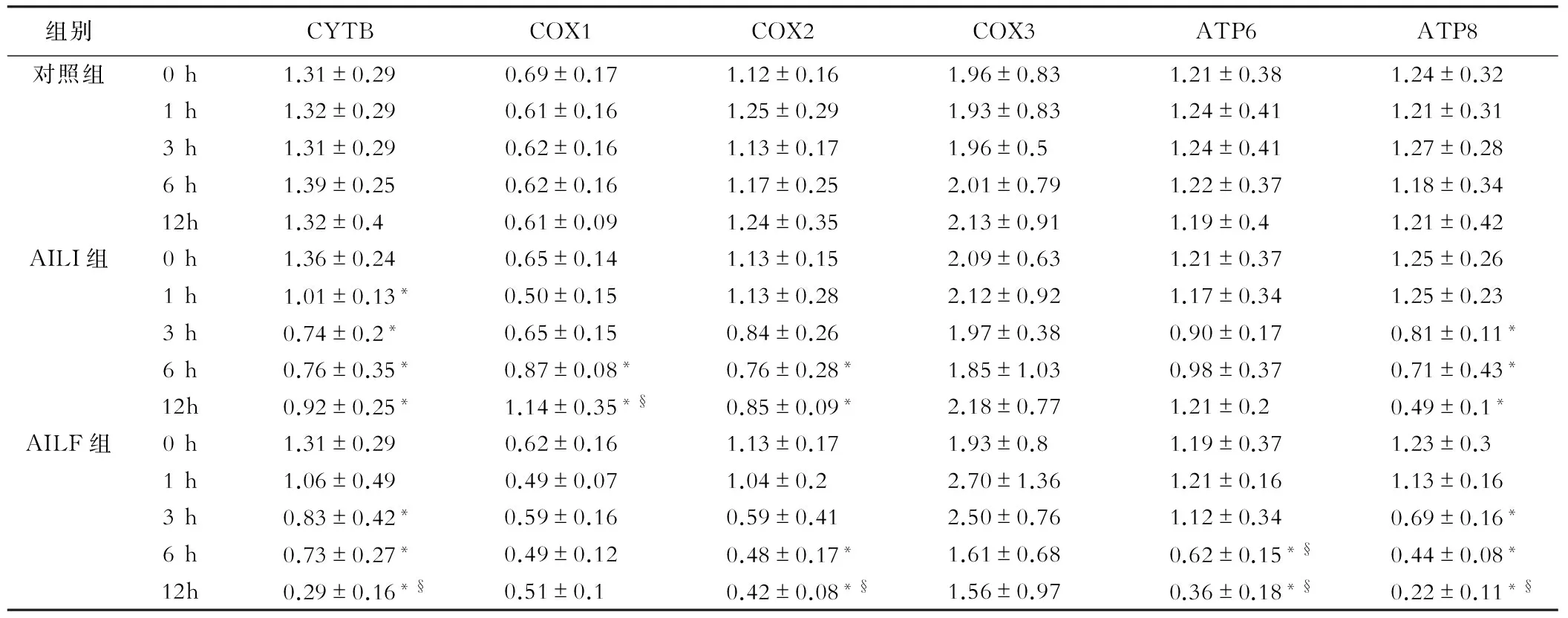
表3 CYTB、COX以及ATP等线粒体编码基因的转录水平
注:*:与对照组比较,P<0.05;§:与AILI组比较,P<0.05
本研究结果显示,腹腔注射不同剂量APAP后,早期即可见ROS明显升高,且衰竭组明显高于损伤组。线粒体是ROS产生的主要细胞器,mtDNA由于无组蛋白保护,而且相对于细胞核DNA对复制和转录过程中出现各种错误后完善的修复机制,线粒体DNA的修复机制并不完善,过量ROS使其更易受损伤。Suliman等[14]的研究发现,氧化应激可能是mtDNA损伤的原因,严重的mtDNA损伤,会进一步减少ATP的生成,并增加ROS在肝内的累积,因此,本研究中观察到的AILI和AILF组中ROS明显增加,与既往的研究报道一致。
从mtDNA的转录水平变化来看,除AILI组COX1的转录升高,其他差异表达的mtDNA的转录水平均表现为降低趋势,并随时间延长更为明显。本研究中发现肝脏组织中mtDNA转录水平明显降低可能与预后不良密切相关,推测急性肝衰竭时肝脏组织中mtDNA大量释放入血后导致的血浆mtDNA明显升高,可以作为预测预后的潜在生物标记,循环中高水平的mtDNA与AILI患者预后不良密切相关。
有研究发现,mtDNA降低20%~40%就会直接影响线粒体功能,持续mtDNA减少能够降低线粒体呼吸链NAD+ FAD的再生功能,最终影响脂质和丙酮酸盐氧化并产生大量的ROS[15,16]。
长期高脂饮食以及急性酒精性肝损伤模型均出现mtDNA减少和损伤[17,18]。ROS对mtDNA的直接损伤导致正常mtDNA发生突变,以及细胞膜裂解后将mtDNA释放到血液循环可能是导致AILI动物模型中mtDNA减少的重要因素。释放的mtDNA可作为配体活化TLR9,引起NASH肝脏的炎性反应[19]。另外有研究认为,mtDNA作为损伤相关模式分子激活DILI的无菌性炎性反应[20]。从上述针对其他肝病的证据来看,mtDNA的减少不仅是疾病发生早期重要的机制,也可能是导致疾病进展的关键因素之一。相反,通过药物改善肝脏内mtDNA的水平可能阻断毒物引起的肝衰竭[21]。
本研究仅对AILI和AILF发生过程中mtDNA的转录水平改变进行相应研究,并未对其原因进行深入的探讨,而mtDNA转录降低是由于数量的减少还是刺激导致其异常突变,还需要通过更多的实验提供证据。此外,肝脏组织中mtDNA转录水平降低的程度对于预测疾病预后具有潜在的价值,但尚需要更多研究加以验证。COX1在AILI组的变化趋势与其他mtDNA明显不一致,可能与线粒体及肝细胞的适应性反应有关,对于COX1在不同疾病和生理过程中的作用和意义还需进一步的实验证实。
本研究描述了AILI和AILF发生和发展过程中mtDNA转录水平随时间变化的特点,结果提示,mtDNA转录水平变化差异可能具有预测预后的价值,但其转录改变机制还需要更多的基础和临床研究加以证实。
参考文献
[ 1 ]Lee WM. Drug-induced hepatotoxicity. N Engl J Med, 2003,349:474-485.
[ 2 ]Lee KK, Imaizumi N, Chamberland SR, et al. Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology,2015,61:326-336.
[ 3 ]Inoue JG, Miya M, Tsukamoto K, et al. Complete mitochondrial DNA sequence of Conger myriaster (Teleostei: Anguilliformes): novel gene order for vertebrate mitochondrial genomes and the phylogenetic implications for anguilliform families. J Mol Evol,2001,52:311-320.
[ 4 ]Dianov GL, Souza-Pinto N, Nyaga SG, et al. Base excision repair in nuclear and mitochondrial DNA. Prog Nucleic Acid Res Mol Biol,2001,68:285-297.
[ 5 ]Demeilliers C, Maisonneuve C, Grodet A, et al. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology,2002,123:1278-1290.
[ 6 ]McGill MR, Staggs VS, Sharpe MR, et al. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology,2014,60:1336-1345.
[ 7 ]Larsen FS, Wendon J. Understanding paracetamol-induced liver failure. Intensive Care Med,2014,40:888-890.
[ 8 ]Ramsay RR, Rashed MS, Nelson SD. In vitro effects of acetaminophen metabolites and analogs on the respiration of mouse liver mitochondria. Arch Biochem Biophys,1989,273:449-457.
[ 9 ]Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem 1991;266:5049-5054.
[10]Arduini A, Serviddio G, Escobar J, et al. Mitochondrial biogenesis fails in secondary biliary cirrhosis in rats leading to mitochondrial DNA depletion and deletions. Am J Physiol Gastrointest Liver Physiol,2011,301:G119-127.
[11]Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev,2012,44:88-106.
[12]Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol,2010,369-405.
[13]Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J,1999,341 ( Pt 2):233-249.
[14]Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med 2003;167:570-579.
[15]Igoudjil ABK, Pessayre D, Fromenty B. Mitochondrial, metabolic and genotoxic effects of antiretroviral nucleoside reverse-transcriptase inhibitors. Curr Med Chem,2006,5.273-292.
[16]Velsor LW, Kovacevic M, Goldstein M, et al. Mitochondrial oxidative stress in human hepatoma cells exposed to stavudine. Toxicol Appl Pharmacol,2004,199:10-19.
[17]Yuzefovych LV, Musiyenko SI, Wilson GL, et al. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One,2013,8:e54059.
[18]Mansouri A, Demeilliers C, Amsellem S, et al. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther,2001,298:737-743.
[19]Garcia-Martinez I, Santoro N, Chen Y, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest,2016,126:859-864.
[20]Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology, 2012,143:1158-1172.
[21]Bottani E, Giordano C, Civiletto G, et al. AAV-mediated liver-specific MPV17 expression restores mtDNA levels and prevents diet-induced liver failure. Mol Ther,2014,22:10-17.
(本文编辑:钱燕)
基金项目:“十二五”科技重大专项(2012ZX09303-001,2012ZX09401004)
通信作者:茅益民,Email:maoym11968@163.com
Corresponding author:MAO Yi-min, Email: maoym11968@163.com
(收稿日期:2016-04-13)
The transcriptional change of mitochondrial genome in mice models for acetaminophen-induced acute liver injury and failure
MINGYa-nan,LIChun-min,ZHANGJing-yi,LIUXiao-lin,MAOYi-Min.DivisionofGastroenterologyandHepatology,RenjiHospital,SchoolofMedicine,ShanghaiJiaoTongUniversity,ShanghaiInstituteofDigestiveDisease,Shanghai200001,China
【Abstract】ObjectiveTo explore the relationship between transcription level of mitochondrial DNA (mtDNA) and disease progression in the mice models of acetaminophen (APAP)- induced acute liver injury (AILI) and acute liver failure (ALF), and to find the relative new biomarkers for outcome. MethodsNinety mice were randomly divided into three groups, including control group, AILI group (300 mg/kg) and ALF group (750 mg/kg). After fasting 16 h, all mice were intraperitoneally injected with same volume of saline or different doses of APAP. At different time points of 0, 1, 3, 6 and 12 h, 6 mice randomly selected from each group were sacrificed for blood and liver, respectively. Plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST) and reactive oxygen (ROS) levels were detected, and liver total RNA was extracted for RT-PCR to detect changes in transcription of mitochondrial genome. ResultsCompared with the control group, ALT and AST levels in AILI and ALF group were significantly increased. In AILI group, ALT peaked at 6-12 h (5000-10000 IU/L), and ALT exceeded 10000 IU/L at 12 h in ALF group, which showed significantly difference (P<0.05). Microbubbles steatosis in three zones was observed in both AILI and ALF groups, and massive hepatic necrosis (MHN) were found merely in ALF group. In 1 h after APAP injection, ROS in ALF group was about 2.5 times as much as that in AILI group, which significantly increased at all time points in both group. Contrasting with control and ALIF groups, COX1 transcriptional level in AILI group increased significantly at 6 h. In AILI and ALF group, CYTB, COX2 and ATP8 reduced significantly at 3 h (P<0.05), COX1,ND1,ND5 and ATP8 significantly decreased at 6 h (P<0.05), and transcription level of other subunits of NADH significantly decreased at 12 h (P<0.05) comparing with those in control group. Furthermore, ATP6 at 6h in ALF group was obviously lower than that in AILI and control group (P<0.05). At 12 h, the majority of mtDNA (CYTB, COX2, ATP8, ND2, ND3, ND5 and ND6 ) had significant differences between AILI and ALF group. ConclusionThere are significant difference in mtDNA transcription between AILI and ALF groups. Besides COX1, other mtDNA showed significant decreases in AILI and ALF group compared with those in control group, especially in ALF group. mtDNA transcriptional changes may have great potential as biological makers to predict outcomes of AILI.
【Key words】APAP; Liver injury; Liver failure; Mitochondrial; Transcription

