Hematology of Wild Caught Hoplobatrachus rugulosus in Northern Thailand
Suthirote MEESAWAT, Noppadon KITANA, 2and Jirarach KITANA, 2*
1Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand2Center of Excellence in Biodiversity, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
Hematology of Wild Caught Hoplobatrachus rugulosus in Northern Thailand
Suthirote MEESAWAT1, Noppadon KITANA1, 2and Jirarach KITANA1, 2*
1Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
2Center of Excellence in Biodiversity, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
Abstract The rice feld frog Hoplobatrachus rugulosus is an important anuran species found in wetlands throughout Thailand.At present, the hematological parameters of wild populations are unknown.Therefore, hematological and morphological characteristics of peripheral blood cells of wild-caught H.rugulosus were examined.Thirty-three adult frogs (17 male and 16 female frogs) were collected from a natural population in Nan Province, northern Thailand during the wet season of 2014.Blood samples were analyzed by packed cell volume (PCV) and blood cell counts from hemocytometer and Giemsa-stained blood smears.The mean PCV of male frogs (30.70% ± 6.07%) was signifcantly higher than that of the female frogs (25.09% ± 4.85%).The mean number of lymphocytes and neutrophils also showed signifcant sex-related differences.Moreover, the morphometric analysis of blood cells revealed dimensions as follows: erythrocytes (17.96 ± 1.44 µm length × 11.50 ± 1.09 µm width), immature erythrocytes (14.91 ± 2.20 µm diameter),thrombocytes (13.93 ± 3.14 µm length × 7.05 ± 1.31 µm width), lymphocytes (11.01 ± 2.69 µm diameter), monocytes (12.04 ± 2.40 µm diameter), neutrophils (12.58 ± 2.08 µm diameter), basophils (13.60 ± 2.17 µm diameter) and eosinophils (12.33 ± 2.95 µm diameter).Overall, the hematological parameters obtained in this study could be regarded as the frst report and a crucial baseline data of wild H.rugulosus in Thailand that can be used for monitoring the health status of this anuran.
Keywords Rice feld frog, erythrocyte, leukocyte, packed cell volume, morphometry, Nan Province
1.Introduction
Monitoring the health of wildlife is very important for their conservation and management.Among general health assessment approaches, hematological analyses are viewed as reliable methods to determine the health status in mammals and other vertebrates.Changes in some hematological parameters compared with the reference values may be used as evidence of physiological disturbances, such as xenobiotic exposure,and diseases or stress, in vertebrates (Bloom and Brandt,2008; Davis et al., 2008).Among the hematological parameters of peripheral blood that have been frequently used to assess the health status of animals are the packed cell volume (PCV) or hematocrit, erythrocyte count,leukocyte count and differential leukocyte count.Data on the hematological parameters of human and domestic mammals are well documented, but remain limited for amphibians and reptiles.With respect to amphibians the use of hematological parameters for health assessment has been reported in some species, such as the common toad (Bufo arenarum) in agricultural areas in Argentina (Cabagna et al., 2005), the marsh frog (Rana ridibunda)in an industrial area in Bulgaria (Zhelev et al., 2006),the eastern hellbender (Cryptobranchus alleganiensis alleganiensis) in a pesticide contaminated river in southern Indiana, USA (Burgmeier et al., 2011) and the northern leopard frog (Lithobates pipiens) in pesticide contaminated areas in Canada (Shutler and Marcogliese,2011).However, reference data on the hematological parameters of wild populations of frog species is scarce compared with the diversity of animals in this taxonomic group, even though it is crucial for their health assessment.The rice field frog (Hoplobatrachus rugulosus,Wiegmann, 1835) is an anuran amphibian in the family Dicroglossidae with a widespread distribution from central China and Myanmar to Thailand and peninsular Malaysia, where they are commonly found in wetlands and paddy felds (Diesmos et al., 2004).Even though their natural habitats in Thailand have been disturbed and are very limited, natural populations can still be found in Nan Province, northern Thailand, and lowland sites in other provinces from central to southern Thailand (Pansook et al., 2012).This frog is economically important in Thailand because it is used as food by the local people.It also has the potential to be used as a model for research in many felds, such as physiology, reproductive biology and ecotoxicology.At present, wild populations of H.rugulosus are of concern due to human-mediated over harvesting, habitat disturbances, stress and diseases, but the health status of these natural populations is unknown.Until now, the hematological parameters in the natural populations of H.rugulosus are still undocumented.Only the hematological aspects of a closely related species,the common Indian frog (Rana tigrina) have been reported (Singh, 1977a, 1977b, 1978).Therefore, the purpose of this study was to identify the morphological characteristics of different peripheral blood cells and to determine the hematological parameters of the peripheral blood in H.rugulosus.Data from this study would then form the fundamental basis for monitoring of the health status of this natural anuran population.
2.Materials and Methods
2.1 Frog collection and blood sampling Adult H.rugulosus were collected from organic rice felds (UTM 0686779 2047187, zone line 47Q) with no history of herbicide usage for more than 10 years in Wiang Sa District, Nan Province, northern Thailand during the rainy season (July to August 2014).Thirty-three adult frogs (17 males, 16 females) were collected by the visual encounter survey method (Crump and Scott, 1994) from this natural habitat.Blood samples (0.5 mL per 100 g body weight)were collected from the frogs by cardiac puncture (Heatley and Johnson, 2009) under cold anesthesia using 25-gauge needle with heparinized tuberculin syringes and transferred to microcentrifuge tubes.The samples were stored in ice bucket during an hour of PCV,hemocytometer counting, and blood smear processes.
2.2 Hematology The PCV was determined after the blood sample had been transferred to a microcapillary tube and centrifuged at 8,700×g for 10 min.Erythrocyte and leukocyte counts were determined manually using a hemocytometer (Tharp and Woodman, 2002) after the blood sample was diluted with Natt and Herrick's solution (Natt and Herrick, 1951).Blood smears were prepared on glass slides immediately and fixed with absolute methanol.Giemsa staining (Bain and Lewis,2012) was used to study the frog blood cell morphology and determination of the hematological parameters using light microscopy at 40× objective lens.On blood smear slide of each frog, a total of 200 leukocytes were counted for the differential leukocyte count.Immature and mature erythrocytes were counted in 10 fields with an aid of grid ocular micrometer and the percentage of immature erythrocyte was calculated (Briggs and Bain, 2012).For morphometric study of blood cell size, blood smears from 10 frogs (5 frogs per sex) were randomly selected out of 33 frogs.On each blood smear slide of a frog, the length and width of 30 randomly selected mature erythrocytes and their nuclei, and 30 randomly selected thrombocytes were measured using the Image-Pro Plus (ver.6.00 software) from their digital images taken with a digital camera (Cannon EOS 550D).Selection of the areas from good spread blood flm with no overlap of the cells and the cell number (30 cells) used in these measurements were based on a previous study by Kuramoto (1981).Likewise, the diameters of 30 randomly selected cells for each of lymphocytes, monocytes, neutrophils, basophils,eosinophils and immature erythrocytes, were also measured in the same manner.The mean value of each morphometric parameter in each frog was calculated from 30 cells, and the grand mean from 5 frogs was finally calculated.The erythrocyte cell and nuclear areas (EA and NA, respectively) were calculated according to the formula of the area of an elliptical shape (length × width × π/4), and the nucleocytoplasmic ratio (NA/EA) was calculated (Arikan and Cicek, 2010).In the same way the immature erythrocyte cell and nuclear areas (IEA and NIEA, respectively) were calculated according to the formula of the area of a circular shape [π × (diameter/2)2],and used to derive the nucleocytoplasmic ratio (NIEA/ IEA).
2.3 Statistical analysis All hematological parameters of each sex were summarized as the mean, standard deviation (SD) and range.Statistical analyses were performed according to Zar (1998) using the SigmaPlot (ver.11.00) statistical software.If the distribution of data was not signifcantly different from a normal distribution (Kolmogorov-Smirnov test, P > 0.05), the significance of differences in the means were compared using the Student's t-test otherwise they were compared using theMann-Whitney U test.Signifcance was accepted at the P ≤ 0.05 level.
3.Results
The hematological parameters of the peripheral blood of H.rugulosus are summarized in Table 1, where the mean erythrocyte count (derived from hemocytometer counting)of male frogs was numerically slightly (1.08-fold) higher but not signifcantly different from that of female frogs (t = 0.38, df = 31, P = 0.71).Likewise, the leukocyte count was numerically (1.25-fold) but not significantly higher in male frogs than in female frogs (Mann-Whitney U test,T = 261.5, P = 0.71).However, a signifcant difference in the mean PCV between the sexes was found, being 1.22-fold higher in male than in female frogs (t = 2.84, df = 31,P = 0.01).
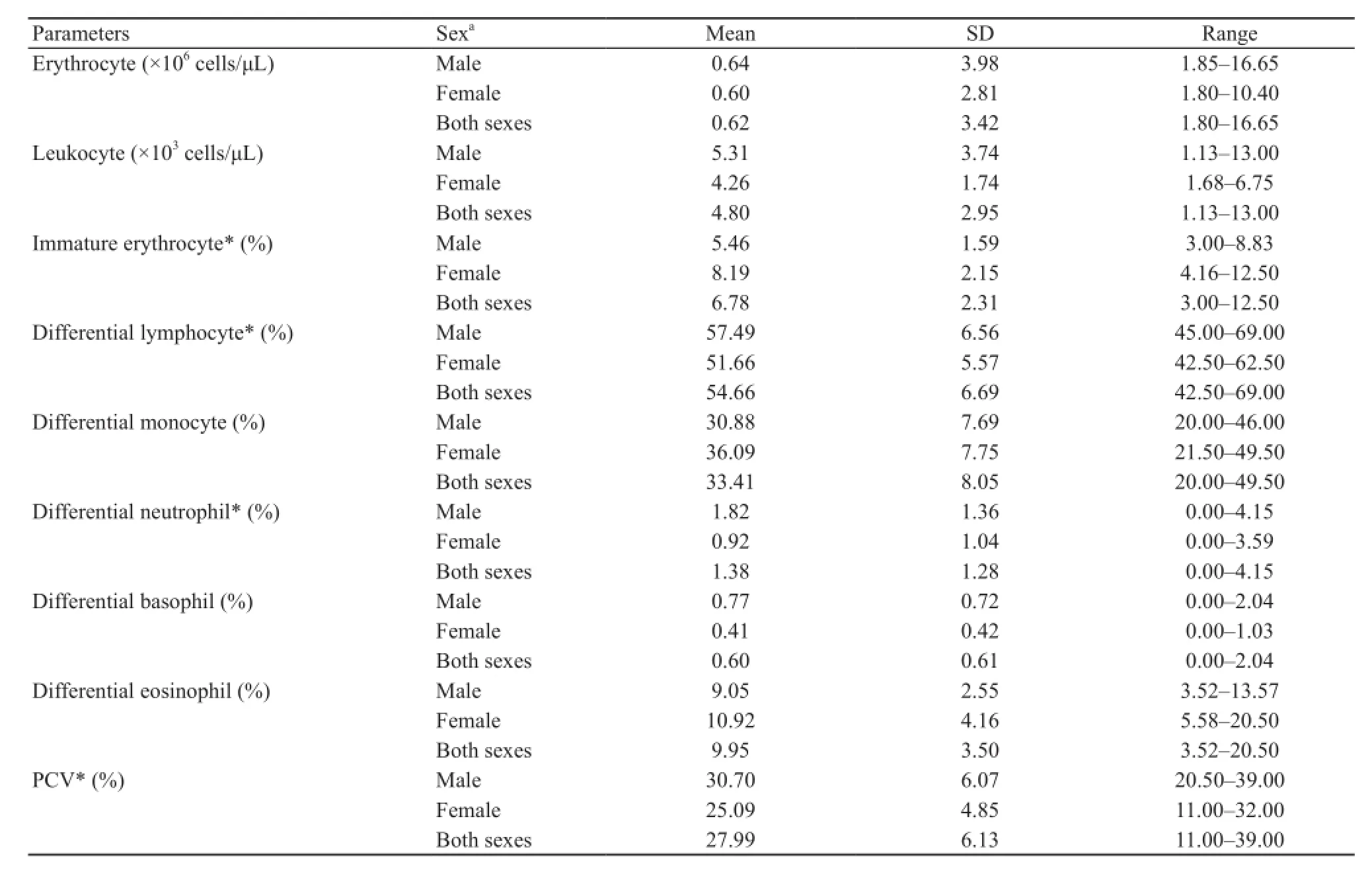
Table 1 Blood cell counts and packed cell volume (PCV) of H.rugulosus collected from Nan Province, Thailand during July to August 2014.
Hematological examination of the peripheral blood smear slides revealed sex-related differences in some leukocyte parameters (Table 1), where the mean values of differential lymphocyte count of male frogs is signifcantly higher than that of the female frogs (1.1-fold,t = 2.74, df = 31, P = 0.01).Mean values of differential neutrophil count was significantly higher in male frogs than in female frogs (2.0-fold, t = 2.12, df = 31, P = 0.04).
Based on their morphological characteristics under light microscopy, the peripheral blood cells of H.rugulosus were classified into erythrocytes, immature erythrocytes, thrombocytes and leukocytes.The cytomorphological analyses revealed no significant differences between the sexes in the size of each cell type, allowing an average size of both sexes combined to be evaluated (Tables 2 and 3).Mature erythrocytes were large elliptically shaped cells with a centrally located elliptical shaped nucleus with dense basophilic chromatin.The nuclear membrane was very prominent under light microscopy and the cytoplasm stained light blue to colorless (Figure 1A).Immature (polychromatic)erythrocytes were found in the circulating blood of this frog species.They were spherical with a centrally located round nucleus that was larger and less basophilic staining than that of mature erythrocytes, while the cytoplasmstained light blue or colorless (Figure 1A).Comparison of the nucleocytoplasmic ratios (Table 2) showed that the immature erythrocytes had a significantly higher (1.69-fold) nucleocytoplasmic ratio than that of the mature erythrocytes.
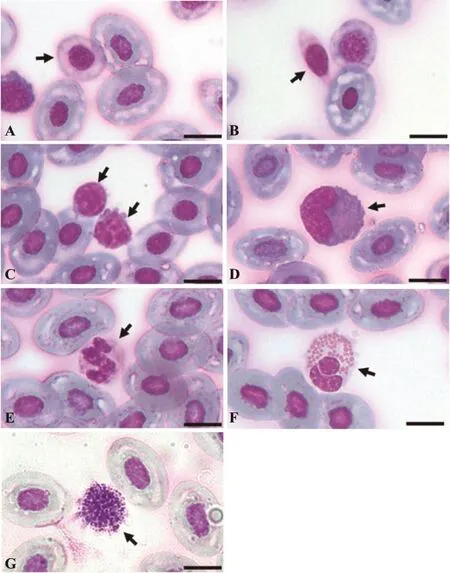
Figure 1 Light micrographs showing the different types of blood cells in H.rugulosus.Erythrocytes were classifed as (A) mature and immature (arrows) erythrocytes.(B) Thrombocytes.Leukocytes were classifed as (C) lymphocytes (arrow), (D) monocytes (arrows), (E)neutrophils (arrow), (F) eosinophils (arrow), (G) basophils (arrow).Bar = 10 µm; Giemsa stain.
Thrombocytes were round to elliptical-shaped cells of a smaller size (1.29- and 1.93-fold in length and width,respectively) than mature erythrocytes and contained anelliptical nucleus.The clear cytoplasm was scarce and stained light blue or colorless (Figure 1B).The cells were sometimes found grouped together on the blood smears.Leukocytes were separated into agranulocytes and granulocytes and then further classifed into two and three cell types, respectively, based on the morphological characteristics of the Giemsa stained blood smears.Agranulocytes were classified into lymphocytes and monocytes.Lymphocytes were round or slightly elliptical shaped cells that contained small amount of cytoplasm.The nucleus was compact and dark stained, positioned centrally in the cell and covered by a light-blue stained cytoplasm (Figure 1C).Monocytes had a relatively higher cytoplasmic to nuclear ratio than large lymphocytes and contained a round, kidney- or horseshoe-shaped nucleus adjacent to the cell edge.The nucleus had less intensely
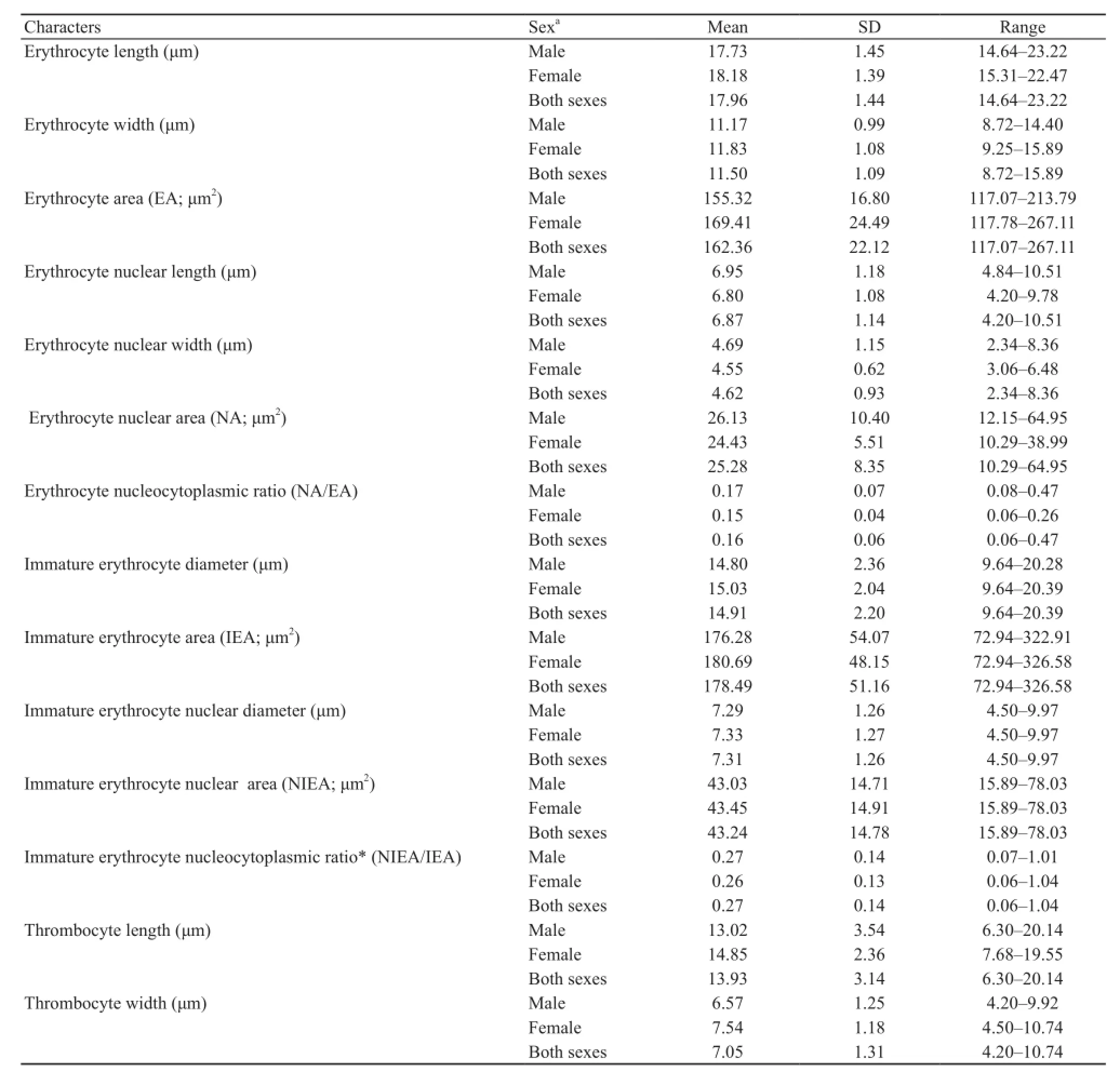
Table 2 Morphometric data of mature and immature erythrocytes and thrombocytes of H.rugulosus collected from Nan Province, Thailand during July to August 2014.
stained chromatin than that of the lymphocytes (Figure 1D).
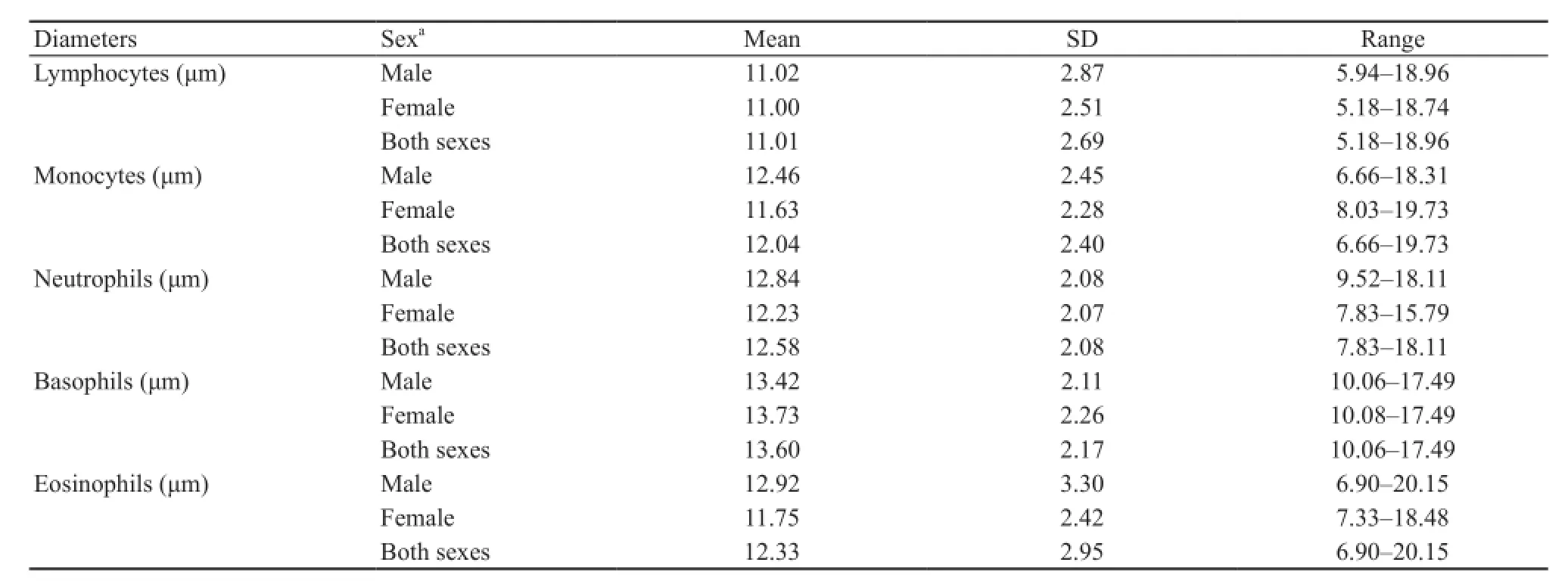
Table 3 Morphometric data of leukocytes (cell diameter) of H.rugulosus collected from Nan Province, Thailand during July to August 2014.
Granulocytes were classified into three cell types (neutrophils, eosinophils and basophils) based on the characteristics of their nuclei and cytoplasmic granules.Neutrophils were round cells, marginally larger (1.04-fold) than monocytes and had a multiple-lobed nucleus,like in humans.However, some cells contained nuclei that looked U-shaped.The cytoplasm contained fine granules and stained light purple (Figure 1E).Eosinophils were sized in between neutrophils and monocytes with a less segmented nucleus, and relatively large cytoplasmic granules of a round to elliptical shape that stained red brown (Figure 1F).Basophils were fairly large cells,slightly (1.03-fold) larger than large lymphocytes, and were characterized by the presence of round highly basophilic (dark blue) granules of various sizes in the cytoplasm.The inconspicuous round nucleus was positioned in the center of the cell (Figure 1G).
4.Discussion
Data on the hematological parameters of amphibians in Asia is still limited.This study is the first report of the hematological parameters in H.rugulosus in Southeast Asia.The number of erythrocytes, as determined by hemocytometer counts, in wild-caught H.rugulosus was higher than that previously reported for other anurans,including that of a closely related species, R.tigrina (Singh, 1977a).
The presence of immature erythrocytes in the circulating blood can occur under normal (healthy)conditions in vertebrates, but if this reaches above the normal value it indicates stress-related condition in erythropoiesis (Briggs and Bain, 2012).In general, an abnormal increase in the number of immature erythrocytes in the circulating blood indicates a compensatory regenerative response due to anemia or loss of circulating erythrocytes (Allender and Fry, 2008).Because of the lack of baseline data of circulating immature erythrocyte levels in H.rugulosus it was not possible to determine if these are normal or abnormal levels of circulating immature erythrocytes, but the external appearance of the frogs were normal and healthy.
Sex-related differences in some blood parameters are known to exist in mammals and other vertebrates (Golemi et al., 2013; Nemeth et al., 2010; Xie et al.,2013).From the blood smear examinations of H.rugulosus, the differential lymphocyte and neutrophil counts showed sex-related difference with higher values in males.However, very few researches have reported sex-related differences of these parameters previously in amphibians.A study in wild-caught Indian tree frog (Polypedates maculatus) reported significantly higher monocyte and eosinophil counts in males than in females (Mahapatra et al., 2012), while the neutrophil/lymphocyte ratio was significantly higher in wild-caught female mole salamanders (Ambystoma talpoideum), which was attributed to reproductive stress (Davis and Maerz,2008).Given that the sampling period used in this study was with the reproductive period of H.rugulosus this may have been the cause of the observed sex-related differences in the blood parameters detected in this study.
From the differential leukocyte counts, agranulocytes were found to comprise a seven-fold higher proportion of the leukocytes than granulocytes (88% and 12% of allleukocytes, respectively).The most abundant leukocytes in H.rugulosus peripheral blood were lymphocytes,which is similar to previous reports in other frog species (Arikan and Cicek, 2014; Cabagna et al., 2005; Cathers et al., 1997; Das and Mahapatra, 2012; Singh, 1977b).
The PCV of these H.rugulosus also showed a signifcant sex-related difference with a higher PCV value in males.The PCV value has been reported to indicate the erythrocyte mass in amphibians (Allender and Fry, 2008).However, the mean PCV value of H.rugulosus regardless of the gender (28%) was similar to those reported in other frogs (Cabagna et al., 2005; Cathers et al., 1997;Donmez et al., 2009; Gul et al., 2011; Mahapatra et al.,2012; Sinha, 1983; Wojtaszek and Adamowicz, 2003), but lower than those of salamander (Solis et al., 2007).The difference in PCV values among amphibians is believed to depend on differences in sex, season, habitat and natural history of each species.
The morphology of blood cells in amphibians is similar in terms of their general characteristics, such as cell shape, nuclear shape and granules in cytoplasm.But some specific characteristics may vary among species,especially the size of each cell type.In this study, the erythrocyte size, determined as dimensions and area,fell within the ranges reported for other anurans (Arikan and Cicek, 2010).The significant difference between the nucleocytoplasmic ratios of circulating mature and immature erythrocytes in H.rugulosus confirmed the identification criteria of immature cells, where a higher nucleocytoplasmic ratio indicate less mature cells.The mean nucleocytoplasmic ratio of circulating erythrocytes in these H.rugulosus was in the range reported in other anurans (Arikan and Cicek, 2014).However, the mean length and width of thrombocytes in these H.rugulosus suggested that this cell type is more ellipsoid than other anurans, while leukocytes also showed a difference in size (diameter) of each leukocyte type compared with that in other anuran species (Arikan and Cicek, 2010).The largest circulating leukocytes in H.rugulosus were basophils and the smallest were lymphocytes.
5.Conclusion
Based on their morphological characteristics, cells in the peripheral blood of H.rugulosus were classified into mature and immature erythrocytes, leukocytes (lymphocytes, monocytes, neutrophils, basophils and eosinophils) and thrombocytes.The presence of immature erythrocytes, even though frequently reported in vertebrates, is interesting since an increase in their circulating level can indicate a disturbance of the erythron.Thus, the data obtained in this study will be useful in assessing abnormality in the erythron of H.rugulosus in the future.Moreover, these results revealed that the PCV and the differential lymphocyte and neutrophil counts of male frogs were signifcantly higher than those of the females.However, the size of each blood cell type was not signifcantly different between sexes of H.rugulosus.The hematological parameters presented in this study are the frst report for H.rugulosus and so represent the crucial baseline data for wild H.rugulosus in Thailand that can be expanded upon to use for monitoring the health status of this anuran in the future.
Acknowledgements We thank Dr.Wichase Khonsue, Mr.Eakkachai Panyain, Mr.Srinun Kumsrikaew, Mr.Rachata Maneein and Mr.Khattapan Jantawongsri for help in the feld and Dr.Robert J.D.Butcher (Publication Counseling Unit, Faculty of Science, Chulalongkorn University) for revision of English language of this manuscript.This research was supported by the Sponsorship of Graduate Student Research under the CU Academic Network in the region (CU-ANR-57-01), Chulalongkorn University and the 90thAnniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund).The authors assure that all portions of this research involving animal subjects had been approved by the Chulalongkorn University Animal Care and Use Committee (Protocol Review Number 1423009).
References
Allender M.C., Fry M.M.2008.Amphibian hematology.Vet Clin Exot Anim Pract, 11: 463-480
Arikan H., Cicek K.2010.Morphology of peripheral blood cells from various species of Turkish herpetofauna.Acta Herpetol, 5: 179-198
Arikan H., Cicek K.2014.Haematology of amphibians and reptiles: A review.North-West J Zool, 10: 190-209
Bain B.J., Lewis M.2012.Preparation and staining methods for blood and bone marrow films.57-68.In Bain B.J., Bates I.,Laffan M.A., Lewis S.M.(Eds.), Dacie and Lewis Practical Haematology, 11thEd.London: Churchill Livingstone.
Briggs C., Bain B.J.2012.Basic haematological techniques.23-56.In Bain B.J., Bates I., Laffan M.A., Lewis S.M.(Eds.),Dacie and Lewis Practical Haematology, 11thEd.London: Churchill Livingstone.
Bloom J.C., Brandt J.T.2008.Toxic responses of the blood.455-484.In Klaassen, C.D.(Ed.), Casarett and Doull's Toxicology: The Basic Science of Poisons, 7thEd.New York: McGraw-Hill.
Burgmeier N.G., Unger S.D., Meyer J.L., Sutton T.M.,Williams R.N.2011.Health and habitat quality assessment for the eastern hellbender (Cryptobranchus alleganiensisalleganiensis) in Indiana, USA.J Wildl Dis, 47: 836-848
Cabagna M.C., Lajmanovich R.C., Stringhini G., Sanchez-Hernandez J.C., Peltzer P.M.2005.Hematological parameters of health status in the common toad Bufo arenarum in agroecosystems of Santa Fe Province, Argentina.Appl Herpetol,2: 373-380
Cathers T., Lewbart G.A., Correa M., Stevens J.B.1997.Serum chemistry and hematology values for anesthetized American bullfrog (Rana catesbeiana).J Zoo Wildl Med, 28: 171-174
Crump M.L., Scott Jr N.J.1994.Visual Encounter Surveys.84-92.In Heyer W.R., Donnelly M.A., McDiarmid R.W., Hayek L.C., Foster M.S.(Eds.), Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians.Washington, D.C.: Smithsonian Institution Press.
Das M., Mahapatra P.K.2012.Blood cell profles of the tadpoles of the Dubois's tree frog, Polypedates teraiensis Dubois, 1986 (Anura: Rhacophoridae).Sci World J, 2012: 701-746
Davis A.K., Maerz J.C.2008.Sex-related differences in hematological stress indices of breeding paedomorphic mole salamanders.J Herpetol, 42: 197-201
Davis A.K., Maney D.L., Maerz J.C.2008.The use of leukocyte profles to measure stress in vertebrates: a review for ecologists.Funct Ecol, 22: 760-772
Diesmos A., Van Dijk P.P., Inger R., Iskandar D., Wai Neng Lau M., Ermi Z., Shunqing L., Baorong G., Kuangyang L., Zhigang Y., Huiqing G., Haitao S., Wenhao C.2004.Hoplobatrachus rugulosus.In: The IUCN Red List of Threatened Species.Version 2014.3.Internet references.Retrieved from http://www.iucnredlist.org/details/full/58300/0 14/05/2015
Donmez F., Tosunoglu M., Gul C.2009.Hematological values in hermaphrodite, Bufo bufo (Linnaeus, 1758).North-West J Zool,5: 97-103
Golemi S., Medja N., Lacej D.2013.Influence of sex on the hematological and morphometric parameters of Cyprinus carpio (Linnaeus, 1758) from Shkodra Lake.Acad J Interdiscip Stud, 2: 45-49
Gul C., Tosunoglu M., Erdogan D., Ozdamar D.2011.Changes in the blood composition of some anurans.Acta Herpetol, 6: 137-147
Heatley J.J., Johnson M.2009.Clinical technique: Amphibian hematology: A practitioner's guide.J Exot Pet Med, 18: 14-19
Kuramoto M.1981.Relationships between number, size and shape of red blood cells in amphibians.Comp Biochem Physiol, 69A: 771-775
Mahapatra B.B., Das M., Dutta S.K., Mahapatra P.K.2012.Hematology of Indian rhacophorid tree frog Polypedates maculatus Gray, 1833 (Anura: Rhacophoridae).Comp Clin Pathol, 21: 453-460
Natt M.P., Herrick C.A.1951.A new blood diluents for counting the erythrocytes and leukocytes of the chicken.Poult Sci, 31: 735-738
Nemeth N., Kiss F., Furka I, Miko I.2010.Gender differences of blood rheological parameters in laboratory animals.Clin Hemorheol Micro, 45: 263-272
Pansook A., Khonsue W., Piyapattanakorn S., Pariyanonth P.2012.Phylogenetic relationships among Hoplobatrachus rugulosus in Thailand as inferred from mitochondrial DNA sequences of the cytochrome-b gene (Amphibia, Anura,Dicroglossidae).Zool Sci, 29: 54-59
Shutler D., Marcogliese D.J.2011.Leukocyte profles of northern leopard frog, Lithobates pipiens, exposed to pesticides and hematozoa in agricultural wetlands.Copeia, 2011: 301-307
Singh K.1977a.Haematology of the common Indian frog Rana tigrina.I.erythrocytes.Anat Anz, 141: 280-284
Singh K.1977b.Hematology of the common Indian frog Rana tigrina.II.Leucocytes.Anat Anz, 141: 445-449
Singh K.1978.Hematology of the common Indian frog Rana tigrina.III.Hemoglobin and hematocrit.Anat Anz, 143: 161-166
Sinha R.C.1983.Haematological studies on the prewintering and wintering frog, Rana esculenta.Comp Biochem Physiol, 74: 311-314
Solis M.E., Bandeff J.M., Huang Y.2007.Hematology and serum chemistry of Ozark and eastern hellbenders (Cryptobranchus alleganiensis).Herpetologica, 63: 285-292
Tharp G.D., Woodman D.A.2002.Experiments in Physiology,8thEd.New Jersey: Prentice Hall.
Wojtaszek J., Adamowicz A.2003.Haematology of the fre-bellied toad, Bombina bombina L.Comp Clin Path, 12: 129-134
Xie L., Xu F., Liu S., Ji Y., Zhou Q., Wu Q., Gong W., Cheng K., Li J., Li L., Fang L., Zhou L., Xie P.2013.Age- and sexbased hematological and biochemical parameters for Macaca fascicularis.PLoS ONE, 8: e64892.Doi: 10.1371/journal.pone.0064892
Zar J.H.1998.Biostatistical Analysis, 4thEd.New Jersey: Prentice Hall.
Zhelev Z.M., Angelov M.V., Mollov I.A.2006.A study of some metric parameters of the erythrocytes in Rana ridibunda (Amphibia: Anura) derived from an area of highly developed chemical industry.Acta Zool Bulg, 58: 235-244
E-mail: jirarach.s@chula.ac.th
Received: 23 May 2015 Accepted: 11 March 2016
DOI:10.16373/j.cnki.ahr.150037
*Corresponding author:Dr.Jirarach Kitana, Department of Biology,Faculty of Science, Chulalongkorn University, Bangkok, Thailand, with her research focusing on microanatomy, environmental biology and ecotoxicology of fsh, amphibians and reptiles.
 Asian Herpetological Research2016年2期
Asian Herpetological Research2016年2期
- Asian Herpetological Research的其它文章
- Tracing the Origin of the Black-spotted Frog, Pelophylax nigromaculatus, in the Xinjiang Uyghur Autonomous Region
- Genetic Diversity and Population Structure for the Conservation of Giant Spiny Frog (Quasipaa spinosa) Using Microsatellite Loci and Mitochondrial DNA
- Coevolution of Male and Female Response Preferences to Sexual Signals in Music Frogs
- Ecological Niche Divergence between Trapelus ruderatus (Olivier,1807) and T.persicus (Blanford, 1881) (Sauria: Agamidae) in the Middle East
- The Effects of Chronic Hypoxia on Thermoregulation and Metabolism in Phrynocephalus vlangalii
- Comparison of Skull Morphology in Two Species of Genus Liua (Amphibia: Urodela: Hynobiidae), L.shihi and L.tsinpaensis
