The Effects of Chronic Hypoxia on Thermoregulation and Metabolism in Phrynocephalus vlangalii
Weixin LI, Shiwei LIANG, Huihui WANG, Ying XIN, Songsong LU, Xiaolong TANG and Qiang CHEN
School of Life Science, Lanzhou University, 222 Tian Shui South Road, Lanzhou, China
The Effects of Chronic Hypoxia on Thermoregulation and Metabolism in Phrynocephalus vlangalii
Weixin LI, Shiwei LIANG, Huihui WANG, Ying XIN, Songsong LU, Xiaolong TANG and Qiang CHEN*
School of Life Science, Lanzhou University, 222 Tian Shui South Road, Lanzhou, China
Abstract Phrynocephalus vlangalii are widely distributed on Tibetan plateau spanning diverse altitudes and habitats.In the present study, P.vlangalii were exposed to 8% oxygen concentration in a hypoxic chamber for 6 weeks.Then the body temperature (Tb), standard metabolic rate (SMR), heart rate and metabolic enzyme activities of the lizards were measured at 20°C and 30°C.The results indicated that hypoxia exposure decreased Tb, SMR and heart rate.Lactate dehydrogenase (LDH) activity of 8% O2group became signifcant elevated in liver and skeletal muscle compared with control group at 20°C, but descended significantly in heart.Using electrophoresis we found that LDH contains five isozymes (LDH1, LDH2, LDH3, LDH4 and LDH5) and are expressed specifcally in liver, skeletal muscle and heart.Citrate synthase (CS) activity in the liver also decreased at 20°C and 30°C.No signifcant difference of CS activity was found between the two groups in skeletal muscle and heart.
Keywords lizard, hypoxia, hypothermia, standard metabolic rate, enzyme activity
1.Introduction
Vertebrates living at high altitude are subjected to hypoxic conditions that challenge their aerobic metabolism.Hypoxia can elicit an array of behavioral and physiological responses in animals ranging from protozoa to mammals.One of the predominant responses is hypothermia that decreases O2demand through reducing body temperature (Steiner and Branco, 2002).It is suggested that hypothermia is a regulatory response that protects tissues against oxygen depletion, particularly in life-sustaining organs such as the heart and brain (Cadena and Tattersall, 2009).Low oxygen could cause reptiles to select lower body temperatures in the laboratory (He et al.,2013a; Hicks and Wood, 1985) as well as in the field (Rollinson et al., 2008).
Generally organisms employ two initial metabolicstrategies during hypoxic conditions: (1) an overall reduction of metabolic rate; and (2) a shift in the aerobic and anaerobic contributions to total metabolism (Hochachka et al., 1996; Hochachka et al., 1997; Storey,1998; Via et al., 1998; Virani and Rees, 2000).Citrate synthase (CS), an indicator of aerobic metabolism, did not change in estuarine fish (Leiostomus xanthurus)(Cooper et al., 2002), but increased in harbor seals (Phoca vitulina) (Fuson et al., 2003) and pikas (Sheafor,2003) under hypoxic conditions.LDH, an indicator of anaerobic metabolism, catalyzes the conversion of pyruvate to lactate with concomitant conversion of NADH to NAD+(Sheafor, 2003).Reports indicate LDH activity is influenced by environmental oxygen concentration in vertebrates.Fuson et al.(2003) found hepatic LDH activity increased in seals during hypoxia.However, lower myocardial LDH activity was observed in yak at high altitude (Kuang et al., 2010).LDH is a tetramer composed of M and H subunits encoded by A and B genes which form fve isozymes LDH1-5 in tissues (Holbrook et al., 1975).LDH1 is composed of only the H subunit, while LDH5 contains only M subunit.LDH-H subunit can facilitate lactate reconversion, while LDH-Msubunit holds the opposite effect (Kuang et al., 2010).
Phrynocephalus vlangalii are widely distributed on the Tibetan plateau covering a large span of altitude (2000-4300 m above sea level) and diverse habitats.Recent phylogenetic studies found that all high altitude species formed a monophyletic group, which nested within the low elevation species (Guo and Wang, 2007).The result suggests that the high altitude species may have evolved from low altitude ancestors.So the comparison between the hypoxia group and control group may provide information regarding the high altitude adaptation.Until recently, the studies on this species mainly consist of the infuence of high altitude on body size (Jin et al., 2007)and the effect of hypoxic acclimatization on anatomical,physiological and biochemical manifestation such as heart weight, hemoglobin concentration and enzyme activities (lactate dehydrogenase and succinate dehydrogenase)(He et al., 2013b).In this study, thermoregulation,standard metabolic rate and metabolic enzymes under hypoxic conditions were investigated to elucidate the physiological response to hypoxia in a high land lizard (P.vlangalii).We also expected to fnd a strong correlation between the physiological indexes and oxygen tension.
2.Materials and Methods
2.1 Lizard collection and hypoxic acclimation Sixty individuals (all males) of P.vlangali were collected from Gonghe (36°27′ N, 100°61′ E) on the North Tibetan (Qinghai) plateau in early July 2013.This area belongs to the plateau subfrigid zone and the altitude is about 2850 m.All the lizards were brought to Lanzhou University within 3 days after capture.The mean body mass and snout vent length were 5.65 ± 0.39 g and 5.35 ± 0.35 cm,respectively.Lizards were randomly divided into two groups (n = 30, all males) and exposed to two different levels of oxygen concentrations (15% O2group as control group and 8% O2group as hypoxic acclimatization group) in the non-pressurized hypoxic chambers for 6 weeks.We chose 15% and 8% oxygen concentration since the oxygen concentration of capture site is about 15%, and 8% oxygen concentration would cause obvious physiological responses of lizards according to previous study (He et al., 2013a).All individuals were housed together in hypoxic chambers within each group.The base of the chambers (1.00 m length, 0.45 m width and 0.40 m height) was covered with 5 cm of sand.The oxygen concentration was maintained by the influx of nitrogen controlled by an oxygen controller (KY-2F,Sanjiang Oxygen Analysis Instruments Plant, China).We controlled the room temperature using air-conditioning and kept at 17 ± 2 °C.One end of the chamber was heated with a 100 W bulb, which provided a thermal gradient.The lizards were exposed to a photoperiod of 12L: 12D.Heat was available from 08:30 h to 18:30 h.The chamber was opened for 10 min to clean and supplement food and water every day.
2.2 Tissue sampling Lizards were euthanized in airtight containers containing ethyl ether in the fuming cupboard at the laboratory (Davor et al., 2008).All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory animals (Ministry of Science and Technology of China, 2006), and were approved by the animal ethics committee of Lanzhou University.Liver, skeletal muscles (all of the hind leg muscles) and heart were removed, washed with saline solution, and then frozen in liquid nitrogen for enzymatic analysis.According to different individuals, the mass of the sample has certain differences, but we used similar sample weight in the experiment.
2.3 TbAfter the 6 weeks acclimation period, Tbwas measured by a digital electronic thermometer every two hours from 1000 h to 1800 h and lasted 6 days.We randomly selected 20 lizards each group every time.The probe was inserted 3 mm into the cloaca of lizards.Alcohol was utilized to scrub their cloaca to prevent infection.In addition, we corrected digital electronic thermometer using mercurial thermometer in the digital circulating water bath every other day.At the same time,the air and sand temperature was measured under the heating source and in the cold section of the hypoxic chamber.
2.4 SMR and heart rate SMR was assessed by a small animal respiratory measurement system (RP1LP, Qubit,Canada).All lizards were fasted 2 days before assessment,but water was complemented as usual.The test of SMR was made in a dark room from 2000 h to 0600 h.In the wild, active body temperatures of the lizards were in the range of about 17°C to 32°C, so the measurements were conducted at 20°C and 30°C with different oxygen concentrations (15% and 8%).For each group the oxygen concentration during measurement remained the same with acclimation.The animals were allowed to adjust to measuring temperature more than 30 min before test and the measurements lasted about 30-50 min.The SMR was expressed in the production of carbon dioxide (CO2).Heart rate was determined by electrocardiograph (ECG)with BL-420E Biological Signal Acquisition and Analysis System (Chengdu Taimeng technology, Ltd, China) at20°C and 30°C with 15% and 8% oxygen concentration.
2.5 Enzyme activities CS and LDH activities in liver, skeletal muscle and heart were measured by UV Spectrophotometer (TU-1901, Beijing Puxi Instrument co., Ltd, China).Thawed samples were homogenized in nine volumes of buffer (w/v) on the ice with a glass homogenizer, using the following composition: 10 mM potassium phosphate buffer, pH 6.5.Homogenates were centrifuged at 3000 g and 4°C for 10 min, and the supernatant kept at 4°C until assay.Enzyme activities were measured by the spectrophotometer at 20°C and 30°C which controlled by the circulating water bath.Soluble protein concentration was measured in all tissue using bovine serum albumin standards.Assay conditions were as follows, CS: 100 mM Tris-HCl (pH 8.0), 0.1 mM DTNB, 0.1 mM acetyl CoA, 150 mM oxaloacetic acid,λ=420 nm; LDH: 100 mM potassium phosphate buffer (pH 7.0), 0.16 mM NADH, 0.4 mM pyruvate, λ=340 nm.Enzyme activities were described as U·mg-1pro (μmol substrate converted min-1per mg tissue protein).Thermal sensitivities of enzymes were expressed as Q10values that were calculated as
Q10= (k2/k1)10/(T2-T1), where ki= reaction rate at temperature Ti.
2.6 LDH isozymes analysis Isozymes of LDH were separated by polyacrylamide gel electrophoresis using a vertical gel electrophoresis system (DYCZ-25D, Beijing Liuyi Instrument Factory, China).The gel was comprised of a separating gel of 7.5% acrylamide in 1.5 M Tris-HCl (pH 8.9) and a stacking gel of 5% acrylamide in 0.5 M Tris-HCl (pH 6.7).Homogenates were diluted with 25% stacking gel buffer, 10% sucrose and 0.25% bromophenol blue.Volume of each sample was 6 µl.Electrophoresis was operated for 4 hours under constant current and then 9 hours under constant voltage (DYY-10C, Beijing Liuyi Instrument Factory, China).Staining solution was as follows: 5 mg ml-1oxidized coenzyme Ι, 1 M sodium lactate, 100 mM NaCl, 1 mg ml-1phenazine methosulfate,1 mg ml-1nitro blue tetrazolium, 100 mM phosphate buffer (pH 7.5).
2.7 Statistical analysis Using SPSS 16.0 software, we analyzed the relationships between Tb, SMR, heart rate,enzyme activity and different oxygen concentrations.The data were tested for normality and homogeneity of variances to meet the assumptions of Para metric testing prior to analysis and no signifcant deviations from these assumptions were evident in the data.The one way analysis of variance was used to evaluate the effect of hypoxia on Tb.Then the remaining results were analyzed by the multi-factor analysis of variance.All results are expressed as means ± SE.P < 0.05 indicates a statistically signifcant difference.P < 0.001 indicated a statistically very signifcant difference.
3.Results
3.1 Effect of chronic hypoxia on behavioral thermoregulation Compared to 15% O2group, Tbof 8% O2group was signifcantly lower at 1000 h (F1, 238= 22.256, P < 0.001), 1600 h (F1, 238= 31.983, P < 0.001) and 1800 h (F1, 238= 44.612, P < 0.001) (Figure 1).There was no difference between two groups at 1200 h and 1400 h (P > 0.05).As shown in Table 1 and Table 2, there was no signifcant difference in the air temperature and sand temperature between two hypoxic chambers.
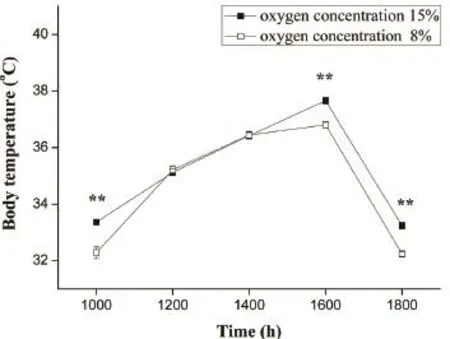
Figure 1 Preferred Tbof lizards exposed to 15% O2and 8% O2for 6 weeks at different times.The errorsbars shows standard error.The square and triangle mean 15% O2group and 8% O2group,respectively.**P < 0.001 when compared between two groups.
3.2 Effect of chronic hypoxia on SMR and heart rate SMR of 8% O2group was signifcantly lower than 15% O2group at 30°C (F1,10= 20.352, P = 0.001).However,no significant reduction in SMR was observed at 20°C (Figure 2).Compared with 15% O2group, 8% O2group had lower heart rate at 20°C (F1,17= 101.323, P <0.001) and 30°C (F1,18= 26.038, P < 0.001) (Figure 3).Similar to SMR, the heart rate increased markedly as the temperature rose.The results of multi-factor analysis of variance indicated in Table 3.The results showed that oxygen concentration and temperature for SMR and enzymes activities have no interaction besides heart rate.
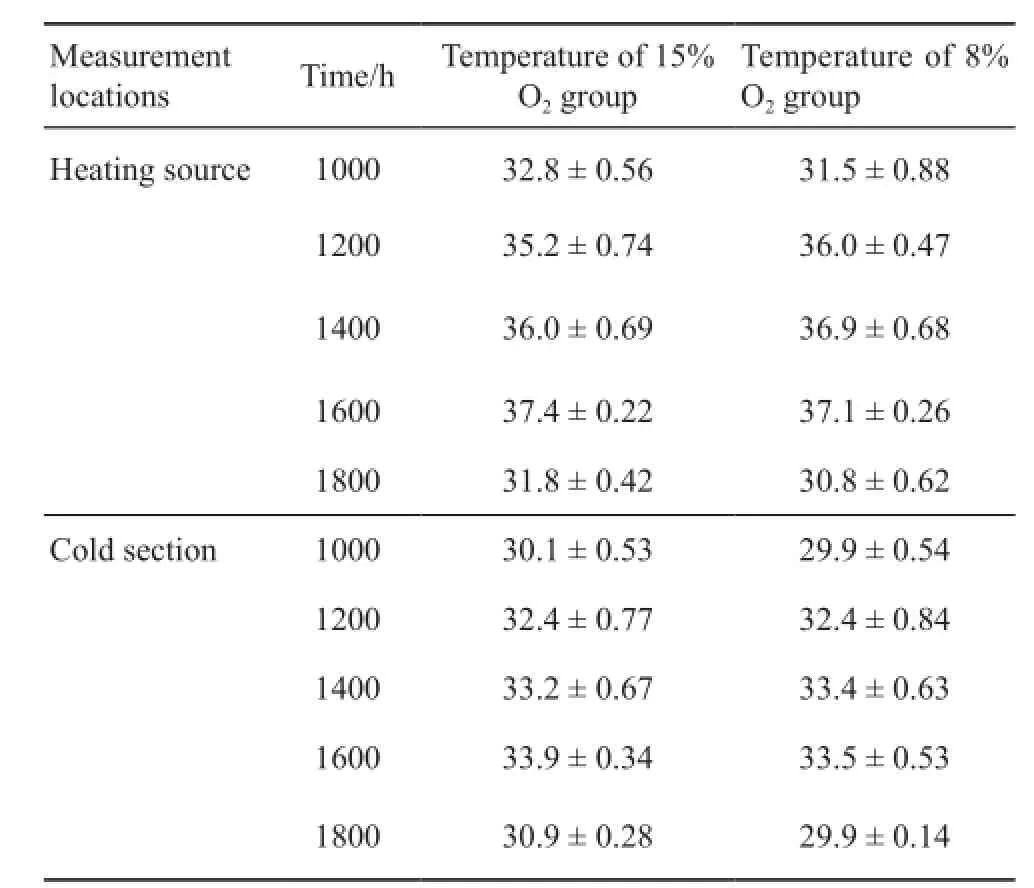
Table 1 The air temperature recorded under the heating source and in the cold section of the hypoxic chamber.
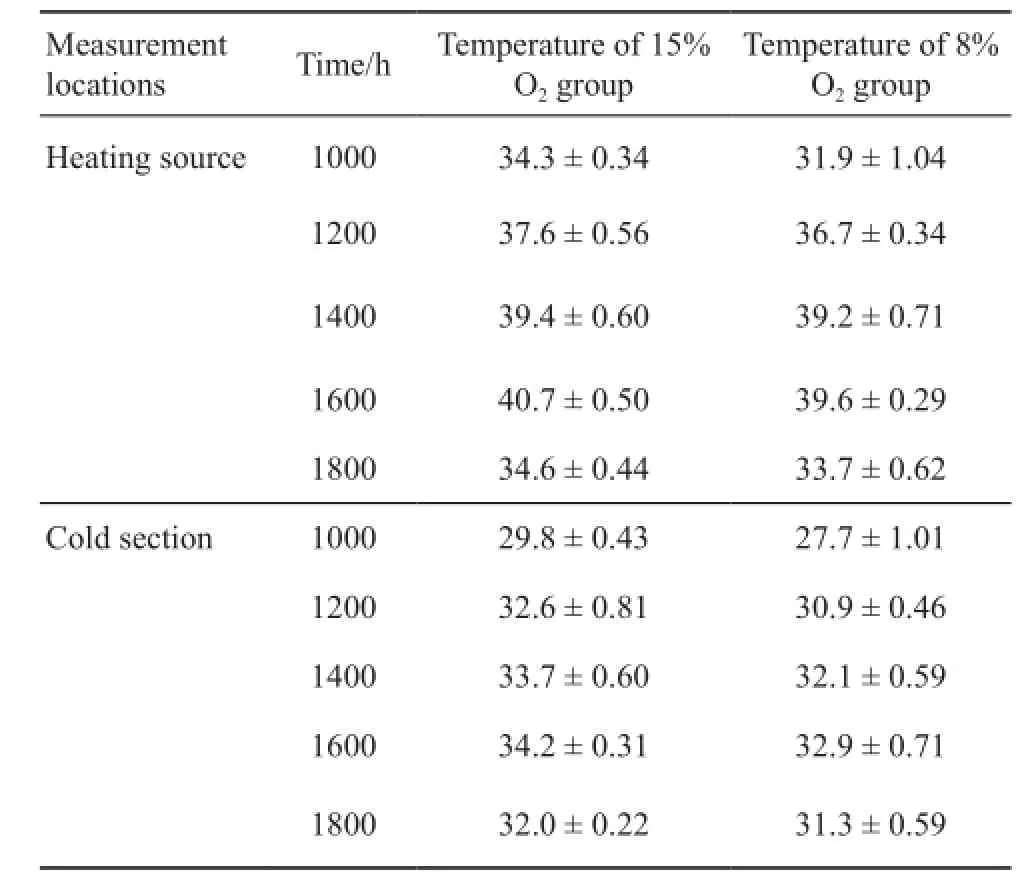
Table 2 The temperature recorded of sand under the heating source and in the cold section of the hypoxic chamber.
3.3 Enzyme activities and isozymes distribution As shown in Figure 4, LDH activity of 8% O2group was significantly higher in liver and skeletal muscle than control (15% O2) group at 20°C (P < 0.05) although there was no significant difference at 30°C.However, after hypoxic acclimatization LDH activity in the heart was significantly lower than control both at 20°C and 30°C (P < 0.001).The CS activity of 8% O2group was lower than 15% O2group in liver at 20°C (F1,12= 7.482, P = 0.018) and 30°C (F1,12= 9.147, P = 0.011) (Figure 5).No significant difference of CS activity was found between two groups in skeletal muscle and heart.The Q10of LDH and CS of these tissues at different oxygen concentrations are summarized in Table 4.The electrophoresis results showed that LDH isozyme pattern of this lizard contained five bands (LDH1, LDH2, LDH3, LDH4 and LDH5).LDH1 and LDH2 were found predominantly in heart,while LDH4 and LDH5 were mainly observed in skeletal muscle and liver (Figure 6).
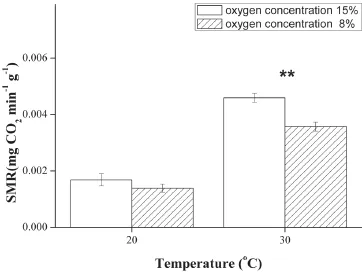
Figure 2 SMR of lizards exposed to different oxygen concentrations at 20°C and 30°C.**P < 0.001 when compared between two groups at the same temperature.
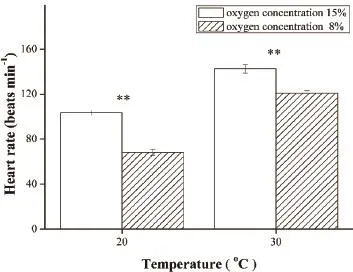
Figure 3 Heart rate of lizards exposed to different oxygen concentrations at 20°C and 30°C.**P < 0.001 when compared between two groups at the same temperature.
4.Discussion
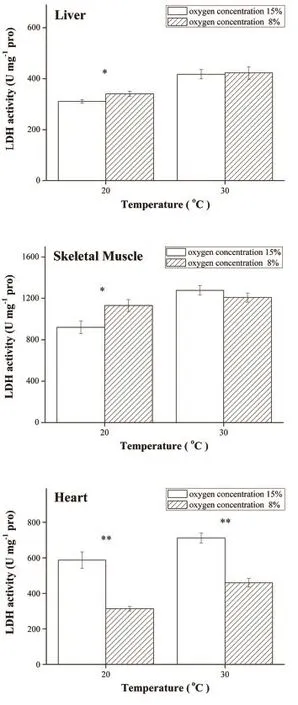
Figure 4 Lactate dehydrogenase (LDH) activity of liver, skeletal muscle and heart was measuredat 20°C and 30°C under different oxygen concentrations.* P < 0.05 when compared between two groups at the same temperature.
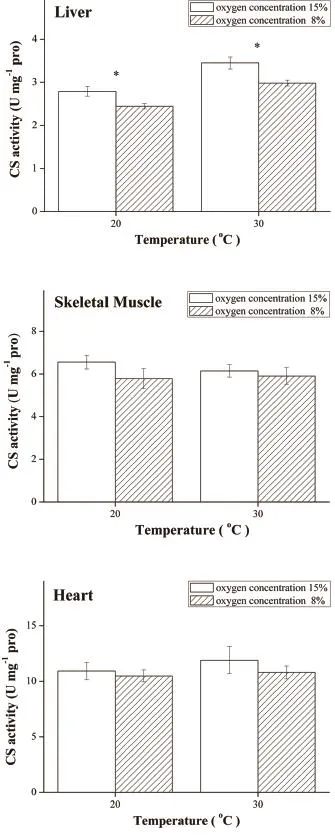
Figure 5 Citrate synthase (CS) activity of liver, skeletal muscle and heart was measured at 20°C and 30°C under different oxygen concentrations.*P < 0.05 when compared between two groups at the same temperature.
Highland vertebrates usually exhibit functional and structural modifications that allow them to cope with the concomitant decrease in oxygen tension.Mammals generally acclimatize to hypoxia by decreasing both body temperature and energy metabolism (Wood, 1991).However, little information is available about the infuence of hypoxia on metabolism in reptiles.In present study, we investigated the effect of chronic hypoxic acclimatization on Tb, standard metabolic rate, heart rateand enzyme activities in P.vlangalii.Our results indicated that hypoxia caused variations on these issues.
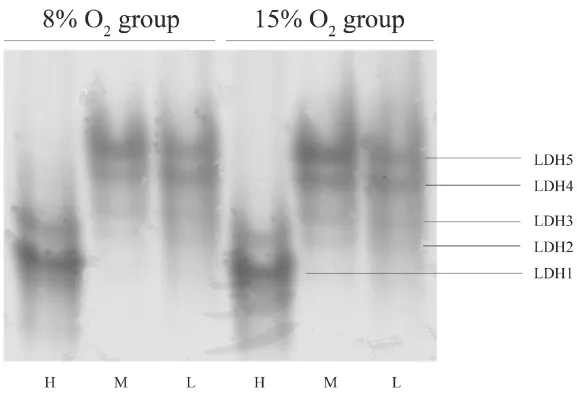
Figure 6 LDH isozyme electrophoresis of lizards acclimated to different oxygen concentrations.LDH contains fve isozymes (LDH1, LDH2,LDH3, LDH4 and LDH5).H: Heart, M: Skeletal muscle, L: Liver.
4.1 Behavioral thermoregulation and SMR Given a choice in the hypoxic environment, a reasonable prediction is animals would move from a warm area to a cooler area and reduce body temperature (Jackson,2007).In this study, P.vlangalii in 8% O2group selected a lower Tbcompared to those in 15% O2group at 1000 h,1600 h and 1800 h (Figure 1).Similar results were also reported in other ectotherms, including lizards (Varanus exanthematicus, V.varivs, Iguana iguana, Ctenosaura pectinata, Phrynocephalus przewalskii) (Hicks and Wood,1985; He et al., 2013a), toads (Bufo marinus) (Wood and Malvin, 1991), and alligators (Alligator mississippiensis)(Branco et al., 1993), which have suggested that the low body temperature could change physiological functions and be in favor of higher survival rates under hypoxic conditions.As shown in Table 1 and Table 2,the temperature was no significant difference between two hypoxic chambers.This result indicated that hypoxia exposure decreased Tb.
In ectothermic vertebrates, several studies document downregulation of cellular metabolism in response to hypoxia; turtles (Trachemys scripta), for example, reduce heat production by 85% during anoxic submergence (Jackson, 1968; Lutz and Nilsson, 1993).Similar result is reported for Rana temporaria (West and Boutilier,1998).In general, an overall reduction in metabolic rate was considered as the initial strategies on metabolism during hypoxia (Cooper et al., 2002).Our data clearly demonstrated that hypoxia was accompanied by a decrease of SMR at 30°C (Figure 2).This result is similar to that of sagebrush lizards (Sceloporus graciosus)at high-altitude (Sears, 2005), but inconsistent to Phrynocephalus przewalskii exposed to acute hypoxia (He et al., 2013a).SMR increase will cause the imbalance of oxygen supply and demand during acute hypoxia, so SMR cannot increase for a long time.So organisms need to reach a steady state during chronic hypoxia and then maintain the balance of metabolism (Hicks and Wang,2004).In most newborn mammals (e.g.rats), chronic exposure to hypoxia also results in hypometabolism (Mortola, 2001).SMR of P.vlangalii did not change at 20°C during hypoxia.It may be related to the attenuation of the effect of hypoxic stress in cold ambient temperature (Jackson, 2007).Moreover, hypoxic acclimatization caused a signifcant lower heart rate at 20°C and 30°C in this study (Figure 3).Similar fndings have been reported in previous studies (Boutilier and Toews, 1977; Stecyk and Farrell, 2007).Taken together, our data showed consistent results for the infuence of chronic hypoxia on Tb, SMR and heart rate in P.vlangalii.
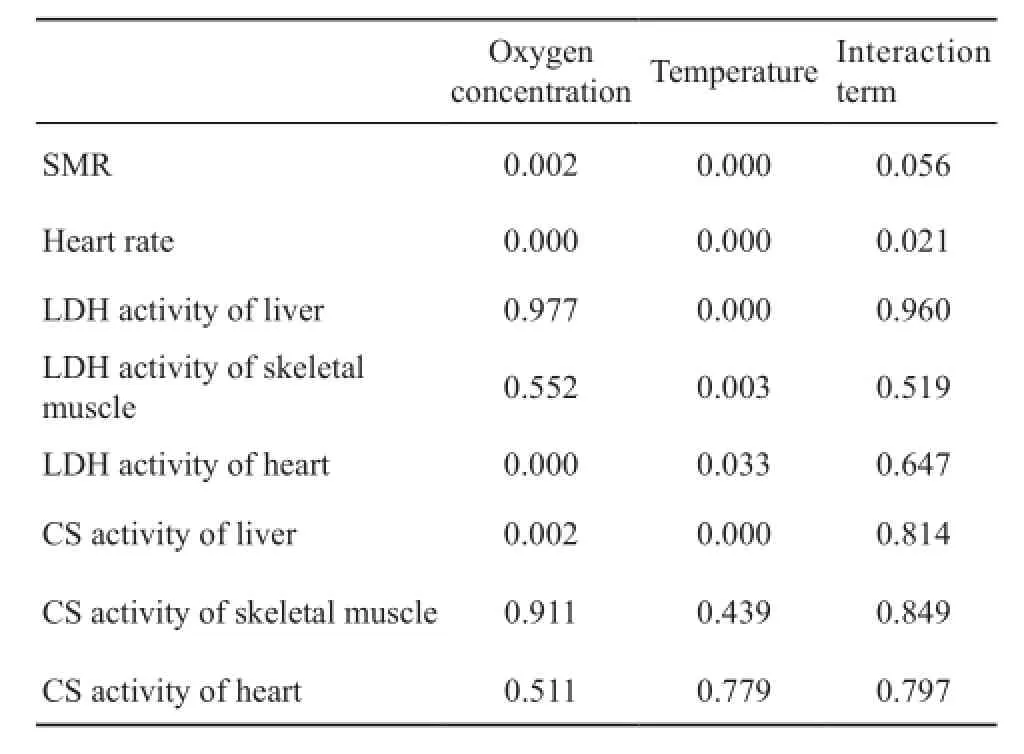
Table 3 The results of multi-factor analysis of variance.
4.2 Enzyme activities A predicted response to hypoxia is an increase of the levels of anaerobic enzymes and a decrease of the levels of oxidative enzymes (Webster,2003).However, our results demonstrated that the effect of hypoxia on the enzyme activity was tissue specifc in P.vlangalii.Individuals housed at 8% O2exhibited higher levels of LDH activity in liver and skeletal muscle at 20°C (Figure 4).Liver is one of major organs converting lactate from other tissues such heart, and lactate is the substrate of gluconeogenesis (Zhou et al., 2000).Glycogen reserves decline during chronic hypoxia, liver gluconeogenesis must be activated to supply glucose required for anaerobic metabolism (Wright et al., 1989).LDH activity of skeletal muscle was the highest among the tested organs, reflecting the capacity to switch on anaerobic and which may be related to locomotion.However, LDH activity in heart of 8% O2group was lower than 15% O2group at both 20°C and 30°C.The results suggested that the ability to generate ATP through anaerobic glycolysis did not ascend in myocardium during chronic hypoxia.This result was inconsistent with the predicted response.Furthermore, LDH-H is the isozyme found predominantly in heart of P.vlangalii and preferentially converts lactate to pyruvate.The high levels of LDH-H in heart would allow lactate to be used as a metabolic substrate, which removes the possibility of cardiac muscle fatigue due to pH imbalances (Hochachka and Somero, 1984).LDH-M is found predominantly in liver and skeletal muscle of P.vlangalii.Long-term development under hypoxia may facilitate a number of physiological or biochemical processes that minimize the need for LDH activation or other anaerobic responses (Crocker et al., 2013).Moreover, a downregulation of anaerobic metabolic capacity is thought to be caused by a more effcient coupling between ATP demand and ATP supply, allowing for a more effective integration between glycolysis and oxidative metabolism (Hochachka et al.,1983; Tang et al., 2013).
Compared with 15% O2group, hypoxic acclimatization group had lower CS activity in liver, but not in skeletal muscle and heart (Figure 5).CS activity in heart of P.vlangalii was the highest among the tested tissues, which implies that the heart prefer aerobic metabolism.CS activity in liver decreased after being acclimatized to chronic hypoxia and this may be caused by two reasons: 1) reduced demand of ATP at low oxygen concentration (He et al., 2013a); 2) liver generated enough ATP for its own requirements from the process of removal of lactic acid.The decreased CS activity in liver of P.vlangalii under hypoxia may limit carbohydrate oxidation,associated with a depression of aerobic metabolism.
In summary, our study demonstrates that chronic hypoxia acclimatization can induce changes in the Tband metabolism of P.vlangalii, a lizard living at high altitude.However, accurate adaptation mechanisms of P.vlangalii through hypoxia acclimatization are not clear, which need further study in the future.
Acknowledgements We appreciate the comments of an anonymous reviewer.Research funding was supported by the National Natural Science Foundation of China (Nos.31272313 and 31472005 to Q.CHEN) and Fundamental Research Funds for the Central Universities (lzujbky-2015-81 to X, L.Tang).We thank Yaojun QIN,Yucheng BAI, Yang ZHANG, Yonggang NIU for their suggestions and assistance in the study.
References
Boutilier R.G., Toews D.P.1977.The effect of progressive hypoxia on respiration in the toad Bufo marinus.J Exp Biol,68: 99-107
Branco L.G.S., Glass M.L., Wang T., Hoffmann A.1993.Temperature and central chemoreceptor drive to ventilation in toad (Bufo paracnemis).Resp Physiol, 93: 337-346
Cadena V., Tattersall G.J.2009.Decreased precision contributes to the hypoxic thermoregulatory response in lizards.J Exp Biol, 212: 137-144
Cooper R.U., Clough L.M., Farwell M.A., West T.L.2002.Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fsh (Leiostomus xanthurus).J Exp Mar Biol Ecol, 279: 1-20
Crocker C.D., Chapman L.J., Martinez M.L.2013.Hypoxiainduced plasticity in the metabolic response of a widespread cichlid.Comp Biochem Phys B, 166: 141-147
Davor V., Luciano A.A., Joaquim J.V., Charles R.B.2008.Helminth parasites of two sympatric lizards, Enyalius iheringii and E.Perditus (Leiosauridae), from an Atlantic Rainforest ares of southeastern Brazil.Acta Parasitol, 53(2): 222-225
Fuson A.L., Cowan D.F., Kanatous S.B., Polasek L.K., Davis R.W.2003.Adaptations to diving hypoxia in the heart,kidneys and splanchnic organs of harbor seals (Phoca vitulina).J Exp Biol, 206: 4139-4154
Guo X., Wang Y.2007.Partitioned Bayesian analyses, dispersalvicariance analysis, and the biogeography of Chinese toadheaded lizards (Agamidae: Phrynocephalus): A re-evaluation.Mol Phylogenet Evol, 45: 643-662
He J., Xiu M., Tang X., Wang N., Xin Y., Li W., Chen Q.2013a.Thermoregulatory and metabolic responses to hypoxia in the oviparous lizard (Phrynocephalus przewalskii).Comp Biochem Phys A, 165: 207-213
He J., Xiu M., Tang X., Yue F., Wang N., Yang S., Chen Q.2013b.The Different Mechanisms of Hypoxic Acclimatization and Adaptation in Lizard Phrynocephalus vlangalii Living on Qinghai-Tibet plateau.J Exp Zool Part A, 319: 117-123
Hicks J.W., Wang T.2004.Hypometabolism in reptiles: behavioural and physiological mechanisms that reduce aerobic demands.Resp Physiol Neurobi, 141: 261-271
Hicks J.W., Wood S.C.1985.Temperature regulation in lizards: effects of hypoxia.Am J Physiol-Reg I, 248: R595-R600
Hochachka P., Buck L., Doll C., Land S.1996.Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack.P Natl A Sci, 93: 9493-9498
Hochachka P., Land S., Buck L.1997.Oxygen sensing and signal transduction in metabolic defense against hypoxia: lessons from vertebrate facultative anaerobes.Comp Biochem Phys A,118: 23-29
Hochachka P., Stanley C., Merkt J., Sumar-Kalinowski J.1983.Metabolic meaning of elevated levels of oxidative enzymes in high altitude adapted animals: An interpretive hypothesis.Resp Physiol, 52: 303-313
Hochachka P.W., Somero G.N.1984.Biochemical adaptation.Princeton University Press Princeton
Holbrook J.J., Liljas A., Steindel S.J., Rossmann M.G.1975.Lactate dehydrogenase.The enzymes, 11: 191-292
Jackson D.C.1968.Metabolic depression and oxygen depletion in the diving turtle.J Appl Physiol, 24: 503-509
Jackson D.C.2007.Temperature and hypoxia in ectothermic tetrapods.J Therm Biol, 32: 125-133
Jin Y., Liu N., Li J.2007.Elevational variation in body size of Phrynocephalus vlangalii in the North Qinghai-Xizang (Tibetan)plateau.Belg J Zool, 137: 197
Kuang L., Zheng Y., Lin Y., Xu Y., Jin S., Li Y., Dong F., Jiang Z.2010.High-altitude adaptation of yak based on genetic variants and activity of lactate dehydrogenase-1.Biochem genet, 48: 418-427
Lutz P.L., Nilsson G.1993.Metabolic transitions to anoxia in the turtle brain: role of neurotransmitters.In: The Vertebrate Gas Transport Cascade: Adaptations to Environment and Mode of Life, edited by J.Eduardo and P.W.Bicudo.Boca Raton, FL,CRC: 323-329
Mortola J.P.2001.Respiratory physiology of newborn mammals: a comparative perspective.Baltimore, MD: Johns Hopkins University Press
Rollinson N., Tattersall G.J., Brooks R.J.2008.Overwintering habitats of a northern population of Painted Turtles (Chrysemys picta): winter temperature selection and dissolved oxygen concentrations.J Herpetol, 42: 312-321
Sears M.W.2005.Resting metabolic expenditure as a potential source of variation in growth rates of the sagebrush lizard.Comp Biochem Phys A, 140: 171-177
Sheafor B.A.2003.Metabolic enzyme activities across an altitudinal gradient: an examination of pikas (genus Ochotona).J Exp Biol, 206: 1241-1249
Stecyk J.A., Farrell A.P.2007.Effects of extracellular changes on spontaneous heart rate of normoxia-and anoxia-acclimated turtles (Trachemys scripta).J Exp Biol, 210: 421-431
Steiner A.A., Branco L.G.2002.Hypoxia-induced anapyrexia: implications and putative mediators.Annu Rev Physiol, 64: 263-288
Storey K.B.1998.Survival under stress: molecular mechanisms of metabolic rate depression in animals.S Afr J Zool, 33: 55-64
Tang X., Xin Y., Wang H., Li W., Zhang Y., Liang S., He J.,Wang N., Ma M., Chen Q.2013.Metabolic Characteristics and Response to High Altitude in Phrynocephalus erythrurus (Lacertilia: Agamidae), a Lizard Dwell at Altitudes Higher Than Any Other Living Lizards in the World.PloS one 8,e71976
Via J.D., Van den Thillart G., Cattani O., Cortesi P.1998.Behavioural responses and biochemical correlates in Solea solea to gradual hypoxic exposure.Can J Zool, 76: 2108-2113
Virani N.A., Rees B.B.2000.Oxygen consumption, blood lactate and inter-individual variation in the gulf killifish (Fundulus grandis), during hypoxia and recovery.Comp Biochem Phys A, 126: 397-405
Webster K.A.2003.Evolution of the coordinate regulation of glycolytic enzyme genes by hypoxia.J Exp Biol, 206: 2911-2922
West T.G., Boutilier R.G.1998.Metabolic suppression in anoxic frog muscle.J Comp Physiol B, 168: 273-280
Wood S.C.1991.Interactions between hypoxia and hypothermia.Annu Rev Physiol, 53: 71-85
Wood S.C., Malvin G.M.1991.Physiological significance of behavioral hypothermia in hypoxic toads (Bufo marinus).J Exp Biol, 159: 203-215
Wright P.A., Perry S.F., Moon T.W.1989.Regulation of hepatic gluconeogenesis and glycogenolysis by catecholamines in rainbow trout during environmental hypoxia.J Exp Biol, 147: 169-188
Zhou B., Randall D., Lam P., Ip Y., Chew S.2000.Metabolic adjustments in the common carp during prolonged hypoxia.J Fish Biol, 57: 1160-1171
E-mail: chenq@lzu.edu.cn
Received: 12 February 2015 Accepted: 11 January 2016
DOI:10.16373/j.cnki.ahr.150010
*Corresponding author:Prof.Qiang CHEN, from School of Life Science, Lanzhou University, Gansu, China, with his research focusing on physio-ecology of reptiles, peptide biochemistry and pharmacology.
 Asian Herpetological Research2016年2期
Asian Herpetological Research2016年2期
- Asian Herpetological Research的其它文章
- Tracing the Origin of the Black-spotted Frog, Pelophylax nigromaculatus, in the Xinjiang Uyghur Autonomous Region
- Genetic Diversity and Population Structure for the Conservation of Giant Spiny Frog (Quasipaa spinosa) Using Microsatellite Loci and Mitochondrial DNA
- Coevolution of Male and Female Response Preferences to Sexual Signals in Music Frogs
- Ecological Niche Divergence between Trapelus ruderatus (Olivier,1807) and T.persicus (Blanford, 1881) (Sauria: Agamidae) in the Middle East
- Comparison of Skull Morphology in Two Species of Genus Liua (Amphibia: Urodela: Hynobiidae), L.shihi and L.tsinpaensis
- Discovery of Female Laudakia papenfussi Zhao, 1998, with Insights into its Phylogenetic Relationships
