Ecological Niche Divergence between Trapelus ruderatus (Olivier,1807) and T.persicus (Blanford, 1881) (Sauria: Agamidae) in the Middle East
Seyyed Saeed HOSSEINIAN YOUSEFKHANI, Omid MIRSHAMSI, Çetin ILGAZ,Yusuf KUMLUTAŞand Aziz AVCI
1Department of Biology, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran2Zoological Innovations Research Department, Institute of Applied Zoology, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran3Dokuz Eylül University, Faculty of Science, Department of Biology, 35160 Buca, İzmir, Turkey4Adnan Menderes University, Faculty of Science and Arts, Department of Biology, 09010 Aydın, Turkey
Ecological Niche Divergence between Trapelus ruderatus (Olivier,1807) and T.persicus (Blanford, 1881) (Sauria: Agamidae) in the Middle East
Seyyed Saeed HOSSEINIAN YOUSEFKHANI1*, Omid MIRSHAMSI1,2, Çetin ILGAZ3,Yusuf KUMLUTAŞ3and Aziz AVCI4
1Department of Biology, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran
2Zoological Innovations Research Department, Institute of Applied Zoology, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran
3Dokuz Eylül University, Faculty of Science, Department of Biology, 35160 Buca, İzmir, Turkey
4Adnan Menderes University, Faculty of Science and Arts, Department of Biology, 09010 Aydın, Turkey
Abstract Modeling the potential distribution areas for a given species is important in understanding the relationship between the actual distribution and the most suitable habitat for a species.In this study, we obtained all available records of Trapelus ruderatus and Trapelus persicus from museums, literature and feldwork and used them with environmental layers in the Maximum Entropy algorithm to predict highly suitable habitat areas.The distribution model of T.ruderatus and T.persicus showed excellent performance for both models (T.ruderatus AUC = 0.964 ± 0.001 and T.persicus AUC = 0.996 ± 0.003), and predicted suitable regions in Iran, Turkey, Iraq and Syria.Niche overlap was measured between the two groups by ENMtools and 13% overlapped.We used a niche identity test to determine differences between the niches of the two species.Finally, by comparing our null hypothesis to the true niche overlap of the two species, we were able to reject our null hypothesis of no difference between the niches.Due to the sympatric distribution pattern of these species, we do not need a background test for niche divergence.
Keywords MaxEnt modeling, habitat suitability, niche differentiation, Trapelus ruderatus, Trapelus persicus, Middle East
1.Introduction
Ecological niche modeling tries to predict suitable habitats for a particular species based on similarities between occurrence point grids and other potential areas not occupied by the species (Graham et al., 2004).Initially, geographic range maps of a given species can be created using museum records (MacArthur, 1972; Guisan and Zimmermann, 2000; Peterson and Vieglais, 2001;Hugall et al., 2002).
Species distribution modeling is one of the most important methods used in recent herpetological studiesto examine the effects of environmental conditions on species distribution.Recent studies have indicated that bioclimatic layers are very useful in predicting the distribution of reptile species (Litvinchuk et al., 2010;Doronin, 2012; Ananjeva and Golynsky, 2013; Bernardes et al., 2013; Ficetola et al., 2013; Hosseinian Yousefkhani et al., 2013; Ananjeva et al., 2014; Fattahi et al., 2014).Ecological niche divergence between some species is clear, but we must use identity and background tests to investigate the level of this divergence.Due to the sympatric distribution of the two species, the identity test is suffcient and must be employed to confrm ecological niche separation (Warren et al., 2010).For allopatric and parapatric species, it is necessary to use the background test for niche divergence as well (Warren et al., 2010).
Trapelus ruderatus (Olivier, 1804) and Trapelus persicus (Blanford, 1881) are two species of thefamily agamidae that are distributed in the Middle East (Anderson, 1999; Smid et al., 2014).Based on the study of holotypes, Rastegar-Pouyani (2000) placed T.persicus in synonymy with T.ruderatus due to morphological similarities between the specimens.Later, Ananjeva et al.(2013) described the differences between these two taxa (T.persicus and T.ruderatus) and resurrected T.persicus as a valid species.Finally, Ananjeva et al.(2013)examined the holotype of T.lessonae with T.ruderatus and, due to the morphological similarity, considered the holotype of T.lessonae, (MZUT R 307) as the neotype of T.ruderatus. These species have been rarely studied ecologically and there is little information on distribution modeling and ecological niche differentiation between them (Anderson, 1999; Smid et al., 2014).
In the present study, we employed both occurrence records of the species in the Middle East and environmental layers to predict the potential distribution modeling and compared the results to calculate the level of niche overlap.The main objectives in this study are: 1)to predict highly suitable areas for T.ruderatus and T.persicus distribution and determine which environmental factors are important for species distribution; and 2) to measure and compare niche divergence between the two species.
2.Material and Methods
2.1 Data collection Distribution records were collected from all literature for T.ruderatus (84 records) (Baran et al., 1989; Frynta et al., 1997; Anderson, 1999; Rastegar-Pouyani, 2000; Torki, 2006; Göçmen et al., 2007;Göçmen et al., 2009) and T.persicus (23 records) (Frynta et al., 1997; Anderson, 1999; Rastegar-Pouyani, 2000;Fathinia and Rastegar-Pouyani, 2011).Other records were gathered from our original feld work in Iran, Syria and Turkey from June 2010 to May 2014 and where we found the species, localities were recorded by GPS.These records cover the whole range of these species in these countries.Some of the records did not have exact coordinates but did have correct localities, so we estimated their coordinates using Google Earth (Figure 1).Additional records were gathered from museums: California Academy of Sciences, San Francisco,California, USA (11 records for T.ruderatus and 13 for T.persicus), Museum of Vertebrate Zoology, Berkeley, USA (two records for T.ruderatus) and Sabzevar University Herpetological Collection, Khorasan Razavi, Iran (10 records for T.ruderatus).Finally, 13 records of T.ruderatus were obtained from our direct feldwork in Iran, Syria and Turkey.120 unique records of T.ruderatus and 36 records of T.persicus compose the dataset.
2.2 Ecological Niche Modeling To avoid problems with highly correlated variables in analysis, we used environmental information from 500 random points across all parts of the species range (http://www.geomidpoint.com /random/).The correlation matrix was calculated for all 19 bioclimate variables.A Pearson correlation coefficient higher than 0.75 shows highly correlated variables and these were eliminated from the main analysis.Present-day bioclimatic variables (downloaded from www.worldclim.org ) in 30 arc-seconds resolution were put as the base for the model predictions.The slope layer was created using ArcGIS 9.2 from the original altitude layer in 30 arc-second resolution.
After collecting the random points, data were imported to the Openmodeller ver.1.0.7 (Muñoz et al., 2011) and were analyzed with all environmental layers.A matrix of bioclimatic values for 500 random points resulted in Openmodeller and this dataset was imported to statistical software (SPSS 20.0) to obtain bivariate correlation.Variables with a Pearson index higher than 0.75 were distinguished and one variable from a bivariate correlation were eliminated, because another one might uncorrelated with other variables.After removing the correlated variables we used four bioclimatic layers for T.ruderatus and seven layers for T.persicus in distribution modeling: BIO4=Temperature Seasonality; BIO13= Precipitation of Wettest Month; BIO18= Precipitation of Warmest Quarter and slope steeper for T.ruderatus and in addition to the previous layers BIO5= Maximum Temperature of Warmest Month; BIO19= Precipitation of Coldest Quarter; BIO11= Mean Temperature of Coldest Quarter for T.persicus.The species potential distribution model was run using maximum entropy method in Maxent 3.3.3e (Phillips et al., 2004; Phillips et al., 2006; Elith et al.,2011) and 25% of data was considered as test samples using a jackknife method to evaluate the informative layer.The robustness of the final model was evaluated based on 10 replications because some point localities did not have precise coordinates and were estimated using GoogleEarth maps directly.The area under receiver operating characteristic curve (AUC) were considered as a measure of accuracy between 0 and 1.A value over 0.5 indicates that the model is better than random and a value closer to 1.0 shows high accuracy of the predicted model (Raes and TerSteege, 2007).
Niche overlap was calculated using ENMtools v 1.3.The D value estimates the local density of a population and allows comparisons between populations.The Ivalue indicates the ability of the model to estimate true suitability of the habitat according to Hellinger distance (Warren et al., 2008; Warren et al., 2010; Kolanowska,2013).The null hypothesis that the two groups have similar niches is accepted if the niche overlap of both groups is outside of the 95% confdence interval (Warren et al., 2008).When niche overlap between two groups does not fall within the 95% confidence interval and Schoener's D and I are less than our assumption value,then the groups are separated (Warren et al., 2008).To evaluate this hypothesis statistically, we used a niche identity test between two species.
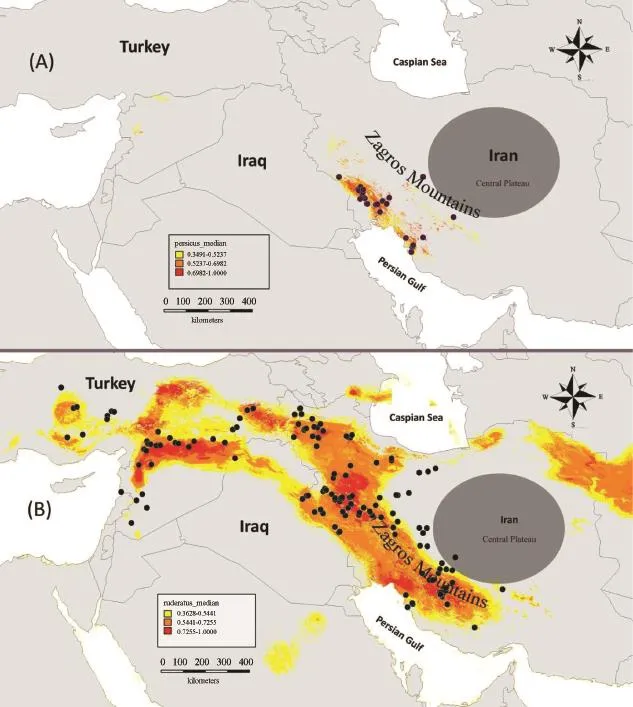
Figure 1 Habitat suitability for Trapelus persicus (A) and Trapelus ruderatus (B).Three main colors show habitat suitability in the map as mentioned in the map legend.Yellow represents suitability of less than 0.52, orange represents suitability between 0.52 and 0.70, and red color represents suitability greater than 0.70.
3.Results
The predicted suitable areas for the two species have high AUC that are 0.964 ± 0.001 for T.ruderatus and 0.996 ± 0.003 for T.persicus (Figure 1).These high AUC values indicate a good model fit and therefore accurate predictions of the distribution of the species.
The variables that highly contributed to the models are as follows.The four variables that were employed for T.ruderatus have important roles in determining species distribution and the relevant contribution levels were: BIO13 Precipitation of wettest month with 34.2%;BIO18 Precipitation of Warmest Quarter with 34%;BIO4 Temperature Seasonality (standard deviation *100)with 18.9%; and slope with 12.8%.For T.persicus, the four most important variables were: BIO18 with 33.6%,BIO19 with 26.3%, BIO11 with 19.3% and BIO13 with 10%.Also the jackknife plot is presented (Figure 2) to verifying the relative importance of variables for species prediction.
Niche overlaps were measured and overlap calculated between T.ruderatus and T.persicus (I = 0.424 and D = 0.135), showing that these species have little overlap in their ecological niche.We ran a niche identity test with both species in a common pool (with shared environmental variables) and the result of this test rejected the null hypothesis.Based on this result, the estimated niche models for both species are DH0= 0.734 ± 0.051 vs.DH1= 0.135 and IH0= 0.809 ± 0.025 vs.IH1= 0.424 (Figure 3).
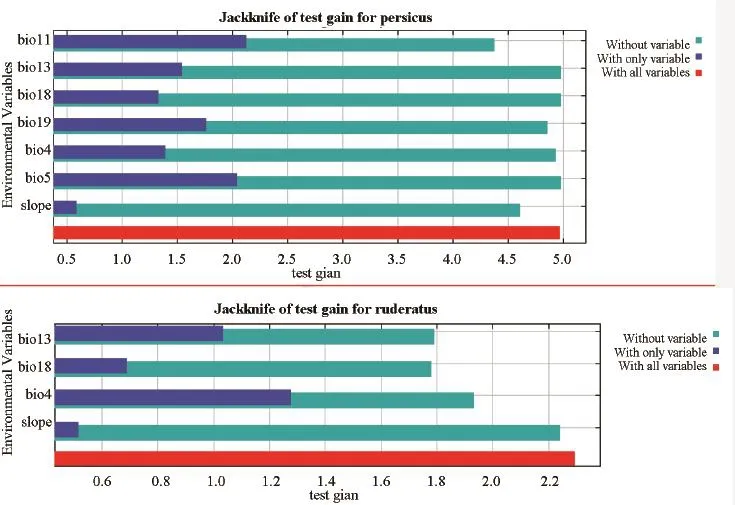
Figure 2 The results of jackknife test of relative importance variables for both species.The upper plot is for T.persicus and the lower plot for T.ruderatus.
4.Discussion
Trapelus ruderatus and T.persicus have not been studied ecologically and the ecological niche differences between them are poorly known.The model results confirm the distribution map and also show some potential areas for distribution where the species have not yet been recorded.Two present models resulted in high AUC scores of over 0.9 that indicate good niche prediction based on presenceonly occurrence points (Renner and Warton, 2013).Trapelus persicus is restricted by the Zagros Mountains and did not expand its range into the Central Plateau (Figure 1).Additionally, the predicted model did not show the suitable regions for this species in central Iran.
Khuzestan in southwestern Iran is the most suitable area for T.persicus.The present model of T.ruderatus confirmed its current distribution in Iran, Turkey and Syria.According to the model, suitability is highest in southeastern Anatolia, where the most known records are found, according to the literature and the results of our observations (Bird, 1936; Schmidt, 1939; Bodenheimer,1944; Başoğlu and Hellmich, 1970; Baran et al., 1989;Baran and Atatür, 1998; Baran et al., 2012).
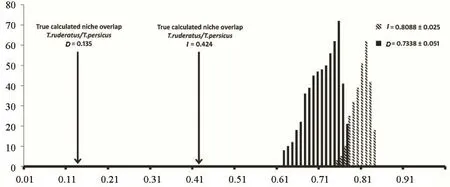
Figure 3 The results of the identity test performed using ENMTools.Black arrows refer to the true calculated niche overlap by ENMTools (D and I).The solid and hachured columns are calculated by replicates with identity test mode.
In this study, we tried to determine importance of variables that are involved in determining species distribution (Table 1).Precipitation is a very important variable for both species' distributions.The warmest quarter precipitation is important variable for T.persicus and the precipitation of the wettest month are the most important variables for T.ruderatus.The highest contribution of these variables indicated that these species are not phytophagous (Ananjeva et al., 2014).Precipitation in the warmest quarter is associated with water availability in arid regions of southern Iran and,according to the model species presence is highly related to this condition. On the other hand, precipitation in the wettest month is the variable with the highest contribution for T.ruderatus and water availability in this month is highly associated with shrub and plant growth.The results of the model show us that increasing the precipitation in the wettest month has a direct impact on species presence and species presence probability will be increased.Water availability in the wettest month is an important factor for growing shrubs, preparing shelters, and for resources needed for insect aggregation.
Warren et al.(2010) established a new method for identifying ecological niches using the ENMTools package and comparing ecological niches belonging to two species using background and identity tests.ASCii fles that are resulted from MaxEnt analysis were employed to calculate niche overlap using ENMTools package and showed that 0.13 and 0.42 niches of two populations are overlapped in D and I indexes respectively.As both species are sympatrically distributed in southern Iran, then it is suffcient to run the identity test.When two studied species are distributed parapatrically or allopatrically, it is necessary to calculate the background test as an additional analysis (Warren et al., 2010).According to our test, the true calculated niche overlap of both species (D= 0.13 and I=0.42) is outside of the 95% confdence interval of the null hypothesis (Figure 2)and confrms the separation between them.
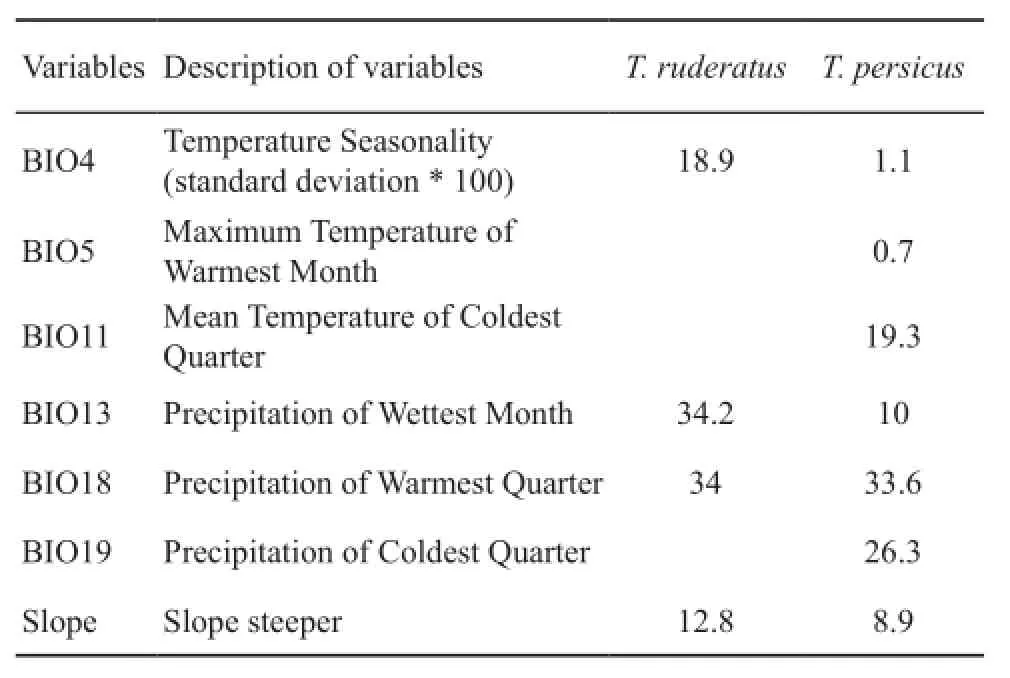
Table 1 Level contribution of variables used in Maxent model for two species of the genus Trapelus.
Our results suggest that T.ruderatus and T.persicus have high separation in their own ecological niches,unless they are distributed sympatrically.Distribution range of T.persicus is restricted to a small area, on the other hand, IUCN status of the species is marked as LC (Least Concern) but the niche modeling suggests that the environmental requirements for the species is limited.Niche modeling studies can aid in conservationassessment, because the model highlights the areas of unknown occurrence as the high suitable area without relying on confrmed species presence.More feld studies will help us to know about the factual distribution range of the species and shape its status clearly than present time.
Acknowledgements We thank Prof.Steven C.ANDERSON and Ann PATERSON who improved early versions of the manuscript and made some helpful comments for the authors.
References
Ananjeva N.B., Golynsky E.A.2013.Analysis of distribution of the Turkestan rock agama, Paralaudakia lehmanni (Nikolsky,1896): Using of MAXENT modelling.Proc Zool Inst, 317(4): 426-437 (In Russian)
Ananjeva N.B., David P., Barabanov A., Dubois A.2013.On the Type Specimens of Trapelus ruderatus (Olivier, 1804) and Some Nomenclatural Problems on Trapelus Cuvier, 1816 (Agamidae,Sauria).Russ J Herpetol, 20: 197-202
Ananjeva N.B., Golynsky E.A., Hosseinian Yousefkhani S.S.,Masroor R.2014.Distribution and Environmental Suitability of the Small-scaled Rock Agama, Paralaudakia microlepis (Sauria: Agamidae) in the Iranian Plateau.Asia Herpetol Res, 5: 161-167
Anderson S.C.1999.The Lizards of Iran.Oxford (Ohio): Society for the Study of Amphibians and Reptiles
Baran İ., Atatür M.K.1998.Turkish Herpetofauna (Amphibians and Reptiles), Çevre Bakanlığı, Ankara
Baran İ., Kasparek M., Öz M.1989.On the distribution of four species of Agama (Agamidae) in Turkey.Zool Middle East, 3: 37-46
Baran İ., Ilgaz Ç., Avcı A., Kumlutaş Y., Olgun K.2012.Türkiye Amfbive Sürüngenleri.TÜBİTAK, Ankara.204 p (ISBN: 978-975-403-703-6)
Başoğlu M., Hellmich W.1970.Amphibien Anadolu und Reptilien aus dem Ostlichen.Ege Üniversitesi Fen Fakültesiİlmi RaporlarSerisi, İzmir, 93: 1-26
Bernardes M., Rödder D., Nguyen T.T., Pham C.T., Nguyen T.Q., Ziegler T.2013.Habitat characterization and potential distribution of Tylototriton vietna mensis in northern Vietnam.J Nat Hist, 47: 1161-1175
Bird C.G.1936.The distribution of reptiles and amphibians in Asiatic Turkey, with notes on a collection from the vilayets of Adana, Gaziantep and Malatya.Ann Mag Nat Hist, 18: 257-281
Bodenheimer F.S.1944.Introduction into the knowledge of the Amphibia and Reptilia of Turkey.Istanbul Üniversitesi Fen Fakültesi Mecmuası, Seri B 9: 1-93
Doronin I.V.2012.The use of GIS for the analysis of the distribution of rock lizards Darevskia (saxicola) complex (Sauria: Lacertidae).sSovrem Gerpeto, 12: 91-122 (In Russian)
Elith J., Phillips S., Hastie T., Dudík M., Chee Y.E., Yates C.2011.A statistical explanation of MaxEnt for ecologists.Divers Distribut, 17: 43-57
Fathinia B., Rastegar-Pouyani N.2011.Sexual dimorphism in Trapelus ruderatus ruderatus (Sauria: Agamidae) with notes on the natural history.Amph Rept Conserv, 5:15-22
Fattahi R., Ficetola G.F., Rastegar-Pouyani N., Avcı A.,Kumlutaş Y., Ilgaz Ç., Hosseinian Yousefkhani S.S.2014.Modelling the potential distribution of the Bridled Skink,Trachylepis vittata (Olivier, 1804), in the Middle East.Zool Middle East, 60: 1-9
Ficetola G.F., Bonardi A., Sindaco R., Padoa-Schioppa E.2013.Estimating patterns of reptile biodiversity in remote regions.J Biogeogr, 40: 1202-1211
Frynta D., Moravec J., Ciháková J., Sádlo J., Hodková Z.,Kaftan M., Kodym P., Král D., Pitule V., Šejna L.1997.Results of the Czech biological expedition to Iran.Part 1.Notes on the distribution of amphibians and reptiles.Acta Soc Zool Bohem, 61: 3-17
Göçmen B., Nilson G., Yildiz M.Z., Arikan H., Yalçinkaya D.,Akman B.2007.On the occurrence of the Black Cat Snake,Telescopus nigriceps (Ahl, 1924) (Serpentes: Colubridae) from the Southeastern Anatolia, Turkey with some taxonomical comments.North-West J Zool, 3: 81-95
Göçmen B., Franzen M., Yildiz M.Z., Akman B., Yalçinkaya D.2009.New locality records of eremial snake species in southeastern Turkey (Ophidia: Colubridae, Elapidae,Typhlopidae, Leptotyphlopidae).Salamandra, 45: 110-114
Graham C.H., Ron S.R., Santos J.C., Schneider C.J., Moritz C.2004.Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs.Evolution, 58: 1781-1793
Guisan A., Zimmermann N.E.2000.Predictive habitat distribution models in ecology.Ecol Model, 135:147-186
Hosseinian Yousefkhani S.S., Ficetola G.F., Rastegar-Pouyani N., Ananjeva N.B., Rastegar-Pouyani E., Masroor R.2013.Environmental suitability for the Caucasian rock agama,Paralaudakia caucasia (Sauria: Agamidae) in Western and Central Asia.Asia Herpetol Res, 4(3): 207-213
Litvinchuk S.N., Kazakov V.I., Pasynkova R.A., Borkin L.J.,Kuranova V.N., Rosanov J.M.2010.Tetraploid Green Toad species (Bufo pewzowi) from the Altay Mountains: The first record for Russia.Russ J Herpetol, 17(4): 290-298
Hugall A., Moritz C., Moussalli A., Stanisic J.2002.Reconciling paleodistribution models and comparative phylogeography in the wet tropics rainforest land snail Gnarosophia bellendenkerensis (Brazier 1875).PNAS, 99: 6112-6117
Kolanowska M.2013.Niche conservatism and the future potential range of Epipactis helleborine (Orchidaceae).PLoS ONE, 8: e77352.doi:10.1371/ journal.pone.0077352
MacArthur R.1972. Geographical Ecology: Patterns in the Distribution of Species.New York: Harper and Row
Muñoz M.E.S., Giovanni R., Siqueira M.F., Sutton T.,Brewer P., Pereira R.S., Canhos D.A.L., Canhos V.P.2011.OpenModeller: a generic approach to species' potential distribution modeling.GeoInformatica, 15: 111-135
Peterson A.T., Vieglais D.A.2001.Predicting species invasions using ecological niche modeling: New approaches from bioinformatics attack a pressing problem: A new approach to ecological niche modeling, based on new tools drawn from biodiversity informatics, is applied to the challenge of predicting potential species' invasions.BioScience, 51: 363-371
Phillips S.J., Dudík M., Schapire R.E.2004.A maximum entropy approach to species distribution modeling.In Proceedings of the twenty-frst international conference on Machine learning.ACM,83 pp
Phillips S.J., Anderson R.P., Schapire R.E.2006.Maximum entropy modeling of species geographic distributions.Ecol Model, 190: 231-259
Raes N., terSteege, H.2007.A null model for signifcance testing of presence only species distribution models.Ecography, 30: 727-736
Rastegar-Pouyani N.2000.Taxonomic status of Trapelus ruderatus (Olivier) and T.persicus (Blanford), and validity of T.lessonae (De Filippi).Amphibia-Reptilia, 21: 91-102
Renner I.W., Warton D.I.2013.Equivalence of MAXENT and Poisson point process models for species distribution modeling in ecology.Biometrics, 69: 274-281
Šmíd J., Moravec J., Kodym P., Kratochvíl L., Hosseinian Yousefkhani S.S., Rastegar-Pouyani E., Frynta D.2014.Annotated checklist and distribution of the lizards of Iran.Zootaxa, 3855: 1- 97
Warren D.L., Glor R.E., Turelli M.2008.Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution.Evolution, 62: 2868-2883
Warren D.L., Glor R.E., Turelli M.2010.ENMTools: a toolbox for comparative studies of environmental niche models.Ecography, 33: 607-611
E-mail: hosseinianyousefkhani@stu.um.ac.ir
Received: 16 May 2015 Accepted: 18 March 2016
DOI:10.16373/j.cnki.ahr.150032
*Corresponding author:Ph.D Seyyed Saeed Hosseinian Yousefkhani from Ferdowsi University of Mashhad, Iran, with his research focusing on the Centre of origin and Phylogeographic patterns of the agamid lizards of the genus Laudakia in Middle East and Iranian Plateau.
 Asian Herpetological Research2016年2期
Asian Herpetological Research2016年2期
- Asian Herpetological Research的其它文章
- Tracing the Origin of the Black-spotted Frog, Pelophylax nigromaculatus, in the Xinjiang Uyghur Autonomous Region
- Genetic Diversity and Population Structure for the Conservation of Giant Spiny Frog (Quasipaa spinosa) Using Microsatellite Loci and Mitochondrial DNA
- Coevolution of Male and Female Response Preferences to Sexual Signals in Music Frogs
- The Effects of Chronic Hypoxia on Thermoregulation and Metabolism in Phrynocephalus vlangalii
- Comparison of Skull Morphology in Two Species of Genus Liua (Amphibia: Urodela: Hynobiidae), L.shihi and L.tsinpaensis
- Discovery of Female Laudakia papenfussi Zhao, 1998, with Insights into its Phylogenetic Relationships
