Coevolution of Male and Female Response Preferences to Sexual Signals in Music Frogs
Jianguo CUI, Jichao WANG, Guangzhan FANG, Xiaowei SONG,3, Steven E.BRAUTHand Yezhong TANG
1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China2Ministry of Education Key Laboratory for Tropical Animal and Plant Ecology, College of Life Sciences, Hainan Normal University, Haikou 571158, Hainan, China3School of Life Sciences, Xinyang Normal University, Xinyang 464000, Henan, China4Department of Psychology, University of Maryland, College Park, MD 20742, USA
Coevolution of Male and Female Response Preferences to Sexual Signals in Music Frogs
Jianguo CUI1*, Jichao WANG2, Guangzhan FANG1, Xiaowei SONG1,3, Steven E.BRAUTH4and Yezhong TANG1*
1Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China
2Ministry of Education Key Laboratory for Tropical Animal and Plant Ecology, College of Life Sciences, Hainan Normal University, Haikou 571158, Hainan, China
3School of Life Sciences, Xinyang Normal University, Xinyang 464000, Henan, China
4Department of Psychology, University of Maryland, College Park, MD 20742, USA
Abstract Male signaling influences both female choice and male-male competition.Although male signaling characteristics and female preferences have been shown to coevolve in many species, few studies have examined whether male signal characteristics and male receiver responses related to male-male competition also coevolve.The present study tested the hypothesis that male and female signal receiver preferences may coevolve in parallel for frogs in the genus Babina by comparing the acoustic structure of male advertisement calls of four closely related and geographically isolated Babina species.Then we assessed the behavioral responses of both male and female B.daunchina (Emei music frog) to male call playbacks from each of the four species.The results support the hypothesis that male and female signal receiver preferences have coevolved in this species.Specifcally, both male and female B.daunchina respond strongly to the heterospecific calls of B.hainanensis, suggesting that preexisting biases exist in both females and males.Both male and female individuals showed a slight response to the calls of B.adenopleura while no response was evoked by the calls of B.lini.The manifestation of similar response profles in male and female B.daunchina to the calls of the four species support the idea that male and female signal receiver preferences evolved in parallel and that the origin of these receiver biases refects adaptations dependent on the same neural and cognitive systems in both sexes.
Keywords sexual selection, acoustic communication, male-male competition, phonotaxis tests, coevolution
1.Introduction
Sexual selection drives divarications in morphological characters, physiological processes and reproductive behaviors between males and females (Andersson, 1994).For instance, males display their sexual signals while females choose mates based on these signals.During the breeding season, male signals used by females for mate recognition may also incite males for male-malecompetition (Gerhardt and Huber 2002; Cui et al., 2010,2012; Xu et al., 2012).Both female mate choice and male-male competition require congruence between the structure of the signal and the response properties of the sensory system that decodes the signal (Ryan and Wilczynski, 1988).Most studies of signal evolution have focused on female preferences for specific signal parameters because preference from female receivers largely determines signal evolution (Bush et al., 1996;Gerhardt et al., 1996; Ryan and Rand 1993; Cui et al.,2012).Only a few studies have considered the relationships between male signals and the responses of male receivers in shaping the evolution of male signals,despite the fact that male-male completion also plays a role in this process (Morris and Ryan, 1996).For example,male signals can evolve under sexual selection as signals used not only for attracting mates but also for deterring rivals (Gerhardt, 1994).Thus it is important to consider the relationship between the receiver preferences of both males and females with regard to male sexual signals.The present study was designed to test the hypothesis that male responses and female preferences to the same male signals may coevolve under the same selection pressures.
The male-female sender-receiver dyad could arise via mechanisms predicted by classical sexual selection models (i.e.the runaway Fisher process, good genes/ indirect benefits, or the direct benefits models) as well as by receiver bias models (i.e.pre-existing bias,sensory exploitation, hidden preferences) as explicated by Andersson (1994) and Schul and Bush (2002).The receiver bias models predict that female preference favoring a male ornament can initially evolve under natural selection for reasons other than mate choice,as, for instance in the context of foraging or predator avoidance.Males who evolve traits that exploit such biases are thus likely to be favored by females during mate choice (Andersson and Simmons, 2006).
The receiver bias models predict that biases in the female's response (female receiver biases) may even favor the evolution of traits that did not previously exist in males of their own species (Ryan, 1998).For example, platyfish and swordtails are both members of the genus Xiphophorus, but only swordtails have sword-like appendages in their tails.Females of two platyfsh species and a species of the closely related and swordless genus Priapella both prefer males of their species for whom swords were appended to their tails,over normal unadorned males (Basolo, 1990, 1995).Thus, a second goal of this study was to determine if preexisting response biases might exist in female or male B.daunchina.This was done by determining first how strongly males and females respond to the calls of the four closely related Babina species and second by assessing if the strengths of either the male or female responses refect the evolutionary relationships between the four species as verifed by the method of neighbor-joining algorithms (see below).
To test these hypotheses, we first compared the acoustic structures of male advertisement calls of these four closely related yet geographically isolated species of the genus Babina (B.daunchina, B.adenopleura,B.hainanensis, B.lini).The advertisement calls of the four species were then played back randomly to male and female B.daunchina to compare the strengths of the male and female responses to each of the calls of the four species.Male responses consisted of calling back to the playback stimuli while female responses were evaluated in phonotaxis experiments.We selected B.daunchina as subject because male and female reproductive behavior in this species has been extensively studied both in the feld and using phonotaxis tests (Cui et al., 2010, 2012).
2.Materials and Methods
2.1 Call recordings The advertisement calls of four closely related yet geographically isolated species of music frogs were recorded from April to July between 2009 and 2011 [B.daunchina, from Mt.Emei (29.36° N and 103.22° E), Sichuan Province; B.adenopleura from Kuankuoshui (28.13° N and 107.09° E), Guizhou Province, B.hainanensis from Mt.Diaoluo (18.44° N and 109.52° E), Hainan Province and B.lini from Jiangcheng (22.40° N and 101.52° E), Yunnan Province].(Temperature ranged from 20°C to 25°C).All vocalizations were recorded using a directional microphone (Sennheiser ME66 with K6 power module)connected to a digital recorder (Marantz PMD 660, 16 bit,44.1 kHz) located approximately 1 m from the subject.The calls of 50 males for B.daunchina, 10 males for B.adenopleura, 8 males for B.hainanensis and 10 males for B.lini were recorded.At least 5 calls were selected from the recordings for each male to measure.For each call,the frst four notes were used for acoustic analysis.
2.2 Female phonotaxis test An open opaque sound attenuated metal tank [270 cm (l) x 95 cm (w) x 100 cm (h)] containing mud and water at the bottom was used for phonotaxis tests.Females were placed in the center of the tank and tested in a two-choice phonotaxis paradigm between 20:00 and 24:00 (21.9-23.2°C).For playback of calls, two Portable Field Speakers (SME-AFS, Saul Mineroff Electronics, Elmont, NY) connected to a computer (Thinkpad, Lenovo) were placed equidistantly from the opposite ends of the tank.The distance of the two speakers was 250 cm, thus the distance between female and each speaker was 125 cm.
Four stimulus pairs were constructed: an advertisement call of each species (B.daunchina, B.adenopleura, B.hainanensis, B.lini) was paired with white noise (as control), respectively.The four stimulus pairs were presented to females (n=21) in a randomized sequence.All calls used in the playback experiments contained fve notes (Figure 1) and were equalized for intensity (75 dB SPL, re 20 μPa, measured at the center of the tank where the female was to be released with a sound level meter (AWA 6291, Hangzhou Aihua Instruments Co.),the distance between the speaker and sound level meter was 125 cm.Stimuli were presented antiphonally with 4-s inter-stimulus intervals, approximately equal to the mean of the inter-call intervals of B.daunchina.The interval between each stimulus pair was fve minutes.
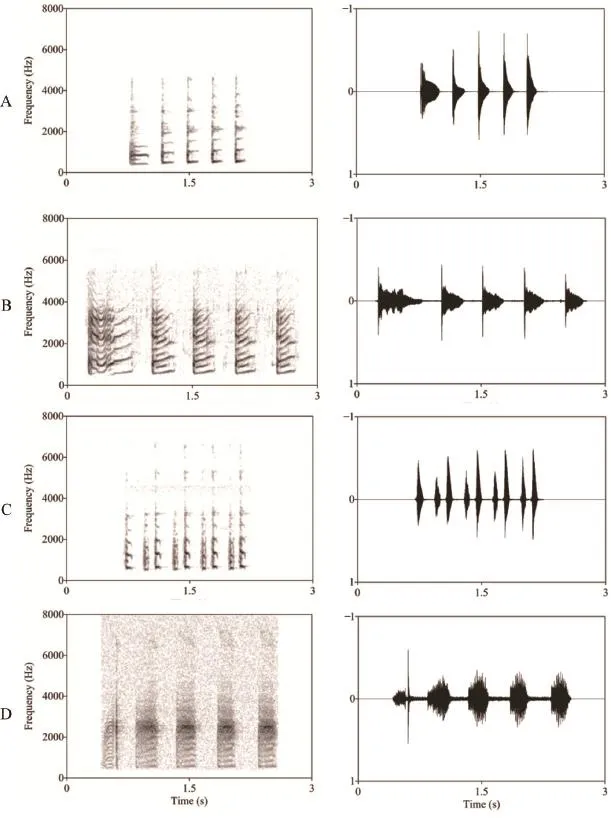
Figure 1 Amplitude-modulated waveforms and spectrograms of a typical advertisement call produced by B.daunchina (A), B.adenopleura (B), B.hainanensis (C) and B.lini (D).
Females with eggs were collected during the reproductive season.The female being tested was monitored via an infrared camera.A positive response was scored if females approached the speaker playing back the advertisement call within 10 cm.If the female failed to make a choice within 10 min or approached the speaker that played back white noise, a negative response was scored and the female was tested with the next stimulus pair.If the female did not respond to all thefour stimulus pairs, the female was excluded from the study.To control for potential side biases, we randomized the speaker assignments for each stimulus pair.After all tests were performed, the frogs were marked with passive integrated transponder (PIT) tags (Hongteng, Inc.China)to avoid recapture, and released to the same ponds within 72 hours after capture.
2.3 Male response measurement To evaluate the responses of male B.daunchina to each of the advertisement calls of the four Babina species, the calls (Figure 1) used in the female phonotaxis tests were broadcast to the subject males living in 18 natural ponds.The stimulus calls were played back repeatedly (with 4 second inter-call intervals) for 5 minutes using a SMEAFS portable field speaker (Saul Mineroff Electronics,Elmont, NY) placed 1.5 m from the subjects.The peak output intensity of the speaker was adjusted to a SPL of 75 dB (measured at the bank of the pond).The interval between each stimulus pair was 15 minutes.The advertisement calls produced by subject males were recorded 5 minutes before, during and after playbacks using a Sennheiser ME66 directional recording microphone (with K6 power module) connected to a Marantz PMD 660 recorder (16 bit, 44.1 kHz) about 1 m from the subject.All vocalizations produced during the playback of the stimulus were recorded although they could not be assigned to individual males.
2.4 Phylogenetic relationships reconstruction To reconstruct the phylogenetic relationships of the four Babina species, we sequenced partial 16S rRNA fragments, i.e.B.daunchina (5 individuals),B.adenopleura (4 individuals), B.hainanensis (3 individuals) and B.lini (4 individuals).In addition, 16S rRNA fragments from Meristogenys poecilus, Pelophylax nigromaculatus and two Odorrana species (Odorrana tormotus and Odorrana exiliversabilis) were selected as outgroups.The reconstruction of phylogenetic trees was performed with MEGA 5 software using the Neighbor-Joining method (Saitou and Nei, 1987).Here we selected the Tamura 3-parameter nucleotide substitution model (Tamura, 1992) and gamma distributed rate variation among sites through a model test in MEGA.The bootstrap method (1000 replicates) was executed to test the robustness of the phylogenetic tree (Felsenstein,1985).
2.5 Ethics Statement All applicable international,national, and/or institutional guidelines for the care and use of animals were followed.All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Care and Use Committee of Chengdu Institute of Biology, CAS.This work was conducted with the permission of the Management Offce of the Mt.Emei Nature Reserve.
2.6 Analysis and statistics Four acoustic properties of the advertisement calls were measured including the fundamental frequency (FF), dominant frequency (i.e.frequency band with greatest energy, DF), note durations (ND) and inter-note intervals (IVL) using Adobe Audition 3.0 software (California, USA); the FFT (Faster fourier transformation) frame is 1024.The amplitude modulated waveforms (oscillograms) and spectrograms of advertisement calls were constructed using PRAAT software (Boersma and Weeninkk, Version 5.1.11,University of Amsterdam).
Data were statistically analyzed using the SigmaPlot 11 software program (Systat Software Inc., San Jose,USA).Prior to the statistical analyses, all data were examined for assumptions of normality and homogeneity of variance, using Shapiro-Wilk and Levene tests,respectively.Kruskal-Wallis One Way ANOVA on Ranks or One Way ANOVA was employed to evaluate the differences between four acoustic properties of the advertisement calls from each species and the Dunn's Method or Holm-Sidak method was used for post hoc comparisons, respectively.Two way repeated measures ANOVA was employed to evaluate the effects of stimulus (calls of four species) and time (before, during and after playback) on the males' response (calls/min and notes/ call), and the Holm-Sidak method was used for post hoc comparisons.The Chi-square and Fisher Exact Test was used to evaluate the phonotaxis data.Data were expressed as Mean ± SD; P < 0.05 was considered to be statistically signifcant.
3.Results
3.1 Acoustic call properties of four Babina species There are some common temporal and spectral parameters in the calls of all four species (Table 1, Figure 1).For all four species, the calls consist of a series of notes, most of which contain 3-5 notes.The fundamental frequencies of the notes are similar, ranging from 438 to 678 Hz (most mean values are 500-600 Hz), and most notes consist of a “musical” harmonic stack for which the frequency bands are nearly integer multiples of the fundamental, although the fundamental frequency may be suppressed in some cases.Interestingly, increases in fundamental frequencies from note to note are apparent for all four species.However, there are clear differences in both the temporaland frequency parameters among the four species (Table 1, Figure 1).
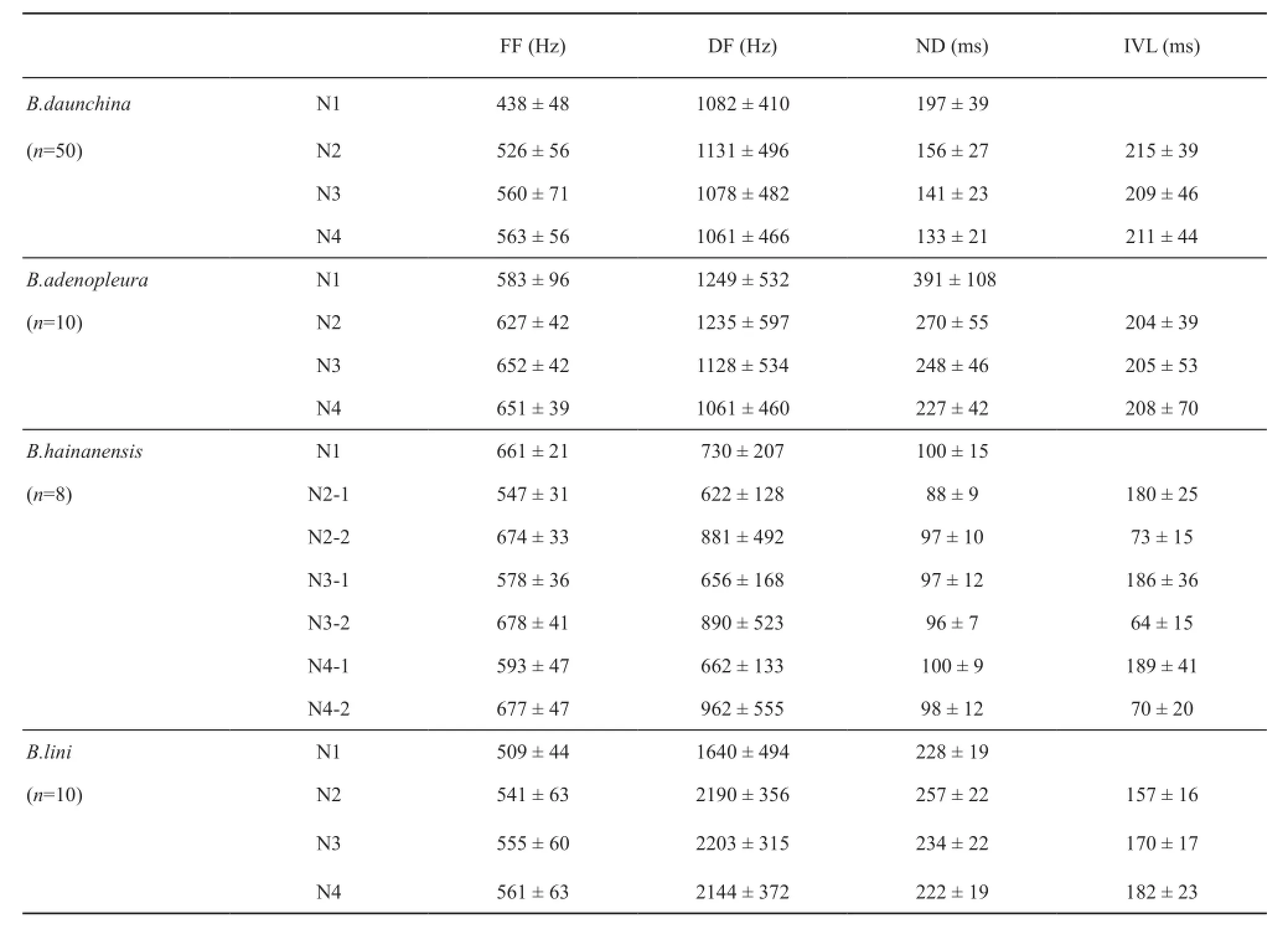
Table 1 Call properties of four closely related species of the genus Babina.
For B.adenopleura, although both the fundamental frequency (FF) and dominant frequency (DF) values are very similar to B.daunchina, the note durations are much longer than for B.daunchina and for the other two species (the first note duration is 391 vs.197 ms in B.adenopleura and B.daunchina, respectively, P <0.05).Furthermore the inter note intervals are similar (P > 0.05) so that the calls of B.adenopleura sound slower and last longer.For B.hainanensis, the FF of the call is very similar to that of B.adenopleura and B.daunchina calls, but the DF is much lower than those of B.adenopleura and B.daunchina calls (about half the value for B.adenopleura and B.daunchina, P < 0.05).The most unique character of B.hainanensis calls is that the successive notes except the first note consist of two phases.The duration of each phase is about 100 ms, and the interval between the two phases ranged from 64-73 ms.The FF and DF of the frst phase are lower than those of the second phase for each note.For B.lini, there is little harmonic structure in the call notes and the DF of the call is higher than those of the other species (P < 0.05)(Table 1 and Figure 1).
3.2 Phylogenetic tree The phylogenetic tree constructed using neighbor-joining algorithms demonstrates that B.daunchina and B.adenopleura possess the closest evolutionary relationship and that B.lini is closest to the common ancestor of the four species (Figure 2).
3.3 Female phonotaxis When the advertisement calls of each species and white noise were broadcast antiphonally,female B.daunchina responded strongly to the conspecifc calls of B.daunchina and heterospecifc calls of B.hainanensis.The percent of the females approaching the speaker broadcasting the advertisement calls was 76.2%, 71.4%, 52.4% and 38.1% for B.daunchina, B.hainanensis, B.adenopleura, B.lini, respectively (n = 21,Table 2).
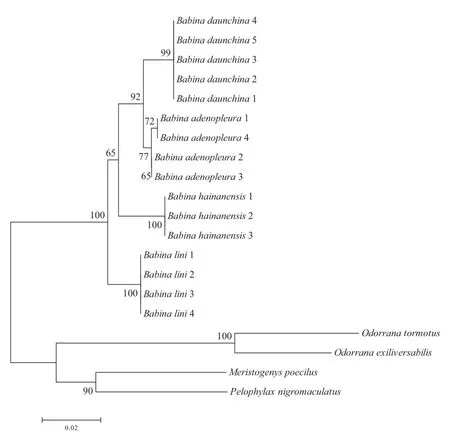
Figure2 Evolutionary relationships of four Babina species.
3.4 Male vocal responses When the calls of all four species were played back repeatedly to male B.daunchina in ponds, the results of Two Way Repeated Measures ANOVA show that male B.daunchina respond signifcantly differently to calls of the four species before,during and after playback (for the parameter of calls/min,Species × Playback time interaction effect: F6,34= 4.161, P< 0.001, Species effect: F3,17= 2.578, P = 0.064, Playback time effect: F2,51= 13.854, P < 0.001, Figure 3A).Male B.daunchina respond strongly to the conspecifc calls of B.daunchina and heterospecifc calls of B.hainanensis.The numbers of calls/min increased signifcantly during playback of the calls of B.daunchina (t = 3.755, P <0.001) and B.hainanensis (t = 5.835, P < 0.001) in contrast to those before playbacks.Calls/min increased but did not reach statistical signifcance during playback of the advertisement calls of B.adenopleura while call rate did not change during playback of the advertisement calls of B.lini compared with call rates before and after playbacks.Notes/call, another parameter refecting male calling activity, exhibited patterns similar to those of calls/ min before, during and after playbacks of calls of four species (for the parameter notes/call, Species × Playback time interaction effect: F6,34= 1.826, P = 0.101, Species effect: F3,17= 4.596, P = 0.006, Playback time effect: F2,51= 3.802, P = 0.032, Figure 3B).Notes/call increased signifcantly during playback of the advertisement calls of B.hainanensis.
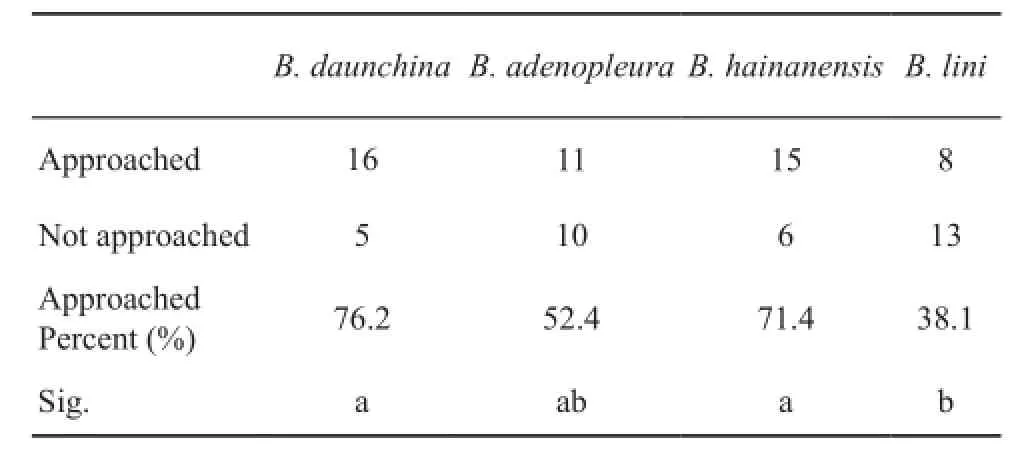
Table 2 Phonotaxis responses to calls of four closely related Babina species.
4.Discussion
The male advertisement calls of the four closely related Babina species studied here share some important acoustic properties.Each consists of a series of notes with similar fundamental frequency (Figure 1, Table 1, Supplementary materials), a feature that might have been derived from the common ancestor (Figure 2).Nevertheless, each species exhibits unique call characters, which could be attributed to geographical and reproductive isolation (McCracken and Sheldon,1997), and the effect of a complex acoustic enviroment with biological and abiological noise.Importantly, the unique character of the calls of B.hainanensis which distinguishes them from those of B.daunchina and the other species is that the notes of B.hainanensis consist of two short phases with 100 Hz frequency-modulation from low to high between the two phases of each note (Figure 1, Table 1, Supplementary materials).The frequency structure of B.adenopleura is very similar to that of B.daunchina but the temporal characteristics are different (Figure 1, Table 1).
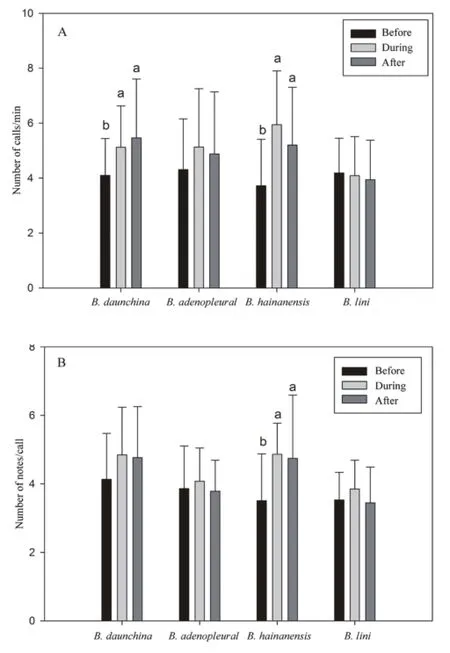
Figure 3 Numbers of advertisement calls per minute (A) and notes per call (B) produced before, during and after playbacks of calls of four species (5 min each) in feld tests (n = 18, mean ± SD).Values without the same superscript letter (a, b) differ signifcantly at P <0.05.
The fact that female B.daunchina are attracted by conspecific calls is consistent with classical sexual selection theories such as the runaway sexual selection and good genes selection models (Andersson, 1994; Ryan,1998).Female responses and male signals can become genetically correlated, if females with the strongest responses mate with males with the strongest signals (Fisher, 1958; Heisler, 1984).Indeed, several sexual selection models rely on a genetic correlation between female preferences (response) and male traits (signal)for the evolution of female preferences (Kirkpatrick andRyan, 1991; Welch et al., 1998; Rodríguez et al., 2006).
Field playback experiments showed that the male B.daunchina responded strongly to the conspecific calls of B.daunchina (Figure 3A).This can be explained in terms of the theory of male-male competition (Andersson,1994).We also found that female B.daunchina are attracted by the calls of B.hainanensis which have unique acoustic characteristics (i.e.double short phases with 100 Hz frequency-modulation) that are absent in the calls of B.daunchina.These results suggest that female B.daunchina possess pre-existing preferences for double short phase notes.This observation adds to the number of known examples consistent with sensory exploitation hypotheses (Ryan, 1990, 1994, 1998; Andersson and Simmons, 2006).
Interestingly, the field playback experiments also show that male B.daunchina respond strongly to the heterospecific calls of B.hainanensis which are also very attractive to female B.daunchina (Figure 3).These results suggest that this perceptual bias is also manifest in male-male interactions (Ryan and Cummings, 2013).For intraspecies competition, males tend to compete with males perceived as most attractive to the female (Fang et al., 2013); thus both males and females respond strongly to attractive male signals.Similar responses of male and female B.daunchina to conspecific calls can be explained as similar signaler (male trait) and receiver (both male and female response) characteristics based on genetic correlation.However, in the present study,we also found that the responses of male and female B.daunchina to the heterospecific calls of B.hainanensis are also similar.This result cannot be explained by genetic correlation between the receiver (response) and signaler (male trait) given that the receiver and signaler are different species.The fact that male and female B.duanchina have similar, apparently pre-existing,preferences (i.e.the double short phase note) suggests that the origin of such preexisting receiver biases in males and females is not random but has resulted from common causes such as responses that have evolved to locate prey or avoid predators, and/or reflect limitations imposed by the more general operating principles of neural and cognitive systems (Ryan et al., 1998).
Xiphophorus helleri use visual cues to communicate.Hence Rosenthal and Evans (1998) used playbacks of video animations to show that females did not exhibit a preference between a swordless male and a sworded male if the total body length of the two were equal.They suggested that males might have evolved a sword to exploit a pre-existing preference for large body size.In present study, the perceptual bias of receivers for calls with double short phase notes may be the result of a general preference for calls with more notes, a preference that is widespread among frogs (Morris and Yoon, 1989;Mcclelland et al., 1996; Gerhardt and Huber, 2002).Notably calls with double short phase notes have a similar duration to calls lacking this character but sound as though they contain more notes.Thus this character might enhance the attractiveness of calls to females and enhance the motivational value of the calls for instigating malemale competition without extra energy cost.
Taken together, we found that female B.daunchina respond strongly to the calls of B.hainanensis, suggesting that pre-existing biases may exist in female B.daunchina.Male B.daunchina not only respond strongly to the heterospecifc calls of B.hainanensis, but respond slightly more strongly than to conspecifc calls (i.e.B.daunchina).These results support the idea that the same pre-existing biases exist in both males and females.
Both male and female B.daunchina exhibit similar overall response patterns to the calls of four geographically isolated Babina species.These patterns include strong responses to conspecific calls and to those of B.hainanensis, slight responses to the calls of B.adenopleura and no response to the calls of B.lini,consistent with the idea that male and female signal receiver characteristics reflect parallel coevolution.These results are also consistent with the idea that an analogous selection pressure for sensory exploitation has acted on both males and females (Ryan, 1990, 1994).Furthermore, the existence of the same perceptual biases in both males and females suggest that the origin of these receiver biases did not result from a random event but from a common specific cause such as improving fitness or from more general operating principles of the underlying neural and cognitive systems (Ryan, 1998;Ryan and Cummings, 2013).These fndings thus provide insights into the evolutionary mechanisms underlying the male's preexisting preferences and how these pre-existing preferences are coupled with those of females.
Acknowledgements We thank Mr.Pengbo GONG,Yayong WU, Longfei ZHAO from CIB, Yunping Fu from Sichuan University, Dr.Wei LIANG from Hainan Normal University for their help during the experiments.This work was supported by the National Natural Science Foundation of China (31270042), Youth Professor Project of CIB (Y3B3011), Youth Innovation Promotion Association of Chinese Academy of Sciences (Y2C3011)and Open Fund of the Hainan Province Key Laboratoryof Tropical Plant and Animal Ecology (Hainan Normal University) to J.C.and National Natural Science Foundation of China (31372217) to G.F.
References
Andersson M.B.1994.Sexual selection.Princeton: Princeton University Press
Andersson M., Simmons L.W.2006.Sexual selection and mate choice.Trends Ecol Evol, 21: 296-302
Basolo A.L.1990.Female preference predates the evolution of the sword in swordtail fsh.Science, 250: 808-810
Basolo A.L.1995.A further examination of a pre-existing bias favouring a sword in the genus Xiphophorus.Anim Behav, 50: 365-375
Bush S.L., Dyson M.L., Halliday T.R.1996.Selective phonotaxis by males in the Majorcan midwife toad.Proc R Soc Lond B, 263: 913-917
Cui J.G., Tang Y.Z., Narins P.M.2012.Real estate ads in Emei music frog vocalizations: female preference for calls emanating from burrows.Biol Lett, 8: 337-340
Cui J.G., Wang Y.S., Brauth S., Tang Y.Z.2010.A novel female call incites male-female interaction and male-male competition in the Emei music frog, Babina daunchina.Anim Behav, 80: 181-187
Fang G.Z., Jiang F., Yang P., Cui J.G., Brauth S.E., Tang Y.Z.2013.Male vocal competition is dynamic and strongly affected by social contexts in music frogs.Anim Cogn, 17: 483-494
Felsenstein J.1985.Confdence limits on phylogenies: An approach using the bootstrap.Evolution, 39: 783-791
Fisher R.A.1958.The Genetical Theory of Natural Selection.New York: Dover
Gerhardt, H.C.1994.The evolution of vocalization in frogs and toads.Annual Review of Ecology and Systematics, 293-324
Gerhardt H.C., Dyson M.L., Tanner S.D.1996.Dynamic acoustic properties of the advertisement calls of gray tree frogs: patterns of variability and female choice.Behav Ecol, 7: 7-18
Gerhardt H.C., Huber F.2002.Acoustic communication in insects and frogs: Common problems and diverse solutions.Chicago: University of Chicago Press
Heisler I.L.1984.Quantitative genetic model for the origin of mating preferences.Evolution, 38: 1283-1295
Kirkpatrick M., Ryan M.J.1991.The evolution of mating preferences and the paradox of the lek.Nature, 350: 33-38
McClelland B.E., Wilczynski W., Ryan M.J.1996.Correlations between call characteristics and morphology in male cricket frogs, Acris crepitans.The J Exp Biol, 99: 1907-1919
McCracken K.G., Sheldon F.H.1997.Avian vocalizations and phylogenetic signal.Proc Natl Acad Sci USA, 94: 3833-3836
Morris M.R., Ryan M.J.1996.Sexual difference in signalreceiver coevolution.Anim Behav, 52: 1017-1024
Morris M.R., Yoon S.L.1989.A mechanism for female choice for large males in the treefrog Hyla chrysoscelis.Behav Ecol Sociobiol, 25: 65-71
Rosenthal G.G., Evans C.S.1998.Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size.Proc Natl Acad Sci USA, 95: 4431-4436
Rodríguez, R.L., Ramaswamy, K., & Cocroft, R.B.2006.Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects.Proceedings of the Royal Society of London B: Biological Sciences, 273(1601), 2585-2593
Ryan M.H., Rand A.S.1993.Sexual selection and signal evolution: the ghost of biases past.Proc R Soc Lond B, 340: 187-195
Ryan M.J.1990.Sensory systems, sexual selection, and sensory exploitation.Oxford Surveys in Evolutionary Biology, 7: 157-195
Ryan M.J.1994.Mechanistic studies in sexual selection.In L.Real (Ed.), Behavioral Mechanisms in Evolution and Ecology.Chicago: University of Chicago Press, 190-125
Ryan M.J.1998.Receiver biases, sexual selection and the evolution of sex differences.Science, 281: 1999-2003
Ryan M.J., Cummings M.E.2013.Perceptual biases and mate choice.Annu Rev Ecol Evol Syst, 44: 437-459
Ryan M.J., Wilczynski W.1988.Coevolution of sender and receiver: effect on local mate preference in cricket frogs.Science, 240: 1786-1788
Saitou N., Nei M.1987.The neighbor-joining method: A new method for reconstructing phylogenetic trees.Mol Biol Evol, 4: 406-425
Schul J., Bush S.L.2002.Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs.Proc R Soc Lond B, 269: 1847-1852
Tamura K.1992.Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases.Mol Biol Evol, 9: 678-687
Tamura K., Peterson D., Peterson N., Stecher G., Nei M.,Kumar S.2011.MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance,and Maximum Parsimony Methods.Mol Biol Evol, 28: 2731-2739
Welch, A.M., Semlitsch, R.D., & Gerhardt, H.C.1998.Call duration as an indicator of genetic quality in male gray tree frogs.Science, 280(5371), 1928-1930
Xu F., Cui J.G., Song J., Brauth S.E., Tang Y.Z.2012.Male competition strategies change when information concerning female receptivity is available.Behav Ecol, 23: 307-312
E-mail: cuijg@cib.ac.cn (J.G.CUI); tangyz@cib.ac.cn (Y.Z.TANG)
Received: 20 August 2015 Accepted: 13 April 2016
DOI:10.16373/j.cnki.ahr.150054
*Corresponding authors: Dr.Jianguo CUI, from Chengdu Institute of Biology (CIB), Chinese Academy of Sciences (CAS), with his research focusing on behavioral ecology of amphibians, and Prof.Yezhong TANG, from CIB, CAS, with his research focusing on behavioral neuroscience of amphibians and reptiles.
 Asian Herpetological Research2016年2期
Asian Herpetological Research2016年2期
- Asian Herpetological Research的其它文章
- Tracing the Origin of the Black-spotted Frog, Pelophylax nigromaculatus, in the Xinjiang Uyghur Autonomous Region
- Genetic Diversity and Population Structure for the Conservation of Giant Spiny Frog (Quasipaa spinosa) Using Microsatellite Loci and Mitochondrial DNA
- Ecological Niche Divergence between Trapelus ruderatus (Olivier,1807) and T.persicus (Blanford, 1881) (Sauria: Agamidae) in the Middle East
- The Effects of Chronic Hypoxia on Thermoregulation and Metabolism in Phrynocephalus vlangalii
- Comparison of Skull Morphology in Two Species of Genus Liua (Amphibia: Urodela: Hynobiidae), L.shihi and L.tsinpaensis
- Discovery of Female Laudakia papenfussi Zhao, 1998, with Insights into its Phylogenetic Relationships
