紫色不结球白菜花色苷合酶基因BrcANS的克隆与表达分析
许玉超侯喜林徐玮玮沈露露张仕林刘世拓胡春梅,*南京农业大学作物遗传与种质创新国家重点实验室,江苏南京 0095;安徽省合肥市肥西县农业委员会,安徽合肥3000
紫色不结球白菜花色苷合酶基因BrcANS的克隆与表达分析
许玉超1侯喜林1徐玮玮1沈露露2张仕林1刘世拓1胡春梅1,*1南京农业大学作物遗传与种质创新国家重点实验室,江苏南京 210095;2安徽省合肥市肥西县农业委员会,安徽合肥230001
摘 要:以不结球白菜紫色品系NJZX1-3和其绿色突变体NJZX1-0及其后代F2的2个株系NJZX2-1和NJZX2-2为材料,研究花色苷合酶基因在紫色不结球白菜叶片花色苷合成途径中的作用。利用同源克隆的方法,分别在NJZX1-3 及NJZX1-0中克隆到花色苷合酶基因;经序列比对发现,花色苷合酶基因的核苷酸和氨基酸序列在2种材料和大白菜中完全一致,长度为1077 bp,编码358个残基,第211~第307肽段具有2OG-Fe(II)双加氧酶家族基因的结构域,被命名为 BrcANS。BrcANS蛋白与同科芥菜的同源性高达99%,进化关系亦与其最相近。在全部4种材料鲜叶中,总花色苷的含量(TAC)与叶片紫色程度是一致的,其中,NJZX1-3叶片中总花色苷含量最高,达到80.15±5.74 mg 100 g-1FW;BrcANS表达量为NJZX1-0 < NJZX2-1 < NJZX2-2 < NJZX1-3,与其总花色苷含量呈正相关。BrcANS的mRNA 在 NJZX1-3和 NJZX1-0两种材料的不同组织中特异性表达∶ 在叶片中高度表达,而在其他组织中表达较弱;另外,在两种材料间的表达亦存在显著差异,在NJZX1-3叶片中的表达丰度显著高于NJZX1-0。随着叶龄的增大,紫色不接球白菜叶片紫色变浅,BrcANS的表达量下降,且在NJZX1-3和NJZX1-0间的表达差异亦明显减小。以上结果表明,BrcANS基因是紫色不结球白菜中花色苷合成的关键基因之一,其mRNA表达量与叶片紫色直接相关,可能在其转录水平上调控叶片中紫色的形成。
关键词:不结球白菜;花色苷合酶;同源克隆;序列分析;总花色苷含量;基因表达
本研究由江苏省农业科技自主创新项目[CX(15)1015]和江苏省科技支撑计划项目(BE2013429)资助。
The study was supported by the Independent Innovation Fund for Agricultural Science and Technology of Jiangsu Province [CX(15)1015],and the Science-technology Support Program of Jiangsu Province (BE2013429).
URL∶ http∶//www.cnki.net/kcms/detail/11.1809.S.20160321.1056.016.html
不结球白菜(Brassica campestris ssp. chinensis Makino)为十字花科芸薹属白菜甘蓝类蔬菜,在中国广泛栽培。近年来,东南亚、日本、欧美等地也引种,逐渐成为一种世界性蔬菜[1]。紫色小白菜作为不结球白菜的一个品种类型,因其色彩鲜艳和富含花色苷而极具营养价值,引起越来越多人的注意。
花色苷,是一类水溶性有色黄酮类化合物,主要存在于液泡中,是植物次生代谢产物三环类黄酮化合物的主要成员[2],广泛分布于种子植物,在植物花、叶片、果实、种子和其他组织中响应深色到蓝色的变化[3-4]。花色苷具有较强的抗氧化活性,其合成路径是植物次生代谢中被最广泛研究的途径之一[5-6]。其中,花色苷合酶(anthocyanidin synthase,ANS,又称leucoanthocyanidin dioxygenase,LDOX)是花色苷合成后期的关键酶,催化无色花色苷转化为有色花色苷[7](图1)。目前,已先后从拟南芥、芥菜、葡萄、马铃薯和洋葱等植物分离了ANS基因[8-12]。此外,R2R3-MYB (MYB)、MYC (bHLH)和WD40三类转录因子通常以WD-repeat/Mybs/bHLH复合物的形式来调节花色苷合成途径中结构基因的表达进而调控花色苷的合成[13]。在拟南芥中,WD-repeat蛋白TTG1通过结合bHLH转录因子(GL3、TT8或EGL3)和R2R3-MYB转录因子(MYB75/PAP1或MYB90/PAP2)形成WD-repeat/Mybs/bHLH复合物来上调花色苷合成后期基因 (DFR、ANS或UF3GT)的表达[7,14-18](图1)。但关于这些转绿因子对紫色不结球白菜中花色苷合成后期关键基因方面的研究,还未见报道。

图1 拟南芥中花色苷的生物合成途径[7]Fig. 1 The biosynthetic pathway of anthocyanidins in Arabidopsis[7]
本研究利用大白菜BrANS1 (Bra013652) (BRAD;http∶//brassi cadb.org/brad/)同源基因设计引物,从紫色不结球白菜和其绿色突变体的叶片中分别得到花色苷合成相关的花色苷合酶基因cDNA全长,利用生物信息学方法分析该基因序列,推测BrcANS基因的功能,为研究紫色不结球白菜中花色苷合成的分子调控机制提供理论依据。
1 材料与方法
1.1 试验材料
以南京农业大学白菜系统生物学实验室提供的紫色不结球白菜品系 NJZX1-3,其绿色突变系NJZX1-0、NJZX2-2 [母本NJZX1-3和父本NJZX1-0杂交的F1代自交得到的深色单株(F2深)]和NJZX2-1[母本NJZX1-3和父本NJZX1-0杂交的F1代自交得到的浅色单株(F2浅)]为材料,将种子用蒸馏水冲洗干净,室温催芽3 d左右,播种至穴盘,移至大棚温室,待材料长到适于本试验时,按试验设计取样,置-80℃保存备用。
大肠杆菌(Escherichia coli)菌株DH5α由本实验室保存;质粒载体PMD19-T easy、Prime STAR GXL DNA聚合酶(高保真聚合酶)、TaKaRa RNAiso Reagent (RNA提取试剂盒)、Prime Script RT Reagent Kit (第 1链 cDNA合成试剂盒)、PrimeScript RT Reagent Kit with gDNA Eraser (单链cDNA的合成试剂盒)、SYBR Premix Ex Taq和DNA胶回收试剂盒等均购自TaKaRa公司,2×HiQPCR MIX购自欧科(南京)生物技术有限公司。
1.2 第1链cDNA的合成
用RNA提取试剂盒(TaKaRa RNAiso Reagent,TaKaRa,大连,中国)分别提取NJZX1-3和NJZX1-0十叶期叶片的总RNA。使用反转录试剂盒(Prime Script RT Reagent Kit,TaKaRa)合成第一链cDNA。
1.3 单链cDNA的合成
用RNA提取试剂盒(同上)分别提取下列材料的总RNA∶ (1) NJZX1-3、NJZX1-0、NJZX2-1和NJZX2-2七叶期叶片;(2) NJZX1-3和NJZX1-0 的根、茎、叶片、心叶和花蕾;(3) NJZX1-3和NJZX1-0四叶期、七叶期、十五叶期和抽薹期叶片。使用反转录试剂盒(PrimeScript RT reagent Kit with gDNA Eraser,Ta-KaRa),合成单链cDNA。
1.4 ANS基因的克隆
以反转录得到的第1链cDNA为模板,PCR 总体系20 μL,包含模板1 μL、dNTPs混合物2 μL、Prime STAR GXL DNA聚合酶 0.5 μL、5×Prime STAR GXL缓冲液5 μL、引物BrcANS-F/R各1 μL (表1)、ddH2O 14.5 μL。反应程序为96℃ 2 min;96℃ 30 s,57℃ 30 s,72℃ 2 min,35个循环;72℃ 10 min。PCR产物经1.2% (w/v)凝胶电泳检测后,用DNA凝胶回收试剂盒(TaKaRa)回收,将回收的目的片段与PMD-19 Teasy载体(TaKaRa)连接,转化大肠杆菌,挑取单克隆,送南京金斯瑞生物技术有限公司测序。
1.5 生物信息学分析
用Cluster1.8和DNAMAN6.0对该基因的核苷酸序列及其推定的氨基酸序列进行序列比对。利用MEGA5.20软件的Neighbor-Joining方法,自展值设定为1000,进行物种间进化树分析。
1.6 半定量PCR
根据BrcANS的cDNA全长序列,Actin基因[19]作为内参,利用Beacon Designer v7.9软件设计引物BrcANS-S/A和Actin-S/A (表1)。以单链cDNA作为模板,体系20 μL,包括2×HiQPCR MIX 10 μL、引物各1 μL、模板1 μL、ddH2O 7 μL。反应程序为96 ℃ 2 min;96℃ 30 s,57℃ 30 s,72℃ 30 s 28个循环;72℃ 10 min。每个反应设3次重复。
1.7 实时荧光定量PCR
采用 SYBR Premix Ex Taq剂盒,以稀释 10 倍的单链cDNA作为模板,体系20 μL,含Taq 10μL、模板2 μL、引物各0.4 μL (表1),相对定量使用参照基因的ΔCT法[19],表达差异等于2-ΔCT,ΔCT = CT目标基因- CTactin。SYBR Premix Ex Taq 10 μL,ddH2O 7.2 μL。每个反应设3次生物学重复。采用Microsoft Excel 2007和IBM SPASS20.0分析基因表达情况及显著性。
1.8 总花色苷含量的测定
总花色苷的提取略有改动[21],将新鲜组织加入液氮研磨成粉末,加入0.1% HCl甲醇(v/v)溶液 15 mL;用超声波(40 kHz,100 W,T < 35℃,KQ-500DE型数控超声波清洗器,中国昆山)浸提 25 min;4℃,10 621 × g离心10 min,上清液经真空浓缩机(Eppendorf Concentrator Plus,中国)富集;加入等体积V (提取液体积)的0.1% HCl水(v/v)溶液,经0.22 μm滤膜过滤,备用。
采用pH值示差法测定[22],取滤液1 mL,分别用pH 1.0的KCl (0.2 mol L-1)缓冲液和pH 4.5的NaAc (0.45 mol L-1)缓冲液,定容至10 mL,分别测定520 nm和700 nm处吸光值A,每个反应设3次重复。
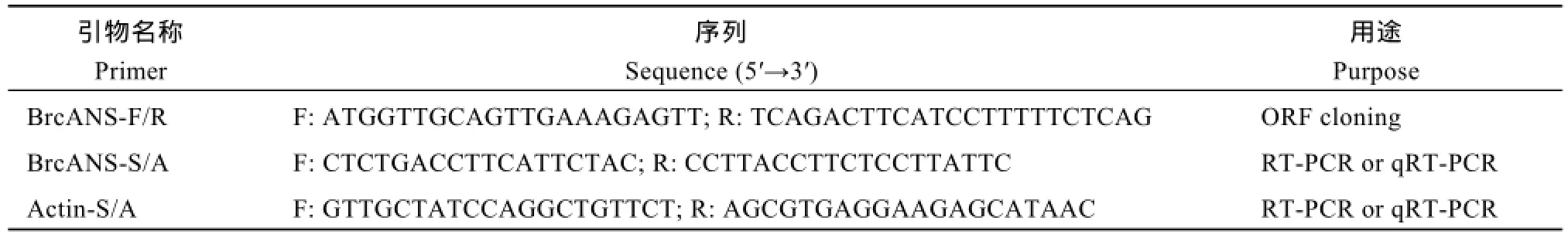
表1 试验引物及其序列Table 1 Sequence of primers used in this study
总花色苷含量(mg 100 g-1) = [(A520-A700) pH1.0-(A520-A700) pH4.5]×449.2×DF×V×100/(26900×1×W)
式中449.2 g mol-1为矢车菊素-3-葡萄糖苷的相对分子质量;26 900为摩尔吸光系数(L mol-1cm-1);DF为稀释倍数;1为比色皿光程(cm);V为提取液体积(L);W为鲜重(g)。
2 结果与分析
2.1 BrcANS的克隆与序列比对
分别以NJZX1-3和NJZX1-0成熟期叶片的cDNA为模板,克隆得到4条片段,片段1和2为NJZX1-3的ANS扩增图谱,片段3和4为NJZX1-0的ANS扩增图谱;测序结果表明,ANS基因在NJZX1-3和NJZX1-0中片段大小一致,长度都为1077 bp(图2)。核苷酸和氨基酸序列比对结果表明,NJZX1-3和NJZX1-0的ANS基因核苷酸碱基和推测的氨基酸序列完全一致,编码358个残基,且与大白菜BrANS1核苷酸和氨基酸序列亦完全一致(图3)。保守域分析(http∶//www.ncbi.nlm.nih.gov/Structure/cdd/-wrpsb.cgi)结果表明,不结球白菜花色苷合酶蛋白属于2OG-Fe(II)双加氧酶超家族,因此被命名为BrcANS基因。
2.2 BrcANS蛋白的同源性比较及进化树分析
将不结球白菜的 BrcANS氨基酸序列与其他物种的ANS氨基酸序列同源比对发现,不同物种花色苷合酶序列之间具有相同的活性中心和较多的高度保守序列,包含典型的 2-酮戊二酸铁依赖型双加氧酶的保守功能域,其中包括与 2-OG特异结合的精氨酸(Arg) 2个(R288,297)和丝氨酸(Ser) 5个(S236,265,278,284,299),与 Fe2+特异结合的组氨酸(His) 4个(H232,243,269,287)和天冬氨酸(Asp) 2个(D234,272),这几个位点在不同物种的ANS序列中高度保守,其中,BrcANS与芥菜(Brassica juncea,ACH58397.1)、结球甘蓝(Brassica oleracea var. capitata,AAO73440.1)、萝卜(Raphanus sativus,AIM48928.1)、拟南芥(Arabidopsis thaliana,AEI99590.1)、苹果(Malus domestica,AAZ79374.1)、蓝莓(Vaccinium corymbosum,AFA53722.1)、马铃薯(Solanum tuberosum,NP_ 001274859.1)、大豆(Glycine max,NP_001239794.1)的ANS基因序列同源性分别为99%、97%、92%、92%、80%、77%、75%和80%,表明花色苷合酶基因序列在进化上具有较高的保守性(图4)。

图2 不结球白菜NJZX1-3和NJZX1-0中BrcANS的全长cDNA扩增结果Fig. 2 Full cDNA amplification of BrcANS in non-heading Chinese cabbage NJZX1-3 and NJZX1-0
系统进化树分析表明,在十字花科的5种主要植物中,不结球白菜与芥菜亲缘关系最近;在不同科属间,不结球白菜BrcANS与蔷薇科的苹果的关系较近(图5)。
2.3 BrcANS在不同叶色材料中的表达
2.3.1 不同材料叶片的表型观察 根据上表皮叶色差异可以把 4个材料分为 3类,即绿色的NJZX1-0、浅紫色的NJZX2-1和深紫色的NJZX2-2 和 NJZX1-3。绿色材料的下表皮呈绿色,浅紫色材料的下表皮中部分靠近叶缘的叶脉为紫色,而深紫色材料的下表皮中靠近叶缘的叶脉和叶肉都呈紫色,特别是叶尖部位(图6)。NJZX2-2和NJZX1-3鲜叶中总花色苷含量较高,分别为 69.30±5.06 mg 100 g-1FW 和 80.15±5.74 mg 100 g-1FW,且极显著高于NJZX2-1和NJZX1-0;NJZX1-0 鲜叶中总花色苷含量最低且极低于 NJZX2-1;提取液颜色的色度与总花色苷含量呈正相关(图7和图8)。
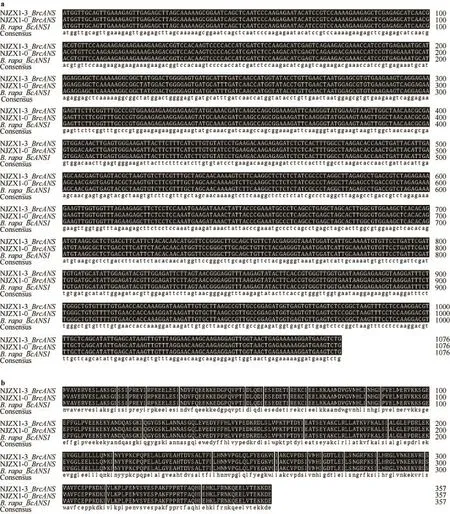
图3 NJZX1-3、NJZX1-0和大白菜ANS的核苷酸(a)和氨基酸(b)序列比对结果Fig. 3 Comparison of ANS nucleotide (a) and amino acid (b) sequences of NJZX1-3,NJZX1-0,and Chinese cabbage
2.3.2 BrcANS的表达分析 实时定量PCR结果表明,BrcANS在不同叶色的叶片中均有表达,在绿色叶片 NJZX1-0中表达量仅为 0.095,而在深紫色叶片NJZX2-2和NJZX1-3中的表达量最高,分别为1.94和 1.99,其次是浅紫色叶片 NJZX2-1,其表达量为0.98。NJZX1-3、NJZX2-2和NJZX2-1中BrcANS表达量分别是NJZX1-0的208.79、203.63和103.01倍(图9)。说明BrcANS的表达量随着叶片紫色的加深而增加。
2.4 BrcANS在NJZX1-3和NJZX1-0材料不同组织中的表达情况

图4 BrcANS和其他已知ANS蛋白同源性比较Fig. 4 Homologous alignment of amino acid sequences of BrcANS and other known ANS

图5 BrcANS与其他植物中ANS蛋白的系统进化分析Fig. 5 Phylogenetic analysis of BrcANS and ANS protein in other species
半定量PCR检测花色苷合酶基因表达的结果表明,2种不结球白菜均为心叶中转录水平最高。NJZX1-3各组织表达丰度为心叶>叶>叶柄>花蕾>茎>根;而 NJZX1-0的表达情况为心叶>根,叶柄>茎>叶>花蕾;其中,BrcANS的表达量在NJZX1-3的叶片中显著高于 NJZX1-0,其他组织中表达量的差异则不明显(图10)。

图6 不同材料不结球白菜叶色间比较Fig. 6 Comparison of leaf color in non-heading Chinese cabbage

图7 不结球白菜叶色中总花色苷提取液颜色Fig. 7 Color of total anthocyanin extracted from non-heading Chinese cabbage leaves

图8 不结球白菜叶中总花色苷含量Fig. 8 Total anthocyanin content of non-heading Chinese cabbage leaves
2.5 BrcANS在NJZX1-3和NJZX1-0两材料不同时期叶片中的表达
花色苷合酶基因BrcANS在2种材料的4个叶期中表达趋势是一致的,四叶期表达量最高,十五叶期其次,七叶期表达量较低,至抽薹期表达量显著降低,即从苗期到抽薹期BrcANS表达量总体呈现降低趋势;在NJZX1-3不同叶期中BrcANS的表达量显著高于 NJZX1-0,其差异随着叶龄的增大而减小,由四叶期为NJZX1-0的633.09倍,降至抽薹期为于NJZX1-0的63.43倍(图11)。

图9 BrcANS基因在不同不结球白菜叶片中表达水平Fig. 9 Relative expression levels of BrcANS gene in different non-heading Chinese cabbage leaves
3 讨论
紫色白菜类叶片中所含色素为花青苷类化合物[23],有研究发现,在包括红叶芥菜、紫红色大白菜、红菜薹、紫色小白菜和紫结球甘蓝5种芸薹属新鲜叶片中总花色苷含量介于13.00~71.90 mg 100 g-1FW[24],在本研究中紫色不结球白菜鲜叶中总花色苷含量介于27.29~80.15 mg 100 g-1FW,说明本研究结果与前人研究具有可比性。并且在紫色不结球白菜NJZX1-3鲜叶中总花色苷含量高于红叶芥菜(71.90 mg 100 g-1FW)。在3种不同叶色的观赏海棠中,常紫叶类品种“王族”中,其 McANS的表达量始终较高,绿叶色类“火焰”中,McANS的表达量始终较低。在新叶有色类品种“绚丽”中,幼叶紫色期其McANS的表达量也较高,功能叶变为绿色后,表达量随之降低[25]。本研究的4种材料中,BrcANS的表达量为NJZX1-0 <NJZX2-1 < NJZX2-2 < NJZX1-3,总花色苷含量也为∶NJZX1-0 < NJZX2-1 < NJZX2-2 < NJZX1-3,说明BrcANS的表达量随着紫色的加深而增加,并且与总花色苷的含量呈正相关;在水母雪莲中,SmANS1在红细胞系的细胞培养物和苗中大量表达,在黄色细胞系的细胞培养物中表达很弱,但在根部不表达[26]。在马铃薯植株中,ScANS 在茎、叶和顶芽中表达较高,在根中表达微弱,在匍匐茎和块茎中无表达[10]。在本研究中,BrcANS在2种材料不同组织中均有表达,但转录水平存在一定差异,说明在不结球白菜中BrcANS基因的表达存在组织特异性;在2种材料心叶中BrcANS的表达丰度较高且基本相同,但在突变体NJZX1-0中BrcANS的表达丰度在心叶中明显高于叶,这与心叶中有花色苷的积累结果一致,说明在突变体中BrcANS的表达水平随着叶片的生长被抑制;另外,在NJZX1-3叶片中BrcANS的表达水平显著高于NJZX1-0叶片。在NJZX1-3和NJZX1-0叶片中,随着叶龄的增加,BrcANS的表达量都在减小,且BrcANS在2种材料叶片中表达量差异亦在减小,特别是在抽薹期时,从外观上,NJZX1-3叶片的紫色褪去很多(图片未展示),这与BrcANS表达量的降低相对应。以上结果表明BrcANS的表达量与不结球白菜花色苷的积累呈正相关,两者关系密切。

图10 BrcANS基因在不结球白菜不同组织中表达量的差异Fig. 10 Expression of BrcANS gene in different tissues of non-heading Chinese cabbage
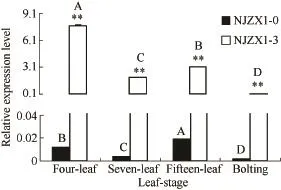
图11 BrcANS基因在不结球白菜叶期中表达水平Fig. 11 Expression levels of BrcANS gene at leaf-stage in non-heading Chinese cabbage
花色苷含量是评价植物花色、叶色、果色等经济器官色泽及营养品质的重要指标,其合成过程复杂。花色苷的生物合成由包括苯丙氨酸解氨酶(PAL),查尔酮合酶(CHS),查尔酮异构酶(CHI),黄烷酮3-羟化酶(F3H)和类黄酮3'-羟化酶(F3'H),NADPH依赖性二氢黄酮醇还原酶(DFR),花色苷合酶/无色花色苷双加氧酶(ANS/LDOX),类黄酮-3-O-葡糖基转移酶(UF3GT)等合成基因参与完成[7,13]。花色苷合酶是花色苷合成途径后期的酶,通过Fe2+和2-酮戊二酸离子将无色花色苷催化为花色苷[27]。本研究在2种材料中克隆得到BrcANS基因,其核苷酸序列和氨基酸序列比对结果完全一致,且与大白菜BrANS1亦完全一致,长度分别为1077 bp和358个残基,BrcANS与同科的芥菜、芜菁和紫菜薹[9,28-29]中ANS基因的大小相同。BrcANS蛋白属于2OG-Fe(II)双加氧酶超家族,该家族被认为参与乙烯和赤霉素等植物激素的合成,也参与色素(如3-羟基花青素和花青素)和次生代谢产物(如黄酮)的羟基化和去饱和步骤[30]。本研究结果表明,不结球白菜BrcANS在紫色材料中高度表达,形成的BrcANS蛋白可能作用于黄烷-3,4-二醇,进而促进3-羟基花青素类次级代谢物的生成,最后,导致紫色不结球白菜中花色苷的积累,这可能是造成叶色泽差异的重要原因。
4 结论
从紫色不结球白菜品系NJZX1-3和其绿色突变系NJZX1-0的叶片中克隆得到的BrcANS基因序列完全一致,但其表达量在 2种材料中存在明显差异,说明 BrcANS基因对花色苷合成积累的影响可能主要发生在其转录水平上,BrcANS蛋白通过调节3-羟基花青素类次级代谢物的生成,使叶片呈现深紫色。
References
[1] 侯喜林. 不结球白菜育种研究新进展. 南京农业大学学报,2003,26(4)∶ 111-115 Hou X L. Advances in breeding of non-heading Chinese cabbage. J Nanjing Agric Univ,2003,26(4)∶ 111-115 (in Chinese with English abstract)
[2] Grotewold E. The genetics and biochemistry of floral pigments.Annu Rev Plant Biol,2006,57∶ 761-780
[3] Strack D,Wray V. The anthocyanidins,In∶ the Flavonoids Advances in Research since 1986 (Chapter 1),Chapman and Hall,London,1994. pp 1-22
[4] Yoshikazu T,Nobuhiro S,Akemi O. Biosynthesis of plant pigments∶ anthocyanidins,betalains and carotenoids. Plant J,2008,54∶ 733-749
[5] Xie D Y,Sharma S B,Paiva N L,Ferreira D,Dixon R A. Role of anthocyanidin reductase,encoded by BANYULS in plant flavornoid biosynthesis. Science,2003,299∶ 396-399
[6] Xie D Y,Dixon R A. Proanthocyanidin biosynthesis still more questions than answers? Phytochemistry,2005,66∶ 2127-2144
[7] Petroni K,Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci,2011,181∶219-229
[8] Wilmouth R C,Turnbull J J,Welford R W D,Clifton I J,Prescott A G,Schofield C J. Structure and mechanism of anthocyanidin synthase from Arabidopsis thaliana. Structure,2002,10∶ 93-103
[9] Yan M L,Liu X J,Guan C Y,Chen X B,Liu Z S. Cloning and expression analysis of an anthocyanidin synthase gene homolog from Brassica juncea. Mol Breed,2011,28∶ 313-322
[10] Samuelian S K,Camps C,Kappel C,Simova E P,Delrot S,Colova V M. Differential screening of overexpressed genes involved in flavonoid biosynthesis in North American native grapes∶‘Noble' muscadinia var. and ‘Cynthiana' aestivalis var. Plant Sci,2009,177∶ 211-221
[11] 王冰,王全逸,印敬明,陈敏,杨清. 野生马铃薯 ANS同源基因的克隆与表达分析. 植物生理学报,2011,47∶ 1103-1108 Wang B,Wang Q Y,Yin J M,Chen M,Yang Q. Molecular cloning and expression analysis of an ANS homologous gene from Solanum cardiphyllum. Plant Physiol J,2011,47∶1103-1108 (in Chinese with English abstract)
[12] 缪军,刘冰江,杨妍妍,霍雨猛,张一卉,霍凤梅,修景润,吴雄. 洋葱花青素合成酶基因的克隆和序列分析. 山东农业科学,2010,(1)∶ 1-5 Miao J,Liu B J,Yang Y Y,Huo Y M,Zhang Y H,Huo F M,Xiu J R,Wu X. Cloning and sequence analysis of anthocyanidin synthase gene in onion. Shandong Agric Sci,2010,(1)∶ 1-5 (in Chinese with English abstract)
[13] Holton T A,Cornish E C. Genetics and biochemistry of anthocyanidin biosynthesis. Plant Cell,1995,7∶ 1071-1083
[14] Walker A R,Davison P A,Bolognesi-Winfield A C,James C M,Srinivasan N,Blundell T L,Esch J J,Marks M D,Gray J C. The TRANSPARENT TESTA GLABRA1 locus,which regulates trichome differentiation and anthocyanidin biosynthesis in Arabidopsis,encodes a WD40 repeat protein. Plant Cell,1999,11∶ 1337-1349
[15] Payne C T,Zhang F,Lloyd A M. GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics,2000,156∶ 1349-1362
[16] Zhang F,Gonzalez A,Zhao M,Payne C T,Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development,2003,130∶ 4859-4869
[17] Baudry A,Caboche M,Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors,allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J,2006,46∶ 768-779
[18] Gonzalez A,Zhao M,Leavitt J M,Lloyd A M. Regulation of the anthocyanidin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J,2008,53∶ 814-827
[19] Lv S W,Zhang C W,Tang J,Li Y X,Wang Z,Hou X L. Genome-wide Analysis and identification of TIR-NBS-LRR genes in Chinese cabbage (Brassica rapa ssp. pekinensis) reveal expression patterns to TuMV infection. Physiol Mol Plant Pathol,2015,90∶ 89-97
[20] 谭国飞,王枫,贾晓玲,李岩,熊爱生. 芹菜甘露醇脱氢酶基因的分离与表达分析. 园艺学报,2013,40∶ 2189-2198 Tan G F,Wang F,Jia X L,Li Y,Xiong A S. Isolation and expression of mannitol dehydrogenase gene in celery. Acta Hort Sin,2013,40∶ 2189-2198 (in Chinese with English abstract)
[21] Guo N,Wu J,Zheng S N,Cheng F,Liu B,Liang J L,Cui Y,Wang X W. Anthocyanin profile characterization and quantitative trait locus mapping in zicaitai (Brassica rapa L. ssp. chinensis var. purpurea). Mol Breed,2015,35∶ 113
[22] Lee J,Durst R W,Wrolstad R E. Determination of total monomeric anthocyanin pigment content of fruit juices,beverages,natural colorants,and wines by the pH differential method∶ collaborative study. J AOAC Int,2005,88∶ 1269-1278
[23] Podsedek A. Natural antioxidants and antioxidant capacity of Brassica vegetables∶ a review. LWT-Food Sci Technol,2007,40∶1-11
[24] 张淑江,马越,徐学玲,钱伟,章时蕃,李菲,张慧,孙日飞.芸薹属 5种紫红色蔬菜花青素苷含量及组分分析. 园艺学报,2014,41∶ 1451-1460 Zhang S J,Ma Y,Xu X L,Qian W,Zhang S F,Li F,Zhang H,Sun R F. Components and amounts of anthocyanidins in several Brassica vegetables. Acta Hort Sin,2014,41∶ 1451-1460 (in Chinese with English abstract)
[25] 田佶,沈红香,张杰,姚允聪,宋婷婷,耿慧. 苹果属观赏海棠 McANS 基因克隆与不同叶色品种间表达差异分析. 园艺学报,2010,37∶ 939-948 Tian J,Shen H X,Zhang J,Yao Y C,Song T T,Geng H. Cloning of McANS gene in Malus crabapple and expression analysis in different cultivars. Acta Hort Sin,2010,37∶ 939-948 (in Chinese with English abstract)
[26] Cheng L Q,Xu Y J,Grotewold E,Jin Z P,Wu F Y,Fu C X,Zhao D X. Characterization of anthocyanidin synthase (ANS)gene and anthocyanidin in rare medicinal plant-Saussurea medusa. Plant Cell Tiss Organ Cult,2007,89∶ 63-73
[27] Xie D Y,Jackson L A,Cooper J D,Ferreira D,Paiva N L. Molecular and biochemical analysis of two cDNA clones encoding dihydroflavonol-4-reductase from Medicago truncatula. Plant Physiol,2004,134∶ 979-994
[28] 许志茹,李春雷,崔国新,孙燕,李玉花. 芜菁花青素合成酶基因的克隆、序列分析及表达. 生物技术通讯,2009,20∶ 66-68 Xu Z R,Li C L,Cui G X,Sun Y,Li Y H. Cloning,sequence analysis and expression of anthocyanidin synthase dene in turnip. Lett Biotechnol,2009,20∶ 66-68 (in Chinese with English abstract)
[29] 蒋明,陈孝赏,李金枝. 紫菜薹花青素合成酶基因BcANS的克隆、表达与序列分析. 浙江大学学报(农业与生命科学版),2011,37∶ 393-398
Jiang M,Chen X S,Li J Z. Cloning,expression and sequence analysis of anthocyanidin synthase gene BcANS in Brassica campestris var. purpurea. J Zhejiang Univ (Agric & Life Sci),2011,37∶ 393-398 (in Chinese with English abstract)
[30] Lepiniec L,Debeaujon I,Routaboul J M,Baudry A,Pourcel L,Nesi N,Caboche M. Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol,2006,57∶ 405-430
Cloning and Expression Analysis of Anthocyanidin Synthase Gene BrcANS from Purple Non-heading Chinese Cabbage
XU Yu-Chao1,HOU Xi-Lin1,XU Wei-Wei1,SHEN Lu-Lu2,ZHANG Shi-Lin1,LIU Shi-Tuo1,and HU Chun-Mei1,*1State Key Laboratory of Crop Genetics and Germplasm Enhancement,Nanjing Agricultural University,Nanjing 210095,China;2Agriculture Committee of Feixi County,Hefei 230001,China
Abstract:Purple non-heading Chinese cabbage cultivar NJZX1-3,its green leaf mutant line NJZX1-0,and their progeny F2∶NJZX2-1 and NJZX2-2 were used to study the function of anthocyanidin synthase gene in the anthocyanin biosynthesis of non-heading Chinese cabbage leaf. Homology-based cloning was used and anthocyanidin synthase gene was respectively cloned from two cultivars (NJZX1-3 and NJZX1-0). The gene nucleotides and amino acids sequences found in the two materials and Chinese cabbage were exactly the same,with a length of 1077 bp and encoding a peptide with 358 residues. Furthermore,a 2OG-Fe(II) dioxygenase super family domain was found in the amino acid sequence from the 211th to the 307th amino acids and the gene was named as BrcANS. The homology between BrcANS protein and BjANS protein was up to 99%,in accordance with the close relationship between them. Their total anthocyanin content (TAC) was consistent with the degree of purple in fresh leaves of the four materials,of which total anthocyanin content in cultivar NJZX1-3 leaves was the highest,up to 80.15±5.74 mg 100 g-1FW. Simultaneously,the expression level of BrcANS (NJZX1-0 < NJZX2-1 < NJZX2-2 < NJZX1-3) was positively correlated with the increasing trend of TAC. The mRNA of BrcANS exhibited tissue-specific expression in both materials,showing high level in leaves and lower level in other organs. In addition,the expression of two materials was significantly different,indicating that the expression of BrcANS in cultivar NJZX1-3 leaves was obviously higher than that in mutant line NJZX1-0. With the in-creasing of leaf age,the leaf color became shallow and the expression of BrcANS reduced. Meanwhile,the difference of expression between NJZX1-3 and NJZX1-0 decreased significantly. These results indicated that BrcANS gene is one of the key genes in the anthocyanin biosynthesis of non-heading Chinese cabbage leaf,and its expression level is directly related to the purple color of leaves,thus the gene might regulate the formation of the purple color in leaves at transcriptional level.
Keywords:Non-heading Chinese cabbage;Anthocyanidin synthase;Homology-based cloning;Sequence analysis;Total anthocyanin content;Gene expression
DOI:10.3724/SP.J.1006.2016.00850
*通讯作者(
Corresponding author)∶ 胡春梅,E-mail∶ jjjhcm@njau.edu.cn
收稿日期Received()∶ 2015-11-11;Accepted(接受日期)∶ 2016-03-14;Published online(网络出版日期)∶ 2016-03-21.

