Inhibition of glucose- and fructose-mediated protein glycation by infusions and ethanolic extracts of ten culinary herbs and spices
Jugjeet Singh Ramkissoon,Mohamad Fawzi Mahomoodally*,Anwar Hussein Subratty,Nessar AhmedDepartment of Health Sciences,Faculty of Science,University of Mauritius,R´eduit,MauritiusSchool of Healthcare Science,Manchester Metropolitan University,Manchester M 5GD,United Kingdom
ABSTRACT
Inhibition of glucose- and fructose-mediated protein glycation by infusions and ethanolic extracts of ten culinary herbs and spices
Jugjeet Singh Ramkissoon1,Mohamad Fawzi Mahomoodally1*,Anwar Hussein Subratty1,Nessar Ahmed21Department of Health Sciences,Faculty of Science,University of Mauritius,R´eduit,Mauritius
2School of Healthcare Science,Manchester Metropolitan University,Manchester M1 5GD,United Kingdom
ABSTRACT
Keywords:
Glycation
Herbs
Spices
Glucose
Fructose
Antioxidant
Phytochemical
ARTICLE INFO
Article history:
Received 25 Nov 2015
Received in revised form 2 Dec,2nd revisedform15 Dec,3rdrevisedform 25 Dec 2015
Accepted 15 Jan 2016
Available online 20 Apr 2016
Original article http://dx.doi.org/10.1016/j.apjtb.2016.01.016
Tel: +230 57327341
E-mail: mahomoodally@uom.ac.mu
Foundation Project: Supported by University of Mauritius and the Tertiary Education Commission for financial support(Project code Q0024).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
Protein glycation is a non-enzymatic reaction between reducing sugars and free amino groups[1],resulting in the formation of reversible intermediates that undergo rearrangements generating advanced glycation end-products(AGEs)[2-5]. The formation of AGEs is also known to result from the action of various metabolites such as fructose,trioses and dicarbonyl compounds[6-9]which participate in the glycation reaction at a much faster rate than glucose[10]. As glucose is the most abundant sugar in blood and elevated in diabetes,most studies have been focused on glycation between glucose and proteins[7]. However,glucose is one of the least reactive sugars[11]and in vitro studies suggest that fructose,compared to glucose,is a more potent initiator of glycation[9-15]. Although direct evidence is limited[7],it is likely that the high reactivity of fructose and its metabolites may substantially contribute to the formation of AGEs in vivo[12-15]. Hence,further insight into its possible role in AGEsmediated pathologies is needed to appraise the significance of fructation in healthy people and in diabetic complicationsespecially where the sorbitol pathway is active[16]. Several studies have shown the role of AGEs in protein modification leading to physiopathological changes such as diabetes [10,17,18]. Much interest has been devoted to using antiglycation agents for alleviating diabetic complications[19,20],however,drugs like aminoguanidine have shown serious side effects[2]. Therefore,much effort has been extended to search for dietary plants that could effectively inhibit AGE formation and also have antioxidant properties[19-24]. A great number of aromatic,spicy,medicinal and other plants contain chemical compounds including tannins,alkaloids,terpenoids,steroids and flavonoids exhibiting antioxidant properties and demonstrate such protective effects[25-30].
However,there are little studies supporting the ability of culinary herbs and spices from Mauritius in the prevention of fluorescent AGE formation. Besides initiating investigation on the inhibitory activity of dietary plants on protein glycation[31]and their antioxidant properties[32],this study also investigated the effects of the extracts against glucose- and fructose-mediated glycation of bovine serum albumin(BSA). Since fluorescence generation through the Maillard reaction has been correlated with long-term complications of diabetes[12]and it is known that fructose is a more potent glycating agent than glucose[7],the participation of glycation by fructose(fructation)deserves further exploration. In addition,the phytochemical contents and supplementary antioxidant activities including the reducing power,metal ion chelating and superoxide radical scavenging activities were also evaluated. Dietary plants might be promising anti-glycation agents for the prevention of diabetic complications via inhibition of AGE and oxidationdependent protein damage.
2. Materials and methods
2.1. Chemicals
BSA(BSA;fraction V,fatty acid free,low endotoxin),D-glucose,sodium azide,phosphate buffered saline(PBS),aminoguanidine,trichloroacetic acid(TCA),ethanol,iron(III)chloride 6-hydrate(FeCl3),concentrated hydrochloric acid (HCl),benzene,ammonia(NH3),sulphuric acid(H2SO4),chloroform,copper acetate,acetic acid,glacial acetic acid,Mayer's reagent,Wagner's reagent,Dragendroff's reagent,Hager's reagent,Molisch's reagent,Millon's reagent,iodine,Fehling A,Fehling B,Benedict's reagent,NaOH,sodium nitroprusside,magnesium ribbon,lead acetate,zinc powder,boric acid,HNO3,ninhydrin,potassium ferricyanide,butylated hydroxytoluene(BHT),ethylenediaminetetracetic acid(EDTA),potassium dihydrogen phosphate,dipotassium hydrogen phosphate,iron(II)chloride,ferrozine,nicotinamide adenine dinucleotide,phenazine methosulphate,nitroblue tetrazolium(NBT),potassium bismuth iodide,picric acid,copper(II)sulphate,potassium sodium tartrate,anhydrous sodium carbonate,sodium citrate,copper(II)sulphate pentahydrate were purchased from Sigma-Aldrich,USA.
2.2. Plant materials and preparation of extracts
Culinary herbs and spices were purchased from a local market,Rose Hill,Mauritius. Ten commercially available herbs and spices were tested,namely,garlic(Allium sativum L.),ginger(Zingiber officinale Rosc.),thyme(Thymus vulgaris L.),parsley(Petroselinum crispum Mill.),curry leaves(Murraya koenigii L. Spreng),peppermint(Mentha piperita L.),turmeric (Curcuma longa L.),onion(Allium cepa L.),green onion scallion(Allium fistulosum L.)and coriander(Coriandrum sativum L.). The study was limited to plants that are widely available to the public and are in routine use for daily food cooking in Mauritius. Fresh culinary herbs and spices were ground into a paste and 5 g of the latter were extracted with ethanol(50%)at a ratio of 10 mL/g at room temperature(28±2)°C for 1 week. The extracts were centrifuged at 1 000 g for 10 min to remove precipitate. For preparing infusions 5 g of the ground paste were infused into boiling distilled water for 30 min at a ratio of 10 mL/g and filtered through Whatman No. 4 paper[23].
2.3. In vitro glycation of BSA with fructose and glucose
BSA was glycated as described previously with minor modifications[33]. Briefly,BSA(1 mg/mL)was incubated with 200 mmol/L fructose or glucose in 0.2 mol/L PBS,pH 7.4 containing 0.01%sodium azide in darkness at 37°C for 3 weeks in the absence or presence of the ten plants extracts separately. The assay was conducted using 100 μL of the plants of either ethanolic extracts or infusions in a final volume of 5 mL. Aminoguanidine was used as a positive control. After three weeks incubation,the reactions were stopped by adding 10 μL of 100%(w/v)TCA and after 10 min the mixture was centrifuged at 2 000 r/min for 15 min. The precipitate was re-dissolved in alkaline PBS. The fluorescent AGEs were determined spectrofluorometrically(PerkinElmer)using fluorescence intensity at an excitation wavelength 370 nm and emission wavelength 440 nm. The inhibitory effect of the extracts and aminoguanidine was evaluated by the calculation of percentage inhibition compared with maximum glycation elicited by BSA-glucose or BSA-fructose[3].
2.4. Reducing power assay
The reducing power of samples was determined by the method of Oyaizu[34]. An aliquot of the sample(1.0 mL)was mixed with phosphate buffer(0.2 mol/L,pH 6.6,2.5 mL)and 1%potassium ferricyanide(2.5 mL). The mixture was incubated at 50°C for 20 min. After adding 10%TCA (2.5 mL),the mixture was centrifuged at 650 r/min for 10 min. The supernatant(2.5 mL)was mixed with distilled water(2.5 mL)and 0.1%iron(III)chloride(0.5 mL),and the absorbance was measured at 700 nm using an appropriate blank. Assays were carried out in triplicate. BHT was used as a control.
2.5. Metal ion chelating assay
The ability of samples to chelate iron(II)ions was estimated using the method reported by Gulcin(2006)[35]and compared with that of the reference chelator agent EDTA. Samples were added to a solution of 2 mmol/L iron(II)chloride(1 mL). The reaction was initiated by the addition of 5 mmol/L ferrozine(1 mL)and the volume of the mixture was finally adjusted to 4 mL with ethanol,shaken vigorously and left standing at room temperature for 10 min. After the mixture had reached equilibrium,the absorbance of the solution wasmeasured spectrophotometrically at 562 nm. All assays were done in triplicate.
2.6. Superoxide radical scavenging activity
Superoxide anions were generated using phenazine methosulphate-nicotinamide adenine dinucleotide(PMS-NADH)system. The superoxide anions were subsequently made to reduce NBT which yields a chromogenic product,which is measured at 560 nm[36]. Test solution(1 mL)in 0.1 mol/L phosphate buffer pH 7.4,1 mL of 468 μmol/L NADH solution,1 mL of 150 μmol/L NBT solution and 1 mL of 60 μmol/L phenazine methosulphate solution were added to a tube and incubated at room temperature for 5 min. The absorbance was read at 560 nm. All assays were done in triplicate. Gallic acid was used as a positive control.
2.7. Qualitative phytochemical analysis
The plant extracts were subjected to qualitative chemical screening for the identification of various classes of chemical compounds using standard methods[20,37-40]. Preliminary phytochemical screening of the ten plant materials for secondary metabolites was performed as follows.
2.7.1. Determination of tannins
Two millilitre of the plant extracts was stirred with equal volume of distilled water. A few drops of 2%FeCl3solution was added. The presence of tannins was indicated by the formation of a green precipitate.
2.7.2. Determination of saponins
In foam test,5 mL of extract was shaken vigorously with 5 mL of distilled water in a test tube and warmed. The formation of stable foam was taken as an indication for the presence of saponins.
In froth test,2 mL of extract was shaken vigorously with distilled water to froth and was then allowed to stand for 10-15 min. The persistent froth was considered as presence of saponins.
2.7.3. Determination of phlobatannins
Two millilitre of extract was added to 2 mL of 1%HCl and the mixture was boiled. Deposition of a red precipitate was taken as evidence for the presence of phlobatannins.
2.7.4. Determination of anthraquinones
In Borntrager's test,3 mL of plant extracts were treated with 3 mL of benzene. The mixture was then filtered. The filtrate was then mixed with 5 mL of 10%ammonia solution. The presence of free anthraquinones was indicated by the presence of a pink,red or violet colour in the lower ammonical phase.
Three millilitre of H2SO4was added to 3 mL of extracts and the mixture was boiled then filtered. To the filtrate,3 mL of benzene was added and shaken. Three millilitre of 10%NH3was added to the benzene layer. Anthraquinone derivatives were revealed by a pink,red or violet colouration in the lower ammonical phase.
2.7.5. Determination of terpenoids
Two millilitre of extract was mixed with 2 mL of chloroform and evaporated to dryness. Two millilitre of concentrated H2SO4was then added and heated for about 2 min. A grayish colour indicates the presences of terpenoids.
2.7.6. Determination of diterpenes
Two millilitre of extract were treated with 3-4 drops of copper acetate solution. Formation of emerald green colour indicates the presence of diterpenes.
2.7.7. Determination of steroids
Two millilitre of extract was mixed with 2 mL of chloroform and 2 mL of concentrated H2SO4. A red colour indicates the presence of steroids.
Two millilitre of extract was mixed with 2 mL of chloroform and treated with H2SO4and acetic acid. The development of a greenish colour indicates the presence of steroids.
2.7.8. Determination of alkaloids
2.7.8.1. Mayer's test
Extracts were mixed with HCl and filtered. Filtrates were treated with Mayer's reagent. Formation of a yellow coloured precipitate indicates the presence of alkaloids.
2.7.8.2. Wagner's test
Extracts were mixed with HCl and filtered. Filtrates were treated with Wagner's reagent. Formation of brown/reddish precipitate indicates the presence of alkaloids.
2.7.8.3. Dragendroff's test
Extracts were mixed with HCl and filtered. Filtrates were treated with Dragendroff's reagent. Formation of red precipitate indicates the presence of alkaloids.
2.7.8.4. Hager's test
Extracts were mixed with HCl and filtered. Filtrates were treated with Hager's reagent. Presence of alkaloids was confirmed by the formation of yellow precipitate.
2.7.9. Determination of carbohydrates and reducing sugars
2.7.9.1. Molisch's test
Two millilitre of Molisch's reagent was shaken with 3 mL of extract. Then 2 mL of concentrated H2SO4was added carefully down the side of the test tube. The presence of carbohydrates was indicated by a violet ring at the interphase.
2.7.9.2. Iodine test
Three millilitre of extract and 1 mL of iodine were mixed together. Presence of carbohydrates was shown by a dark blue or purple colouration.
2.7.9.3. Fehling's test
Equal volume of Fehling A and Fehling B reagents were mixed together and 2 mL of it was added to the extract and gently boiled. A brick red precipitate at the bottom of the test tube indicated the presence of reducing sugars.
2.7.9.4. Benedict's test
Two millilitre of extract was mixed with 2 mL of Benedict's reagent and boiled. A reddish brown precipitate indicated the presence of reducing sugars.
2.7.10. Determination of cardiac glycosides (cardenolides)
2.7.10.1. Liebermann's test
Two millilitre of extract was mixed with 2 mL of chloroform and 2 mL of acetic acid and the solution was cooled on ice. H2SO4was then added carefully. A colour change from violet to blue to green indicates the presence of a steroidal nucleus. That is a glycone portion of glycoside.
2.7.10.2. Salkowski's test(detection of phytosterols)
Two millilitre of each plant extract was added to 2 mL of chloroform. The mixture was then shaken gently with 2 mL of H2SO4. The presence of a steroidal ring;the glycone portion of glycoside was indicated by a reddish brown colour.
2.7.10.3. Keller-Kilani test
One drop of FeCl3solution was added to a mixture of 2 mL of extract and 2 mL glacial acetic acid. One millimetre of concentrated H2SO4was then added to the mixture. The presence of a deoxy sugar characteristic of cardenolides was indicated by a brown ring.
Two millilitre of extract was mixed with 10%NaOH and 0.3%solution of nitroprusside. Appearance of transient pinkish red colouration indicates the presence of cardenolides.
2.7.11. Determination of flavonoids and different types of flavonoids
2.7.11.1. Shinoda's test
A piece of magnesium ribbon and HCl were added to the extracts. Purple,red,pink or orange colour confirmed the presence of flavonoids.
2.7.11.2. Alkaline reagent test
Two millilitre of extract was mixed with 2 mL of 2%solution of NaOH. An intense yellow colour which turned colourless on addition of a few drops of dilute acid indicated the presence of flavonoids.
2.7.11.3. Lead acetate test
Extracts were treated with few drops of lead acetate solution. Formation of yellow precipitate indicates the presence of flavonoids.
2.7.11.4. Test for flavononols
Zinc powder was added with HCl. Development of a deep magenta colour confirmed presence of flavanonols.
2.7.11.5. Test for flavonols
A pinch of boric acid and a few drops of acetic acid were added to the extract. Bright yellow colour with green fluorescence indicated flavonols.
2.7.11.6. Test for flavones and flavanols
H2SO4was added to the extract. A yellow colouration was taken as evidence for the presence of flavones and flavanols. Lively orange to crimson colours indicated the presence of flavanones.
2.7.11.7. Test for flavanones
Few drops of concentrated HNO3were added to the extract. Brilliant blue colour confirmed the presence of phloroglucinol derived flavanones.
2.7.12. Determination of simple phenolics
One millilitre of extract was mixed with 1-2 drops of 1% FeCl3. Development of blue-green or black colouration was indicative of the presence of phenol.
2.7.13. Determination of proteins and amino acids
2.7.13.1. Millon's test
Two millilitre of extract was mixed with Millon's reagent. A white precipitate which turned red upon gentle heating confirmed the presence of protein.
2.7.13.2. Ninhydrin test
Two millilitre of extract was boiled with 2 mL of 0.2%solution of ninhydrin. Appearance of a violet colour suggested the presence of amino acids.
2.7.13.3. Xanthoproteic test
The extracts were treated with few drops of concentrated HNO3. Formation of yellow colour indicates the presence of proteins.
2.8. Statistical analysis
Results were presented as mean±SD. Difference between groups were compared using paired or unpaired t-test with onetailed or two-tail test. In each analysis P<0.05 was considered statistically significant.
3. Results
3.1. The effects of the extracts on glycation
Formationof AGEswasdeterminedbymeasuringfluorescence intensity of BSA-glucose or fructose solutions in presence or absence of the extracts(Figure 1). Our findings indicate that glycationof BSAoccurredatasignificantlyhigher(P<0.05)ratewith fructose(95 AU)than glucose(84 AU)for the controls and also in presenceoftheextracts.TherewassignificantlylessBSA-glycation in the presence of the control aminoguanidine(P<0.05)when comparedtoBSA-glucoseand BSA-fructosesolutions.Inpresence of the extracts,a significantly lower(P<0.05)fluorescence intensity was noted when compared to BSA-sugar solutions.
Aminoguanidine inhibited the formation of AGEs in BSA/ glucose and BSA/fructose by 64.7%and 74.0%,respectively. The percentage inhibition of the ten extracts ranged from 23.8% to 67.8%and 20.2%to 59.4 for the ethanolic extracts and infusions respectively in the BSA/glucose system and from 24.0% to 53.7%and 18.5%-63.2%,respectively for the BSA/fructose system. No significant difference(P>0.05)was found in the inhibitory effects of the ethanolic extracts compared to the infusions and in the percentage inhibitory capacity of the extracts on glycation with glucose compared to fructose.
3.2. Antioxidant activity of the extracts
3.2.1. Reducing power assay
The reducing power assay showed that all the extracts tested exhibited reducing capacity(Figure 2)in the order of turmeric,coriander,scallion,garlic,curry leaves,ginger,pepper mint,parsley,onion and thyme.
3.2.2. Metal ion chelating assay
The results obtained for metal ion chelating assay is shown in Figure 3. Onion,scallion and thyme showed the highest activity;curry leaves,pepper mint and coriander showed average activity;garlic,ginger and parsley showed very little activity whereas only a slight activity was observed with turmeric.
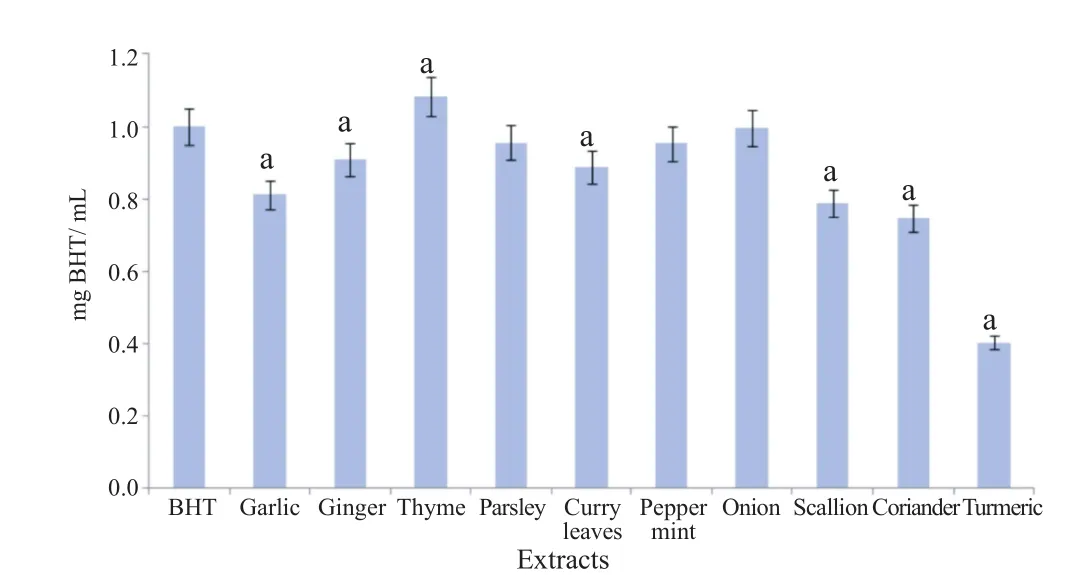
Figure 2. Reducing power of the ten extracts.Results are presented as mean±SD(n = 3).a: P<0.05 when compared to control BHT.
3.2.3. Superoxide radical scavenging activity
The result for superoxide radical scavenging activity is shown in Figure 3. All the extracts showed scavenging activity in the order of garlic<turmeric<ginger<parsley <coriander<pepper mint<thyme<scallion<curry leaves<onion.

Figure 3. Metal ion chelating assay and superoxide scavenging activity of the ten extracts.A: EDTA;B: Gallic acid;C: Garlic;D: Ginger;E: Thyme;F: Parsley;G: Curry leaves;H: Pepper mint;I: Onion;J: Scallion;K: Coriander;L: Turmeric;Results are presented as mean±SD(n = 3);a: P<0.05 when compared to control EDTA;b: P<0.05 when compared to control gallic acid.
3.3. Phytochemical analysis of the extracts
Qualitative evaluation of the chemical constituents of the selected extracts showed the presence of various secondarymetabolites(Table 1). Phytochemical analysis showed the presence of bioactive constituents such as carbohydrates,proteins,steroids,alkaloids,anthraquinones,cardiac glycosides,flavonoids,saponins,phlobatannins,tannins,terpenoids and diterpene.

Table 1Phytochemical profile of ten extracts.
4. Discussion
The present study was undertaken to compare the inhibitory effect of ten culinary herbs and spices on glucose- and fructosemediated BSA glycation. Based on the fluorescence property,we studied the influence of ten plant extracts on the formation of AGEs in vitro. Fructose was found to be significantly more effective than glucose in the glycation of BSA in vitro. Our results showed that the culinary herbs and spices efficiently inhibited AGE formation. Glycation of proteins is a major cause of chemical modification[3]disrupting molecular conformation of polypeptides involved in pathogenesis of age-related disorders including Alzheimer's disease and diabetic complications [2,10,17]. In principle,all reducing sugars whether aldoses or ketoses and even molecules related to sugars can initiate the reaction in vivo[17]. As in the case of glucose,the reducing free carbonyl group of fructose may react non-enzymatically with amino groups in a process known as fructation or fructosylation[3,12]. The fact that elevated fructose and fructose metabolites can initiate deleterious glycation involved in development of metabolic diseases is of much concern[14,41]as long-term consumption of fructose has been associated with hyperlipidaemia,impaired glucose tolerance and increased insulin resistance[12]. Thus,there are some evidence to highlight the importance of fructose as an effective glycating agent with respect to AGE formation and should be given due consideration. Though extracellular concentration of fructose is normally lower than that of glucose;its high reactivity suggests that fructose is a strong candidate for fructation in vivo[13,42]. Fructose and its metabolites are believed to be important precursors in intracellular formation of AGEs and participate in glycation at a much faster rate than glucose[2]. The rate of glycation is directly proportional to the percentage of sugar in the open-chain(acyclic)form[14]and fructose open chain population is approximately 8 times that of glucose [13]. In the context of AGE formation,the formation of glyceraldehyde from fructose is very reactive in the Maillard reaction as compared with that of glyceraldehyde-3-phosphate from glucose[13]. Also,fructose is formed from sorbitol through glucose by the polyol pathway[42],one of the mechanisms for the formation of AGEs of particular relevance in diabetics[43]. Intracellularly,fructose is elevated in a number of tissues of diabetic patients in which the polyol pathway is active[41]. In the cells of these tissues,the concentrations of fructose and glucose are of the same magnitude[14]. Fructose consumption has increased during the past decades despite evidence implicating fructose in the development of metabolic disorders,obesity and diabetic complications[7]. Although dietary fructose has some adverse side effects,it is still advocated as a preferred sweetener and as a glucose substitute for diabetics[7]. Fructose foundnaturally in honey,fruits,used as sweeteners and as a constituent of sucrose in food products forms a main component of human diets[12]. Consequently,the possibility that increased fructose intake might result in increased tissue concentration potentiating fructose derived glycation reaction should be stressed[14]. Further insight into these processes is needed to properly estimate the relevance of such alterations in healthy and diabetic people.
Non-enzymatic reactions of fructose compared with glucose are believed to cause higher production of reactive carbonyls and oxygen species(ROS)[7]. Fructose through the polyol pathway when activated by hyperglycemia may lead to increased oxidative stress and formation of AGEs[14]. Glycation is not only a major cause of AGE-mediated protein modification,but it also induces oxidation-dependent tissue damage[2]. ROS including free radicals and reactive carbonyl groups are also generated during glycation and glycoxidation [7]. In addition,transition metals also catalyse auto-oxidation of glucose and further generate reactive carbonyl compounds to form AGEs[18]. Therefore,scavenging free radicals through antioxidants can alleviate oxidative stress and reduce oxidative damage-mediated glycation and glycoxidation[17,44]. Also,metal chelators may retard the process of AGEs by preventing further oxidation of Amadori products and metal-catalysed glucose oxidation[41].
Various methods of accessing antioxidant capacities of the edible plants studied have been reported previously[45]. Here the reducing power assay,metal ion chelating assay and superoxide radical scavenging assay provide additional evidence of their ability to decrease ROS through free radical scavenging properties of the phytochemical compounds detected in vitro. The reducing ability of a compound generally depends on the presence of reductones[46]. The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity[47]. The antioxidant principles present in the ten extracts caused reduction of Fe3+/ferricyanide complex to the ferrous form,and thus proved the reducing power aptitude. The metal chelating ability of the extracts was measured by the formation of ferrous ion-ferrozine complex. The results of this study demonstrate that the extracts have an effective capacity for iron binding,suggesting their antioxidant potential. Superoxide radical was generated by the PMS-NADH and NBT systems and results revealed that the ten extracts possess antioxidant activity which was similar to previously demonstrated ferric reducing antioxidant power and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity[27,28]. Based on our results,it may be concluded that all the extracts showed strong antioxidant activity;reducing power ability,metal ion chelating ability and free radical scavenging activity when compared to standards such as BHT,EDTA and gallic acid. It has been reported that many antioxidant-containing foods can scavenge free-radicals generated during the glycation process as well as prevent reducing sugars and Amadori products from self-oxidation,thus inhibiting AGE formation [17,48]. Accordingly,the observed anti-glycation activity of the extracts may be explained through mechanisms inhibiting AGE formation by decreasing the ROS formation or by scavenging the ROS formed in vitro by auto-oxidation of sugars and/or oxidative degradation of Amadori products. As the ten extracts exhibited different activities,there may be different percentages of phytochemical constituents present in the extracts as demonstrated by the phytochemical evaluation.
The phytochemical analysis of the ten extracts showed the presence of phenolics,carbohydrates,proteins,steroids,alkaloids,anthraquinones,cardiac glycosides,flavonoids,saponins,phlobatannins,tannins,terpenoids and diterpenes. It has been reported that the antioxidant and protein glycation inhibitory activity of edible plants are related to the free radical scavenging property of phenolic compounds and flavonoids[18]. Our findings indicate that the ten extracts have phenolic compounds and flavonoids which may contribute to the reported antioxidant and anti-glycation activity. Thus,the ability of the extracts to modulate glycation-mediated BSA oxidation might be partly from their antioxidant activity[3]. These in vitro assays indicate that the plant extracts have potent antioxidant properties thus providing evidence in support for their use as significant source of natural antioxidant which might be helpful in preventing the progress of various oxidative stress related diseases. Current scientific evidence demonstrates that inhibition of AGEs formation is a therapeutic strategy for diabetic complications [6,35]. Therefore,much effort has been extended to searching for phytochemical compounds from dietary plants,fruits,and herbal medicines that effectively inhibit AGE formation [2,17].
However,the antioxidant activity of the extracts might not be the only reason for their anti-glycation properties. Several biochemical mechanisms of anti-glycation reactions have been proposed[44]such as breaking the cross-linking structures in the formed AGEs,blocking the carbonyl groups and inhibiting the formation of late-stage Amadori products[17]. Other underlying mechanisms for the anti-glycation activity may be relevant. Further comprehensive studies of the extracts are required to evaluate their anti-glycation mechanisms described above. Nonetheless,information gathered on the plants extracts studied provides evidence that may potentially justify their use as traditional treatment of different ailments. Available data allow to postulate that these dietary agents should be further investigated as possible natural protector of AGE formation in vivo. By virtue of their antioxidants and anti-glycation effects,these edible plants could be a promising natural source of antiglycation agents for preventing AGE-mediated diabetic complications through inhibition of AGEs formation and oxidationdependent protein damage.
In this study,BSA glycation was found to be significantly higher in presence of fructose compared to glucose. Owing to its high reactivity and contribution to AGE formation,fructose should be given due consideration to have further insight to properly estimate the relevance of these properties in diabetic complications. Our findings showed that ethanolic extracts and infusions of culinary herbs and spices have effective inhibitory effect on glucose- and fructose-mediated BSA-glycation in vitro. The presence of phytochemicals tends to suggest potential for pharmacological and other biological relevance of the culinary herbs and spices in drug discovery. The present results suggest that the extracts may act as antioxidants with suppressing effect on the formation of AGEs. Findings from this study indicate that some culinary herbs and spices with both high anti-glycation and high antioxidant activities may offer potential therapeutic treatment prospects for interfering in the glycation pathway and against oxidative stress-related diseases either as dietary interventions or glycation inhibitors. However,further studies are required to isolate the active principle from the crude extract for proper drug development.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We would like to acknowledge the University of Mauritius and the Tertiary Education Commission for financial support (Project code Q0024).
References
[1]Sompong W,Adisakwattana S. Inhibitory effect of herbal medicines and their trapping abilities against methylglyoxal-derived advanced glycation end-products. BMC Complement Altern Med 2015;15: 394.
[2]Jariyapamornkoon N,Yibchok-Anun S,Adisakwattana S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med 2013;13: 171.
[3]Meeprom A,Sompong W,Chan CB,Adisawakttana S. Isoferulic acid,a new anti-glycation agent,inhibits fructose and glucose-mediated protein glycation in vitro. Molecules 2013;18: 6439-54.
[4]Vistoli G,De Maddis D,Cipak A,Zarkovic N,Carini M,Aldini G. Advanced glycoxidation and lipoxidation end products(AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res 2013;47(Suppl 1): 3-27.
[5]Poulsen MW,Hedegaard RV,Andersen JM,de Courten B,B¨ugel S,Nielsen J,et al. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol 2013;60: 10-37.
[6]Rahman A,Choudhary MI,Basha FZ,Abbas G,Khan SN,Shah SA. Science at the interface of chemistry and biology: discoveries of α-glucosidase inhibitors and antiglycation agents. Pure Appl Chem 2007;79: 2263-8.
[7]Semchyshyn HM. Fructation in vivo: detrimental and protective effects of fructose. Biomed Res Int 2013;http://dx.doi.org/10.1155/ 2013/343914.
[8]Muthenna P,Akileshwari C,Saraswat M,Bhanuprakash Reddy G. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. Br J Nutr 2012;107: 941-9.
[9]Aldini G,Vistoli G,Stefek M,Chondrogianni N,Grune T,Sereikaite J,et al. Molecular strategies to prevent,inhibit and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic Res 2013;47(Suppl 1): 93-137.
[10]Singh VP,Bali A,Singh N,Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 2014;18: 1-14.
[11]Krautwald M,M¨unch G. Advanced glycation end products as biomarkers and gerontotoxins-a basis to explore methylglyoxallowering agents for Alzheimer's disease?Exp Gerontol 2010;45: 744-51.
[12]Levi B,Werman MJ. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J Nutr 1998;128: 1442-9.
[13]Kawasaki Y,Fujii J,Miyazawa N,Hoshi A,Okado A,Tano Y,et al. Specific detections of the early process of the glycation reaction by fructose and glucose in diabetic rat lens. FEBS Lett 1998;441: 116-20.
[14]Schalkwijk CG,Stehouwer CD,Van Hinsbergh VW. Fructosemediated non-enzymatic glycation: sweet coupling or bad modification. Diabetes Metab Res Rev 2004;20: 369-82.
[15]Adisakwattana S,Jiphimai P,Prutanopajai P,Chanathong B,Sapwarobol S,Ariyapitipan T. Evaluation of alpha-glucosidase,alpha-amylase and protein glycation inhibitory activities of edible plants. Int J Food Sci Nutr 2010;61: 295-305.
[16]Hou GY,Wang L,Liu S,Songa FR,Liu ZQ. Inhibitory effect of eleven herbal extracts on advanced glycation end-products formation and aldose reductase activity. Chin Chem Lett 2014;25: 1039-43.
[17]Adisakwattana S,Sompong W,Meeprom A,Ngamukote S,Yibchok-Anun S. Cinnamic acid and its derivatives inhibit fructosemediated protein glycation. Int J Mol Sci 2012;13: 1779-89.
[18]Sadowska-Bartosz I,Bartosz G. Prevention of protein glycation by natural compounds. Molecules 2015;20: 3309-34.
[19]Vasu K,Goud JV,Suryam A,Singara Charya MA. Biomolecular and phytochemical analyses of three aquatic angiosperms. Afr J Microbiol Res 2009;3(8): 418-21.
[20]Yadav RNS,Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011;3(12): 10-4.
[21]Molan AL,Faraj AM,Mahdy AS. Antioxidant activity and phenolic content of some medicinal plants traditionally used in Northern Iraq. Phytopharmacology 2012;2(2): 224-33.
[22]Nagai R,Shirakawa J,Fujiwara Y,Ohno R,Moroishi N,Sakata N,et al. Detection of AGEs as markers for carbohydrate metabolism and protein metabolism. J Clin Biochem Nutr 2014;55: 1-6.
[23]Benalla W,Bellahcen S,Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev 2010;6: 247-54.
[24]Chusak C,Thilavech T,Adisakwattana S. Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects. Am J Chin Med 2014;42: 315-36.
[25]Edoga HO,Okwu DE,Mbaebie BO. Phytochemicalsconstituents of some Nigerian medicinal plants. Afr J Biotechnol 2005;4(7): 685-8.
[26]Ahmad H,Khan I,Wahid A. Antiglycation and antioxidation properties of Juglans regia and Calendula officinalis: possible role in reducing diabetic complications and slowing down ageing. J Tradit Chin Med 2012;32: 411-4.
[27]Sham TT,Chan CO,Wang YH,Yang JM,Mok DK,Chan SW. A review on the traditional Chinese medicinal herbs and formulae with hypolipidemic effect. Biomed Res Int 2014;http://dx.doi.org/ 10.1155/2014/925302.
[28]Parikh NH,Parikh PK,Kothari C. Indigenous plant medicines for health care: treatment of diabetes mellitus and hyperlipidemia. Chin J Nat Med 2014;12: 335-44.
[29]Recio MC,Andujar I,Rios JL. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem 2012;19: 2088-103.
[30]Vladimir-Knezevic S,Blazekovic B,Stefan MB,Alegro A,Koszegi T,Petrik J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011;16: 1454-70.
[31]Ramkissoon JS,Mahomoodally MF,Ahmed N,Subratty AH. Relationship between total phenolic content,antioxidant potential and antiglycation abilities of common culinary herbs and spices. J Med Food 2012;15(12): 116-23.
[32]Ramkissoon JS,Mahomoodally MF,Ahmed N,Subratty AH. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med 2013;6(7): 561-9.
[33]Matsuura N,Aradate T,Sasaki C,Kojima H,Ohara M,Hasegawa J,et al. Screening system for the Maillard reaction inhibitor from natural product extracts. J Health Sci 2002;48: 520-6.
[34]Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japn J Nutr Diet 1986;44: 307-15.
[35]Gulcin I. Antioxidant and antiradical activities of L-carnitine. Life Sci 2006;78: 803-11.
[36]Jain PK,Agrawal RK. Antioxidant and free radical scavenging properties of developed mono- and polyherbal formulations. Asian J Exp Sci 2008;22(3): 213-20.
[37]Njoku OV,Obi C. Phytochemical constituents of some selected medicinal plants. Afr J Pure Appl Chem 2009;3(11): 228-33.
[38]Prakash S,Gautam S,Jain A. Phytochemical screening of selected medicinal plants used in skin diseases. Int J Pharm Sci 2011;3(2): 1402-6.
[39]Tiwari P,Kumar B,Kaur M,Kaur G,Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci 2011;1(1): 98-106.
[40]Shabir M,Khan MR,Saeed N. Assessment of phytochemicals,antioxidant,anti-lipid peroxidation and anti-hemolytic activity ofextract and various fractions of Maytenus royleanus leaves. BMC Complement Altern Med 2013;13: 143.
[41]Adisakwattana S,Thilavech T,Chusak C. Mesona chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complement Altern Med 2014;14: 130.
[42]Suarez G,Rajaram R,Oronsky AL,Gawanowicz MA. Non enzymatic glycation of bovine serum albumin by fructose(fructation). J Biol Chem 1989;264(7): 3674-9.
[43]Luevano-Contreras C,Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients 2010;2: 1247-65.
[44]WuCH,HuangSM,LinJA,YenGC.Inhibitionofadvancedglycation end products formation by foodstuffs. Food Funct 2011;2: 224-34.
[45]Ramkissoon JS,Mahomoodally MF,Ahmed N,Subratty AH. Therapeutic potential of common culinary herbs and spices of Mauritius. In: Chemistry:the key to our sustainable future. Amsterdam: Springer Netherlands;2014,p. 147-62.
[46]Umamaheswari M,Chateerjee TK. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Tradit Complement Altern Med 2008;5(1): 61-73.
[47]Chua MT,Tung YT,Chang ST. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour Technol 2008;99: 1918-25.
[48]Elosta A,Ghou T,Ahmed N. Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev 2012;8: 92-108.
Objective:To investigate the inhibitory activity of ten culinary herbs and spices namely on glucose-mediated glycation(GMG)and fructose-mediated glycation(FMG)of bovine serum albumin.
Methods:Fluorescence was used as an index of albumin glycation using glucose and fructose as substrates in the presence of infusions and ethanolic extracts of ten culinary herbs and spices. Antioxidant activity of the extracts was evaluated using reducing power,metal ion chelating and superoxide radical scavenging assays. Phytochemicals profile was analysed using 13 standard methods.
Results:FMG was found to be significantly higher than GMG(95 and 84 AU,respectively;P<0.05). Infusions and ethanolic extracts showed significant(P<0.05)inhibitory activity on both GMG and FMG when compared to appropriate controls. No significant difference(P>0.05)was found in the percentage glycation inhibitory activity of infusions compared to ethanolic extracts. The mean percentage inhibitory activity of the extracts for GMG(45.9%)and for FMG(45.1%)was not significantly different (P>0.05). Qualitative phytochemical analysis showed the presence of alkaloids,flavonoids,tannins,terpenoids,anthraquinones,steroids,reducing sugars,proteins,phenols,saponins,phlobatannins,and cardiac glycosides.
Conclusions:The higher rate of fluorescence generation by fructation suggests that glycation by fructose deserves much attention as a glycating agent. Data herein showed that the extracts inhibited GMG and FMG. Thus,these edible plants could be a natural source of antioxidants and anti-glycation agent for preventing advanced glycation endproducts-mediated complications.
*Corresponding author:Mohamad Fawzi Mahomoodally,Department of Health Sciences,Faculty of Science,University of Mauritius,R´eduit,Mauritius.
 Asian Pacific Journal of Tropical Biomedicine2016年6期
Asian Pacific Journal of Tropical Biomedicine2016年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Potential drug-drug interactions in pediatric wards of Gondar University Hospital,Ethiopia∶A cross sectional study
- Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii(Harms)J. Leonard stem barks(Caesalpiniaceae)in mice and rats
- Prevalence of latent eosinophilia among occupational gardeners at Babcock University,Nigeria
- Biofilm formation in clinical isolates of nosocomial Acinetobacter baumannii and its relationship with multidrug resistance
- Evaluation of the anticonvulsant activity of the essential oil of Myrothamnus moschatus in convulsion induced by pentylenetetrazole and picrotoxin
- Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya Basin,Central Thailand
