Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii(Harms)J. Leonard stem barks(Caesalpiniaceae)in mice and rats
Noreen Orianna Koumba Madingou,Aristide Traore,Alain Souza,Marlaine Michelle Boukandou Mounanga,Raissa Reine Aworet Samseny,Sylvin Ouedraogo,Alfred Sababenedyo TraoreLaboratory of Pharmacology and Toxicology,Pharmacopoeia and Traditional Medicines Institute,BP 95 Libreville,GabonResearch Center in Biological,Food and Nutritional Sciences,University of Ouagadougou,0 BP 79 Ouagadougou,Burkina FasoDepartment of Medicine and Traditional Pharmacopoeia/Pharmacy,Health Science Research Institute,0 BP 79Ouagadougou 0,Burkina FasoDepartment of Biology,Faculty of Sciences,University of Sciences and Technique of Masuku(USTM),BP 9 Franceville,Gabon
ABSTRACT
Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii(Harms)J. Leonard stem barks(Caesalpiniaceae)in mice and rats
Noreen Orianna Koumba Madingou1,2*,Aristide Traore3,Alain Souza4,
Marlaine Michelle Boukandou Mounanga1,Raissa Reine Aworet Samseny1,Sylvin Ouedraogo3,
Alfred Sababenedyo Traore21Laboratory of Pharmacology and Toxicology,Pharmacopoeia and Traditional Medicines Institute,BP 1935 Libreville,Gabon
2Research Center in Biological,Food and Nutritional Sciences,University of Ouagadougou,03 BP 7129 Ouagadougou,
Burkina Faso
3Department of Medicine and Traditional Pharmacopoeia/Pharmacy,Health Science Research Institute,03 BP 7192
Ouagadougou 03,Burkina Faso
4Department of Biology,Faculty of Sciences,University of Sciences and Technique of Masuku(USTM),BP 941 Franceville,Gabon
ABSTRACT
Keywords:
Guibourtia tessmannii Stem barks
Plant extract
Herbal medicine
Toxicity
ARTICLE INFO
Article history:
Received 10 Sep 2015
Received in revised form 19 Sep,2nd revisedform19 Oct,3rdrevisedform 2 Nov,4th revised form 9 Nov,5th revised form 30 Nov 2015
Accepted 20 Dec 2015
Available online 22 Apr 2016
Original article http://dx.doi.org/10.1016/j.apjtb.2016.04.001
Tel: +226 241 06 39 16 83
E-mail: madnoreen01@gmail.com
Foundation Project: Supported by the doctoral training funds no. 84722652 USTM/4802 AC-USTM,of the University of Sciences and Technique of Masuku.
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
It is well known that plants are an important source of drugs worldwide[1-3]. Indeed,over 50%of chemical drugs used for the treatment of various diseases are derived from vegetables [4]. In the case of cardiovascular diseases,drugs such as digitoxin,digoxin,lanatosides A,B,C,are derived from Digitalis purpurea and Digitalis lanata which are traditionally used by indigenous people as poison[5]. However,the traditional usage of plants is not always a guarantee of the plant safety. In accordance with Ashafa et al.[3],it is plausible to assume that a history of a plant usage does not proof its safety.
In Gabon,the use of medicinal plants is claimed to have an important role in health care system. However,several deaths are regularly reported by practitioners using traditional medications due to overdosing. Moreover,in this country,few scientificstudieshavebeenconductedtoinvestigatethepotentialtoxicityor eventual side effects of traditional recipes in experiments. Guibourtia tessmannii(Harms)J. Leonard(G. tessmannii)is one of the most abundantly used medicinal plant in Central Africa for manypurposessuchasthetreatmentofcardiovasculardiseases[6-8],and its aphrodisiac effects in Cameroon[9].
Phytochemical studies performed on G. tessmannii revealed the presence of bioactive compounds such as tannins,phenolic,triterpenoids and alkaloids[6,7]. In order to study the biosafety of this extract in the present study,we determined the acute and sub-acute toxicity effect of the extract of this plant.
2. Materials and methods
2.1. Plant material
Stem barks of G. tessmannii were collected in the south of Gabon in August 2010. The plant was authenticated in the Gabon National Herbarium,the Institute of Pharmacopeia and Traditional Medicine,Libreville(Gabon)where a voucher specimen(SRFG 879 LBV)was deposited.
2.2. Aqueous extract
The stem barks of the plant were sun-dried and crushed into powder using mortar and Culatti micro-crusher. The powder obtained(1 kg)was macerated in 2000 mL of water during 48 h at room temperature and filtered using a Whatman millipore filter. The filtrate was lyophilized at−40°C. The powder obtained(67.7 g)was stored at 5°C until further use.
2.3. Animals
NMRI mice weighing 19-30 g were used in the acute toxicity test. Animals were provided by the Health Science Research Institute(IRSS),Ouagadougou(Burkina Faso). For the subacute toxicity,albino Wistar rats weighing 180-300 g were used. These animals were provided by the Institute of Pharmacopoeia and Traditional Medicine,Libreville,Gabon. All animals were housed under standard laboratory conditions[(25±1)°C]with free access to food and water. Experimental protocols were carried out and followed the Guide for the Care and Use of Laboratory Animals of Gabon.
2.4. Acute toxicity tests
The oral acute toxicity test and the intraperitoneal acute toxicity test were performed. Male and female NMRI mice were randomly distributed into two control groups and 8 treated groups with 10 animals in each group. Among the 8 treated groups,5 groups of animals were subjected to the intraperitoneal acute toxicity test(5 males and 5 females),and 3 groups of animals were subjected to the oral acute toxicity test,in the same proportions. The two control groups received the water orally or by i.p.(0.5 mL vehicle). For the oral acute toxicity test,the treated groups received increasing doses of plant extract(2000,3000 and 5000 mg/kg weight). Regarding the intraperitoneal acute toxicity test,increasing doses of the plant extract(150,250,300,500 and 600 mg/kg weight)were administered.
Animals were deprived of food and water overnight prior to the drug administration. The mice were observed at 0,30,60 and 120 min after treatment. The animals were observed for morbidity and mortality once a day,for up to 14 days,with food and water provided. The number of survivors after 7 days period was recorded[10-12]. The toxicological effect was assessed on the basis of mortality,which was expressed as LD50[13].
2.5. Sub-chronic toxicity
Wistar rats(180-250 g)of both gender were divided into four groupsof6animalseach(3malesand3females)andwerehoused under standard conditions and room temperature[(25±1)°C].
The control group received the vehicle(0.5 mL)and the others received increasing oral doses of the plant extract(150,1500 and 3000 mg/kg weight)by gavage.
Sub-chronic toxicity was evaluated after a single daily administration of extract per os for a period of 28 days. Animals were observed daily. Clinical signs,behavioral pattern,food and water intake,and body weight were monitored. At the end of the 28 days period,animals were deprived of food and water for 15 h and then sacrificed for serum biochemical analyses and organs weighing.
For serum biochemical analyses,blood samples collected from the heart were dispensed into plain tubes and were centrifuged at 3500 r/min for 10 min. The serum samples obtained were then used for biochemical parameters analysis such as: aspartate aminotransferase(AST),alanine aminotransferase (ALT),alkaline phosphate(ALP),total protein,creatinine,urea,total cholesterol,using an automated biochemistry analyzer (Selectra XL Vital Scientific,Elitech Group Company).
Vital organs such as heart,lungs,liver,kidneys,spleen,testes,ovary and uterus were carefully dissected,washed with normal buffer,weighed and examined macroscopically.
2.6. Statistical analysis
Data were expressed as mean±SEM. They were analyzed by GraphPad Prism version 5.0 for Windows. Data were assessed by One-way ANOVAfollowedbyDunnett'smultiplecomparisontest. P values less than 0.05 were considered as statistically significant.
3. Results
3.1. Acute toxicity study in mice
Oral administration of increasing doses of the aqueous extract of G. tessmannii(2000,3000 and 5000 mg/kg)did not produce any abnormal behavioral responses in male and female mice during the 14 days of observation. No mortality was recorded at all dose levels. Orally,the LD50appeared to be>5000 mg/kg.
When administered intraperitoneally,the aqueous extract of G. tessmannii(150 mg/kg body weight to 600 mg/kg body weight)showed a LD50at 328.78 mg/kg body weight. Table 1 summarizes treatment-related responses observed in acute intraperitoneal toxicity study.
3.2. Sub-chronic toxicity study in rats
3.2.1. Mortality and general behavior
Oral ingestion of the aqueous extract of G. tessmannii (150 mg/kg body weight to 3000 mg/kg body weight)for 28Mice were observed daily for signs of toxicity for 14 days. days did not induce any mortality in rats of both genders. No significant difference in food and water intake was recorded in treated and control groups(Table 2). However,abnormal behaviors,such as irritability,diarrhea and piloerection were observed at the 3rd week of the treatment when compared to the control group.
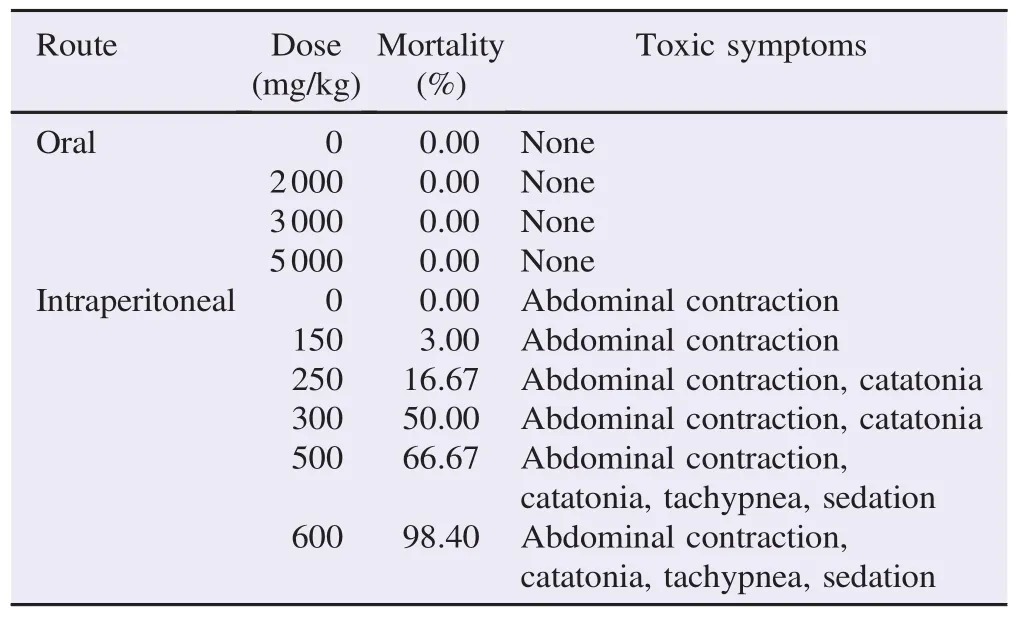
Table 1Acute toxicity of aqueous extract of G. tessmannii stem barks,administered by the oral or intraperitoneal route to mice.
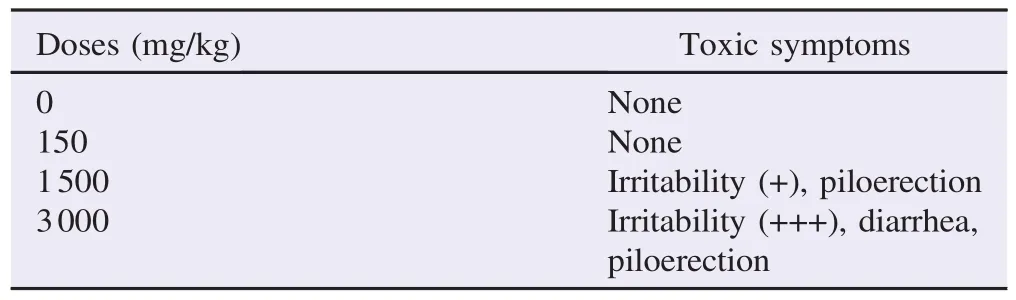
Table 2Sub-chronic toxicity of aqueous extract of G. tessmannii administered by oral route to rats for 28 days.
3.2.2. Body weight alteration in rats
Regarding the body weight,the plant extract(150-3000 mg/ kg)did not induce any significant change in the body weight both in males and females(Table 3).
3.2.3. Biochemical analyses
Table 4 shows the effects of G. tessmannii on biochemical parameters of the serum of rats. A significant decrease in AST(P<0.05 at 3000 mg/kg)and glucose(P<0.01 at 150 mg/kg and 1500 mg/kg)was observed in treated rats. Whereas,no significant change in serum concentration of ALT,ALP,cholesterol,urea,total protein and creatinine was induced by increasing dose of G. tessmannii in both male and female rats.
3.2.4. Effect of G. tessmannii on the weight of body organs
Table 5 shows the effect of G. tessmannii on the weight of body organs during 28 days. No significant change in vital organs weight was induced by the oral administration of increasing doses of G. tessmannii(150-3000 mg/kg).

Table 3Effect of aqueous extract of G. tessmannii stem barks on the weight of rats during 28 days. mg.

Table 4The biochemical parameters of male and female rats orally administered the aqueous extract of G. tessmannii stem barks for 28 days.

Table 5Organ weights of male and female rats orally administered the aqueous extract of G. tessmannii stem barks for 28 days. g.
4. Discussion
The results of the present acute toxicity study indicated,under our experimental conditions,a wide safety range of aqueous extract concentrations of G. tessmannii stem barks. This plant extract at 1000-5000 mg/kg in oral administration exhibited an oral LD50value above 5000 mg/kg and did not induce any mortality. Indeed,based on LD50value and according to the classification of Ou´edraogo et al.[14],the chemical labeling and classification of acute systemic toxicity from Organization for Economic Cooperation and Development[15]and from World Health Organization[16],the plant extract could be assigned as a Class 5 drug and then,recognized as low toxic product. This statement was strengthened by the results obtained in intraperitoneal administration. Furthermore,we observed quickly reversible signs of toxicity,and a LD50estimated at 328.78 mg/kg which suggest a low toxicity of the plant extract according to the classification of Mezui et al.[17]. The difference observed between the LD50values of the oral and intraperitoneal routes may be explained by the low bioavailability of the components that might cause toxicity,the poor absorption from the gastrointestinal tract,or as a result of a high first-pass effect and rapid metabolism to non-toxic metabolites[18].
In the sub-acute toxicity study in rats,the aqueous extract of G. tessmannii(150-3000 mg/kg body weight)did not induce any change in animal behavior,food and water intake,and vital organs weight as well as the body weight gain. It is well known that decrease in body weight gain as well as internal organ weights is a sensitive index of toxicity after exposure to toxic substances[14,19]. In fact,increase or decrease in body weight could be due to adverse effects of drugs[19]. The loss of appetite caused by stress or physiological adaptation to a drug's intake leads to the reduction of caloric intake when the body weight reduces[14]or the body fat accumulation during body weight gain[20].
On the basis of these results,under the same conditions,it has been suggested that the aqueous extract of G. tessmannii did not induce any acute toxicity.
The liver and kidneys are two crucial organs that play a key role in detoxification[21]. The effect of the plant extract was studied on the serum level of ALT,AST,ALP,which are essential for assessing the hepatic function[22]and on urea and creatinine levels in the blood which are usually used to evaluate the kidney function[23]. While a rise of transaminases reflects the liver inflammation or damage[24],any rise in creatinine level suggests damages of nephrons function[25].
Regarding the hepatic function monitoring,the plant extract did not produce deleterious changes in ALT,AST or ALP levels since no increase of these parameters was observed,suggesting no hepatotoxicity effect of this plant extract. However,we observed a decrease in AST level in both males and females treated with the plant extract. The ALP level also significantly decreased in the male group,but not in female group receiving G. tessmannii extract. This result corroborates the ability of the extract of G. tessmannii to restore functional status of liver[7].
No change in creatinine or urea level was obtained suggesting that the plant extract does not affect the renal function.
Regarding other biochemical parameters such as total cholesterol and total protein,no significant difference was observed compared to the control group. Whereas,a decrease in glucose level was recorded indicating a hypoglycemic effect of the plant extract.
Overall,the aqueous extract of G. tessmannii appeared to be low or non-toxic. Studies including hematopoietic system and histology are undertaken to further support the safety of the herbal medicine from G. tessmannii.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors wish to thank Professor Bopassa Jean Chrisostome,Dr Mewono Mewono Ludovick and Bading Bayissi for their kind help regarding the translation of the manuscript in English. We also wish to thank the staff of Health Science Research Institute and Institute of Pharmacopeia and Traditional Medicine and their laboratory personnel,especially Mayinda Ekagba Erica Lorlei,Ada Nguema Sandra Marquise,Wabekota Dagmar Cor`ene for their assistance. This work was supported by the doctoral training funds no. 84722652 USTM/ 4802 AC-USTM,of the University of Sciences and Technique of Masuku.
References
[1]Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014;4: 177.
[2]Dias DA,Urban S,Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012;2: 303-36.
[3]Ashafa AO,Orekoya LO,Yakubu MT. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pac J Trop Biomed 2012;2: 811-7.
[4]Tabassum N,Hamdani M. Plants used to treat skin diseases. Pharmacogn Rev 2014;8: 52-60.
[5]Obeng EA. Guibourtia tessmannii(Harms)J. L´eonard. In: Lemmens RHM,Louppe D,Oteng-Amoako AA,editors. Plant resources of tropical Africa. Wageningen: Wageningen University;2011.
[6]Madingou NOK,Souza A,Lamidi M,Mengome LE,Mba CEM,Bayissi B,et al. Study of medicinal plants used in the management of cardiovascular diseases at Libreville(Gabon): an ethnopharmacological approach. Int J Pharm Sci Res 2012;3: 111-9.
[7]Nyangono BCF,Chakokam Ngangoum RM,Kuate D,Ngondi JL,Enyong Oben J. Effect of Guibourtia tessmannii extracts on blood lipids and oxidative stress markers in triton WR 1339 and high fat diet induced hyperlipidemic rats. Biol Med 2012;4: 1-9.
[8]Fernande NBC,Marthe T,Laure NJ,Enyong OJ. In vitro antioxidant activity of Guibourtia tessmannii Harms,J. Leonard (Cesalpinoidae). J Med Plant Res 2013;7: 3081-8.
[9]Watcho P,Defo PBD,Wankeu-Nya M,Carro-Juarez M,Nguelefack TB,Kamanyi A. Mondia whitei(Periplocaceae)prevents and Guibourtia tessmannii(Caesalpiniaceae)facilitates fictive ejaculation in spinal male rats. BMC Complement Altern Med 2013;13: 4.
[10]El-Said Gad MM. Acute and repeated-doses(28 days)toxicity of thymol formulation in male albino rats. Aust J Basic Appl Sci 2012;7: 915-22.
[11]World Health Organization. WHO Expert Committee on specifications for pharmaceutical preparations-WHO technical report series,No. 863,thirty-fourth report. Geneva: World Health Organisation;1996.[Online]Available from: http://apps.who.int/ medicinedocs/en/d/Js5516e/[Accessed on 25th August,2015]
[12]Ntchapda F,Abakar D,Kom B,Nana P,Hamadjida A,Dimo T. Acute and sub-chronic oral toxicity assessment of the aqueous extract leaves of Ficus glumosa Del.(Moraceae)in rodents. J Intercult Ethnopharmacol 2014;3: 206-13.
[13]Dellai A,Mansour HB,Clary-Laroche A,Deghrigue M,Bouraoui A. Anticonvulsant and analgesic activities of crude extract and its fractions of the defensive secretion from the Mediterranean sponge. Spongia Off Cancer Cell Int 2012;12: 15.
[14]Ou´edraogo S,Som´e N,Ouattara S,Kini FB,Traore A,Bucher B,et al. Acute toxicity and vascular properties of seed of Parkia biglobosa(JACQ)R. Br Gift(Mimosaceae)on rat aorta. Afr J Tradit Complement Altern Med 2011;9(2): 260-5.
[15]Organization for Economic Cooperation and Development. OECD guidelines for the testing of chemicals. Paris: Organization for Economic Cooperation and Development;2008.[Online]Available from: http://www.oecd.org/document/40/0%2C2340%2Cen_ 2649_34377_37051368_1_1_1_1%2C00.html[Accessed on 25th August,2015]
[16]World Health Organization. The WHO recommended classification of pesticides by hazard,and guidelines to classification. Geneva: World Health Organization;2009.[Online]Available from: http:// www.who.int/ipcs/publications/pesticides_hazard_2009.pdf [Accessed on 25th August,2015]
[17]Mezui C,Longo F,Nkenfou C,Sando Z,Ndeme E,Vernyuy Tan P. Evaluation of acute and sub acute toxicity of stem bark aqueous extract of Anthocleista schweinfurthii(Loganiaceae). World J Pharm Pharm Sci 2015;4(3): 197-208.
[18]Ahmad F,Tabassum N. Preliminary phytochemical,acute oral toxicity and antihepatotoxic study of roots of Paeonia officinalis Linn. Asian Pac J Trop Biomed 2013;3: 64-8.
[19]Hayelom K,Mekbeb A,Eyasu M,Wondwossen E,Kelbesa U. MethanoliceffectofClerodendrummyricoidesrootextractonblood,liver and kidney tissues of mice. Afr Health Sci 2012;12: 489-97.
[20]Gautam MK,Goel RK. Toxicological study of Ocimum sanctum Linn leaves: hematological,biochemical,and histopathological studies. J Toxicol 2014;2014: 135654.
[21]Harizal SN,Mansor SM,Hasnan J,Tharakan JK,Abdullah J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in rodent. J Ethnopharmacol 2010;131: 404-9.
[22]Pandith AA,Lateef A,Shahnawaz S,Hussain A,Malla TM,Azad N,et al. GSTP1 gene Ile105Val polymorphism causes an elevated risk for bladder carcinogenesis in smokers. Asian Pac J Cancer Prev 2013;14: 6375-8.
[23]Chang CJ,Tzeng TF,Liou SS,Chang YS,Liu IM. Acute and 28-day subchronic oral toxicity of an ethanol extract of Zingiber zerumbet(L.)Smith in rodents. Evid Based Complement Altern Med 2012;2012: 608284.
[24]Konat´e K,Bassol´e IHN,Hilou A,Aworet-Samseny RRR,Souza A,Barro N,et al. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f. and Sida cordifolia L.(Malvaceae),medicinal plants of Burkina Faso. BMC Complement Altern Med 2012;12: 120.
[25]Mariappan G,Saha BP,Sutharson L,Singh A,Garg S,Pandey L,et al. Analgesic,anti-inflammatory,antipyretic and toxicological evaluation of some newer 3-methyl pyrazolone derivatives. Saudi Pharm J 2011;19(2): 115-22.
Objective:To investigate the toxicity of aqueous extract of Guibourtia tessmannii (Harms)J. Leonard(G. tessmannii)and evaluate its safety.
Methods:NMRI mice were used to determine the acute toxicity of G. tessmannii. Increasing concentrations of the plant extracts were administered intraperitoneally or by force-feeding. General behavior and death were monitored and recorded daily for 7 days. In order to determine the sub-acute toxicity of the extract,several doses were administered by oral gavage daily for 28 days in adult Wistar rats. Different parameters were assessed including body weight,food and water intake,biochemical parameters and several vital organ weights.
Results:LD50of 328.78 mg/kg was obtained by i.p. route and more than 5000 mg/kg was obtained in acute toxicity by oral route. In sub-acute toxicity,no significant alteration was observed in body weight or vital organs,food and water intake,and biochemical parameters.
Conclusions:The results showed that the aqueous extract of G. tessmannii has low toxicity intraperitoneally and no sub-acute toxicity via oral intake.
*Corresponding author:Noreen Orianna Koumba Madingou,Laboratory of Pharmacology and Toxicology,Pharmacopoeia and Traditional Medicines Institute,BP 1935 Libreville,Gabon.
 Asian Pacific Journal of Tropical Biomedicine2016年6期
Asian Pacific Journal of Tropical Biomedicine2016年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Knowledge,attitude and practice of healthcare workers concerning Crimean-Congo hemorrhagic fever in Western Iran
- Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya Basin,Central Thailand
- Potential drug-drug interactions in pediatric wards of Gondar University Hospital,Ethiopia∶A cross sectional study
- Biofilm formation in clinical isolates of nosocomial Acinetobacter baumannii and its relationship with multidrug resistance
- Prevalence of latent eosinophilia among occupational gardeners at Babcock University,Nigeria
- Evaluation of the anticonvulsant activity of the essential oil of Myrothamnus moschatus in convulsion induced by pentylenetetrazole and picrotoxin
