Potential drug-drug interactions in pediatric wards of Gondar University Hospital,Ethiopia∶A cross sectional study
Henok Getachew,Mohammed Assen,Feser Dula,Akshaya Srikanth BhagavathulaDepartment of Clinical Pharmacy,School of Pharmacy,College of Medical and Health Science,University of Gondar,Gondar,EthiopiaDepartment of Hospital Pharmacy,Drug Information Center and Clinical Pharmacy Unit,Gondar University Hospital,Gondar,Ethiopia
ABSTRACT
Potential drug-drug interactions in pediatric wards of Gondar University Hospital,Ethiopia∶A cross sectional study
Henok Getachew1*,Mohammed Assen1,Feser Dula2,Akshaya Srikanth Bhagavathula11Department of Clinical Pharmacy,School of Pharmacy,College of Medical and Health Science,University of Gondar,
Gondar,Ethiopia
2Department of Hospital Pharmacy,Drug Information Center and Clinical Pharmacy Unit,Gondar University Hospital,
Gondar,Ethiopia
ABSTRACT
Keywords:
Potential drug-drug interaction Pediatric ward
Prevalence
Ethiopia
ARTICLE INFO
Article history:
Received 26 Nov 2015
Received in revised form 21 Dec 2015
Accepted 15 Feb 2016
Available online 22 Apr 2016
Original article http://dx.doi.org/10.1016/j.apjtb.2016.04.002
Tel: +251 911438077
E-mail: heniget@gmail.com
The study protocol was performed according to the Helsinki declaration and approved by Institutional Review Board of University of Gondar,School of Pharmacy with the Ref. No. SOP112/02/08.
Foundation Project: Supported by University of Gondar(Grant No. UoG/Re/ Core/Pro/138862/2014).
Peer review under responsibility of Hainan Medical University. The journal implements double-blind peer review practiced by specially invited international editorial board members.
1. Introduction
Selecting a drug for a particular disease is one of the processes for the quality of pharmacotherapy. The possibility of a drug influencing the safety or efficacy of another drug(a drug-drug interaction)isvitalfortheoptimalchoiceforpharmacotherapy[1]. Drug-drug interaction is an important factor for various adverse consequences such as adverse drug events[1-5]. The incidence of adverse drug event was high among pediatric patients hospitalized in Jimma University Specialized Hospital,Ethiopia. The incidences were found to be 9.2 per 100 admissions,1.7 per 1000 medication doses,9.4 per 1000 patient days and 2.8 per 100 medication orders[6].
The mechanism of drug interactions may be due to pharmacokinetic,pharmacodynamics or clinical responses for drugs administered together in which the combined effect is different from the identified effects of individual drugs alone[7]. In terms of severity,drug interactions may be major interactions which may cause threat to life or lasting damages. Moderateinteractions call for additional treatment and mild interactions do not have a significant effect on the therapy. Drug interactions may have favorable or desirable,over and above undesirable or harmful effects[8]. These interactions can augment or lessen the effectiveness of each other or can also make new side effects[9]. As various recent studies reported,rates of potential drug-drug interactions(PDDIs)vary from approximately 19.3%to 78%[10-13].
Polypharmacy is one of the common risk factors for PDDIs which thereby cause adverse drug reactions and may lead to an increased risk of hospitalization and higher health care costs [14-19]. Although prescribing of more drugs for one patient may be logical and necessary practice for patients particularly those with co-morbidity,physicians should take into account that PDDIs incidence approaches 40%for patients taking 5 drugs,and exceeds 80%for patients taking 7 or more medications[19]. In addition to polypharmacy,other risk factors also exist and affect the incidence of PDDIs such as age,acute medical condition,gender,prescriptions from multiple physicians and drugs with narrow therapeutic range [11,19,20].
Hospitalized pediatric patients face higher risk of druginduced problems due to wide-ranging of patient ages and body-weights,limited physiologic reserve,medications dosing errors and inaptitude to properly communicate with healthcare workers[21]. In such situation,PDDIs are more likely to cause adverse outcomes in pediatrics hospital patients,though most of studies are limited to adult patients. Moreover,there have not been published reports on studies that evaluate PDDIs among pediatrics hospital patients in Ethiopia. Therefore,this study was initiated to determine the prevalence,level of severity of PDDIs and the associated factors for PDDIs in hospitalized pediatrics patients of Gondar University Hospital (GUH).
2. Materials and methods
2.1. Study area
The study was conducted in pediatrics ward of GUH,which is one of the oldest and the pioneer teaching hospital located in Amhara region,northwest part of Ethiopia. The hospital is equipped with 550 beds to provide both inpatient and outpatient services for at least 500000 population living in and around Gondar.
2.2. Study design and period
A retrospective cross-sectional study was conducted for a period of 3 months from March to May 2014 in pediatrics ward of GUH,Ethiopia.
2.3. Inclusion and exclusion criteria
The source population was all patients visiting the Pediatrics Department of GUH and the study population was all hospitalized pediatric patients during eight-month period. All pediatric patients' chart containing order sheets with two or more drugs and drugs prescribed at the day of admission were included in the study. Pediatric charts containing order sheets with supplementary diet only,pediatric charts containing order sheets with IV fluids and blood products only,pediatric charts containing order sheets with medical supplies and equipments only and pediatric charts containing only one drug were excluded from the study.
2.4. Sample size determination
The sample size was determined using a formula for estimation of single population proportion with the assumption of 95%confidence level,margin of error of 5%and the prevalence rate of PDDI was 50%.
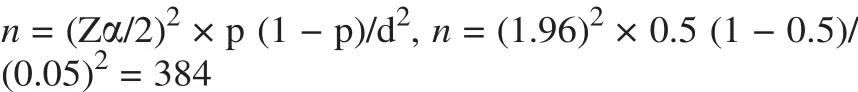
where,d = marginal error,p = proportion of sample population with confidence interval of 95%,Zα/2 = the value under standard normal table for the given value of confidence interval,n = sample size.
Sample size of 384 patient medical records was taken.
2.5. Data collection procedures
Systematic random sampling technique was used to select charts from those all pediatric patient charts with every 7th interval to get sample size of 384,which were documented from September 1,2013 to April 30,2014. Patient charts selected with a 7:1 ratio from the two pediatric wards. An eight-month review of pediatric patient's charts was used as secondary source of data by using data abstraction format. PDDIs were identified from patient prescription at the time of admission. The occurrence and severity of PDDI was analyzed using Micromedex computer-based software. To ensure the quality data,pretest was carried out to check the effectiveness of the data collection instrument and data were collected after standardizing the data collection instruments.
2.6. Ethical clearance
The study protocol was performed according to the Helsinki declaration and approved by Institutional Review Board of University of Gondar,School of Pharmacy with the Ref. No. SOP112/02/08 and confidentiality was kept by using codes rather than stating their names.
2.7. Data analysis
The collected data were checked by using Micromedex 2 software and analyzed by SPSS version 20. The processed data were organized and presented by using frequency distribution tables and hierarchies. Associated factors for the occurrence of PDDIs were assessed using binary logistic regression. Univariate and multivariate analyses were performed to compute crude odds ratio(COR)and adjusted oddsratio(AOR)respectively. Statistical significance was set at P value<0.05.
3. Results
3.1. Patient demographic and clinical information
A total of 384 pediatric patients were enrolled in the study. Among these,219(57.0%)were females. Pediatric patients of various age groups were included with patients younger than 1 month as the least prevalent age group of 2.3%followed by(1 month-2 years)age group of 21.1%. Median age was 7.5 years(range 25 days-16 years). Number of medications prescribed per patient were categorized as 2 (n = 144,37.5%),3 and 4(n = 180,46.9%),and 5 or more (n = 60). Patients with co-morbidity were 212(52.2%)(Table 1).
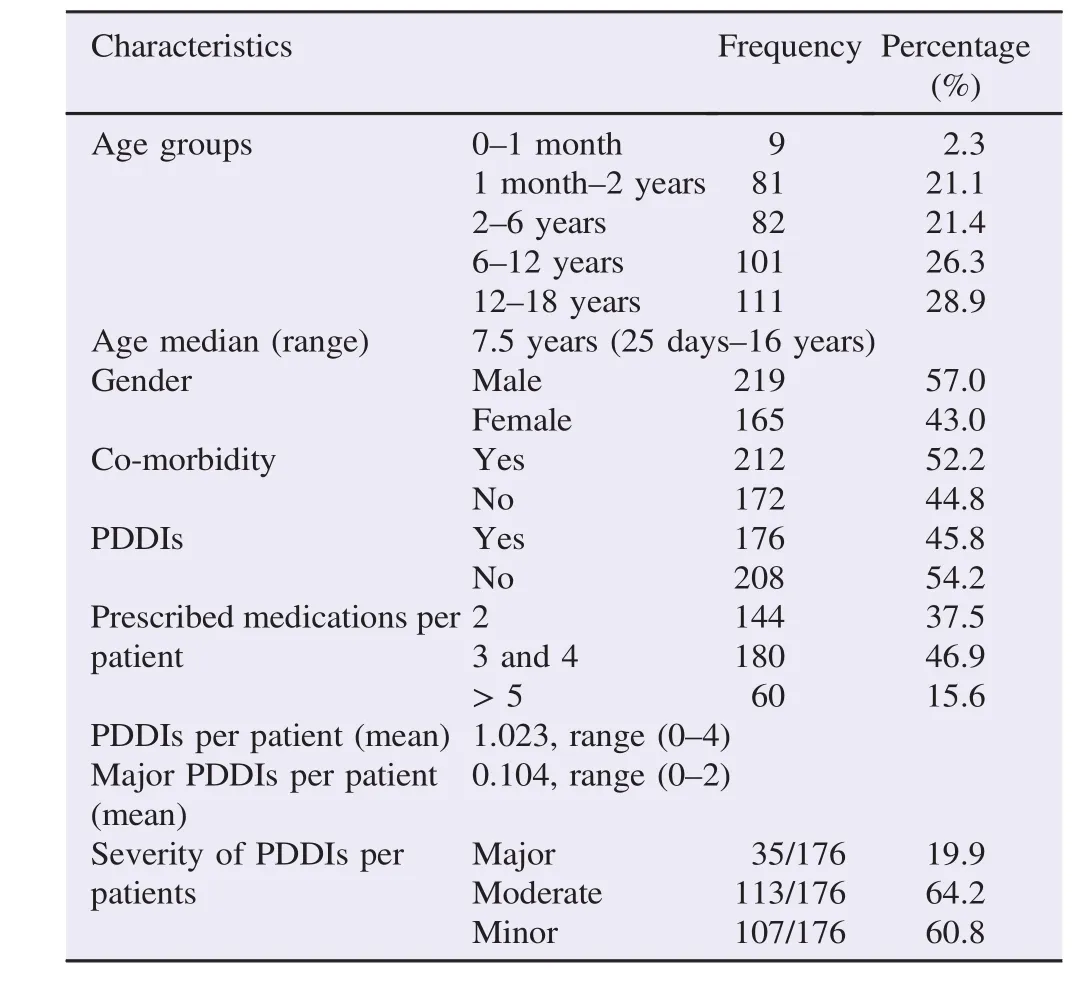
Table 1General demographic and clinical data of the study subjects.
3.2. Prevalence PDDIs
Of the total of 384 pediatric patients,176(45.8%)patients had at least one PDDI regardless of type of severity(Table 1). Of these patients having at least one PDDI,based on the frequency of severity of PDDIs per patient,64.2%(113/176)patients had at least one moderate PDDI followed by minor PDDI occurring in 60.8%(107/176)of patients(Table 1).
A total of 393 PDDIs were identified. Of these,most were moderate severity[201(51%)]followed by minor[152(39%)]and major severity[40(10%)]. In this study,283 types of interacting drug combinations were identified. Frequencies of some widespread interacting drug combinations were listed(Table 2).
Based on their mechanism of the total of 393 PDDIs identified,50%(197/393)of PDDIs were pharmacokinetic with the highest frequency of metabolism subtype(34%)(Figure 1).
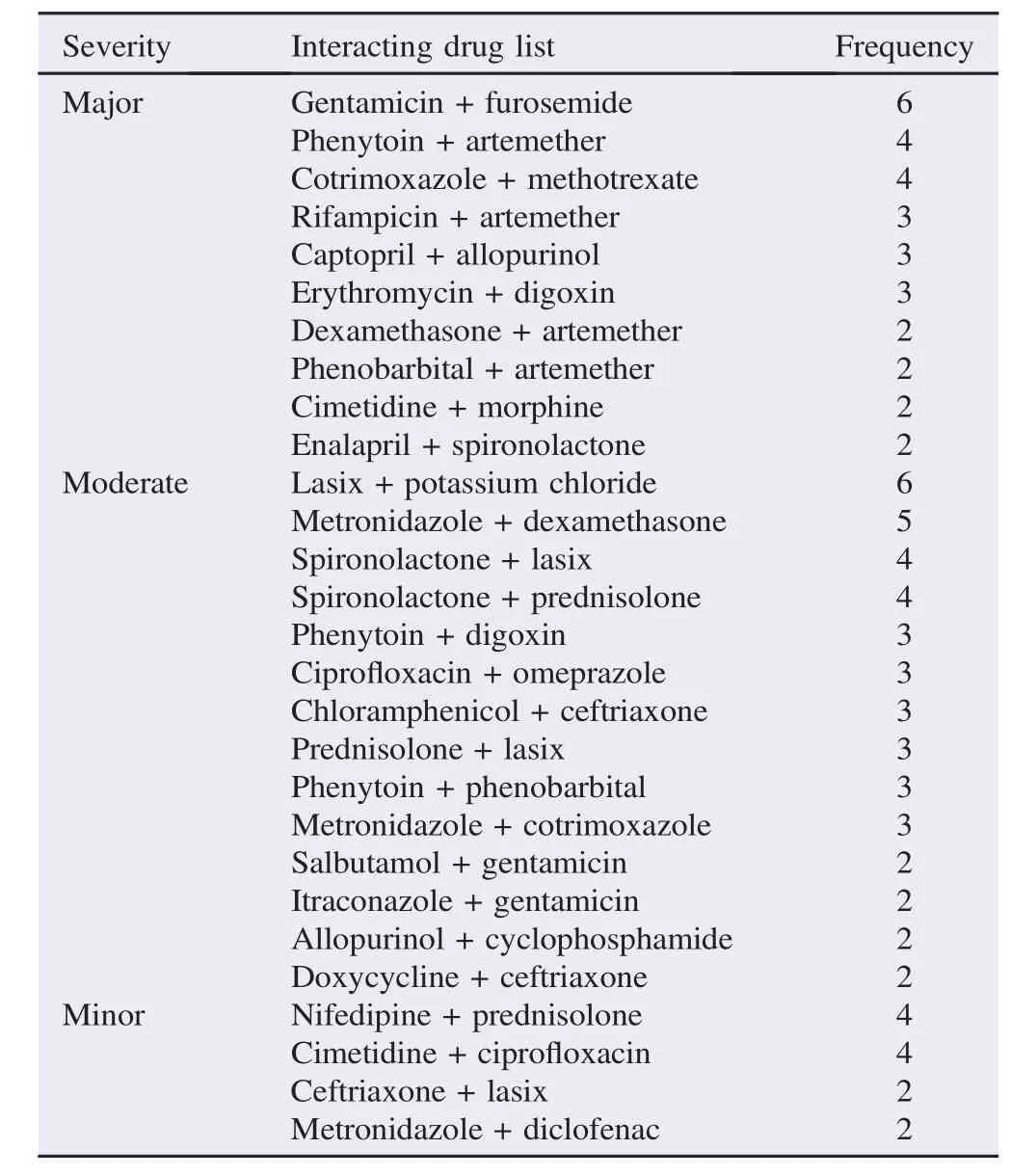
Table 2Frequencies of interacting drug combinations in pediatric wards.
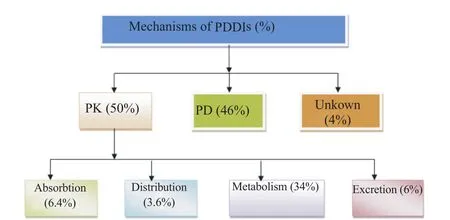
Figure 1. Distribution of PDDIs based on their mechanism in pediatric wards of Gondar University Hospital,Gondar,Ethiopia,May 2014.PK: Pharmacokinetic;PD: Pharmacodynamic.
3.3. Association with predicting factors
On univariate and multivariate analysis(Table 3),COR and AOR revealed that gender and co-morbidity did not show significant difference on the presence of PDDIs per patient. However,the occurrence of PDDIs was significantly associated with age and polypharmacy. This implied that PDDIs were occurring more frequently in age group of 2-6 years than any other age group of pediatric population [AOR = 6.450;95%confidence interval(CI)(1.271-34.353)]. There was also a positive association between the number of medications prescribed and the likelihood of PDDIs occurrence. When three or four medications[AOR = 2.220;95% CI(1.128-5.387)]and five or more medications [AOR = 7.034;95%CI(2.130-11.089)]were prescribed,PDDIs were occurring more frequently than those patients with two medications prescribed.
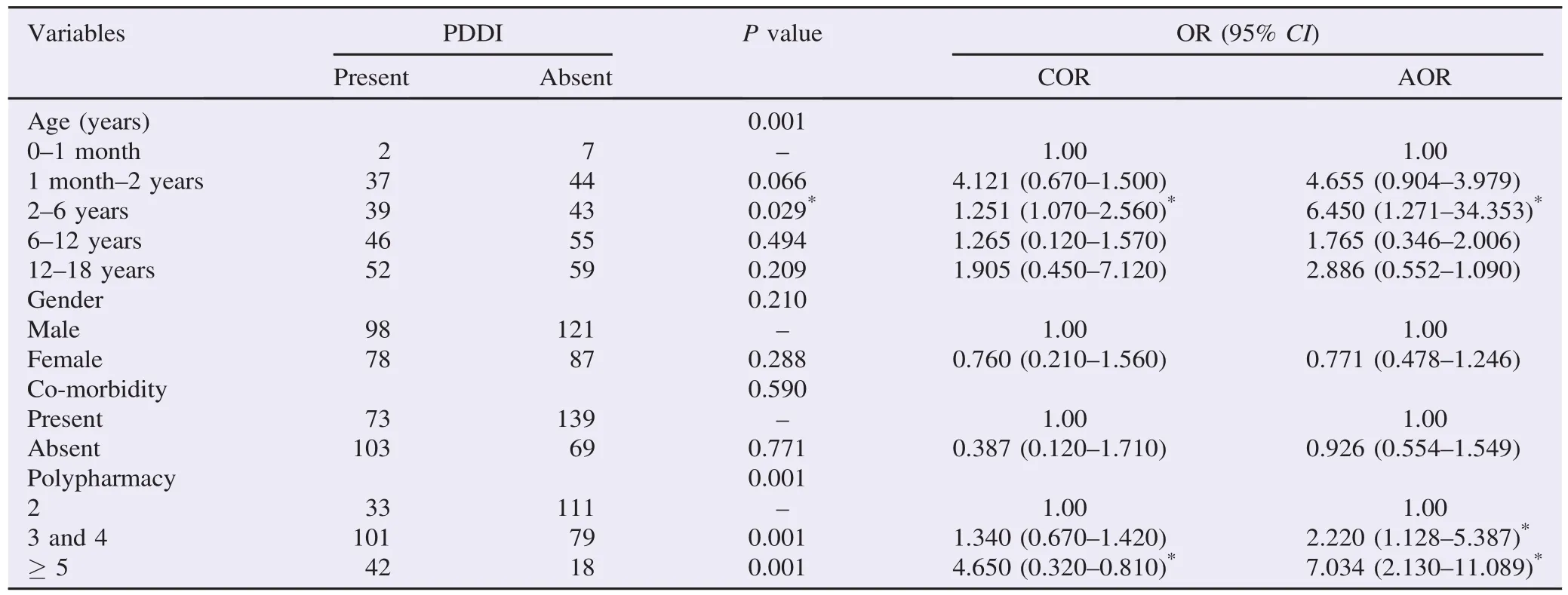
Table 3Predicting factors of PDDIs in pediatric wards.
4. Discussion
This study assessed the occurrence of PDDIs in pediatric population. The prevalence and nature of PDDIs have been reported in 384 pediatric patients. This study revealed that the overall prevalence of at least one PDDI per patient was 45.8% (176/384). It was comparable to Feinstein et al.,which reported that hospitalized pediatric patients exposed to a PDDI were 49%[22]. Of 176 patients having at least one PDDI,major interactions were found in 19.9%(n = 35)of pediatric patients in this study. These findings were higher in comparison to Ismail et al. in which overall interaction was 25%and major interaction was 10.7%(n = 43)[23]. But they were less than the results of Feinstein et al.,which found exposure to the major interaction of PDDIs in 41%of pediatric patients[22]. All PDDIs were not equally detrimental,though,recognition of severity of each PDDIs was central to assess clinical importance and appropriate management[24,25].
As the list of interacting drug combinations,particularly prevailing major and moderate interactions,are useful for health care professionals to monitor patient profiles for PDDIs,this study has also identified a total of 393 PDDIs.
Most interacting drug combinations were moderate severity 201(51%)followed by minor 152(39%)and major severity 40 (10%). In this study,most of the major severity PDDIs were observed with gentamicin and furosemide(6 combinations)which can potentially end up with life threatening of ototoxicity and nephrotoxicity,though it is not common in children[26,27]. Similarly,cotrimoxazole and methotrexate(4 combinations)have a higher risk of toxicity such as cytopenia,mucositis,hepatotoxicity and gastrointestinal symptoms by increasing toxicity of methotrexate by plasma protein binding competition[28,29]. Co-administration of artemether with phenytoin(4 combinations)leads to the loss of antimalarial efficacy as phenytoin causes strong induction of CYP3A4 resulting in decrease of artemether concentration. This finding is different from other studies,such as fentanyl + morphine (13.2%)in Feinstein et al.[22],rifampin + pyrazinamide(14 cases),and phenobarbital + diazepam(14 cases)in Ismail et al.[23],whereas digoxin + furosemide(20.14%)in Yeh et al. were identified as the most common major interactions[30].
Precautions need to be taken while prescribing such medications for pediatric patients. It is also important to monitor all the possible and major PDDIs before prescribing to the patients so as to minimize the potential consequences and manage them accordingly.
The other result of this study showed that age group has statistically significant association with PDDIs which were occurring more frequently in 2-6 years age group than any other age group of pediatric[AOR = 6.450;95%CI(1.271-34.353);P<0.029]. Assessment of the relationship between number of medications prescribed and PDDIs indicated that polypharmacy increases the likelihood of PDDIs occurrence. Bjerrum et al. also described that polypharmacy has been shown to be associated with PDDIs[31]. Polypharmacy could be very detrimental in children because of their physiological eccentricity[32].
This study has some limitations. We could not ascertain with any accurate completeness or reliability of the information obtained as the study design was retrospective. As such,it was possible that we could have under- or over-reported the PDDIs. As some drugs were prescribed to be taken as required,we could not accurately determine whether these drugs were actually taken with others,which is difficult to make the assessment of drug-drug interaction. The findings of this study may not be generalized as it is a single center study.
As the study showed most of the interactions had moderate severity followed by minor severity. Age and polypharmacy were risk factors for the occurrence of PDDIs. Therefore,identifying and preventing potentially harmful PDDIs is a critical component of a pharmacist's mission and the clinical pharmacist must remain vigilant in their monitoring of PDDIs and make appropriate dosage or therapy adjustments. Due to sensitive nature of pediatric population,close monitoring is recommended for detection and management of PDDIs to prevent its negative consequences.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors acknowledge Department of Pediatrics,School of Pharmacy and Drug Information Centre of Gondar University for cooperation in this study based on software. This work was supported by University of Gondar(Grant No. UoG/Re/Core/ Pro/138862/2014).
References
[1]Patel VK,Acharya LD,Rajakannan T,Surulivelrajan M,Guddattu V,Padmakumar R. Potential drug interactions in patients admitted to cardiology wards of a south Indian teaching hospital. Australas Med J 2011;4(1): 9-14.
[2]Rashed AN,Wong IC,Cranswick N,Tomlin S,Rascher W,Neubert A. Risk factors associated with adverse drug reactions in hospitalised children: international multicentre study. Eur J Clin Pharmacol 2012;68(5): 801-10.
[3]Magro L,Moretti U,Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf 2012;11(1): 83-94.
[4]Haji Aghajani M,Sistanizad M,Abbasinazari M,Abiar Ghamsari M,Ayazkhoo L,Safi O,et al. Potential drug-drug interactions in post CCU of a teaching hospital. Iran J Pharm Res 2013;12: 243-8.
[5]Dechanont S,Maphanta S,Butthum B,Kongkaew C. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2014;23(5): 489-97.
[6]Eshetie TC,Hailemeskel B,Mekonnen N,Paulos G,Mekonnen AB,Girma T. Adverse drug events in hospitalized children at Ethiopian University Hospital: a prospective observational study. BMC Pediatr 2015;15: 83.
[7]Martinbiancho J,Zuckermann J,Dos Santos L,Silva MM. Profile of drug interactions in hospitalized children. Pharm Pract(Granada)2007;5(4): 157-61.
[8]Varma MV,Pang KS,Isoherranen N,Zhao P. Dealing with the complex drug-drug interactions: towards mechanistic models. Biopharm Drug Dispos 2015;36: 71-92.
[9]Amirthalingam S,Vaidhun BH. Drug interactions - a view on doctors. Ann Biol Res 2010;1(1): 61-9.
[10]Reimche L,Forster AJ,van Walraven C. Incidence and contributors to potential drug-drug interactions in hospitalized patients. J Clin Pharmacol 2011;51: 1043-50.
[11]Shabani D,Tahiri Z,Bara P,Hudhra K,Malaj L,Jucja B,et al. Prevalence and correlates of drug-drug interactions in the regional hospital of Gjilan,Kosovo. Mater Sociomed 2014;26(4): 268-71.
[12]van Leeuwen RW,Brundel DH,Neef C,van Gelder T,Mathijssen RH,Burger DM,et al. Prevalence of potential drugdrug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer 2013;108: 1071-8.
[13]Bhagavathula AS,Berhanie A,Tigistu H,Abraham Y,Getachew Y,Khan TM,et al. Prevalence of potential drug-drug interactions among internal medicine ward in University of Gondar Teaching Hospital,Ethiopia. Asian Pac J Trop Biomed 2014;4(Suppl 1): S204-8.
[14]Feinstein JA,Feudtner C,Valuck RJ,Kempe A. The depth,duration,and degree of outpatient pediatric polypharmacy in Colorado fee-for-service Medicaid patients. Pharmacoepidemiol Drug Saf 2015;24(10): 1049-57.
[15]Guthrie B,Makubate B,Hernandez-Santiago V,Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med 2015;13: 74.
[16]Lin CF,Wang CY,Bai CH. Polypharmacy,aging and potential drug-drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging 2011;28: 219-25.
[17]Risk R,Naismith H,Burnett A,Moore SE,Cham M,Unger S. Rational prescribing in paediatrics in a resource-limited setting. Arch Dis Child 2013;98(7): 503-9.
[18]Feudtner C,Dai D,Hexem KR,Luan X,Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med 2012;166(1): 9-16.
[19]Grattagliano I,Portincasa P,D'Ambrosio G,Palmieri VO,Palasciano G. Avoiding drug interactions: here's help. J Fam Pract 2010;59: 322-9.
[20]Barrett JS,Patel D,Dombrowsky E,Bajaj G,Skolnik JM. Risk assessment of drug interaction potential and concomitant dosing pattern on targeted toxicities in pediatric cancer patients. AAPS J 2013;15(3): 775-86.
[21]Wang JK,Herzog NS,Kaushal R,Park C,Mochizuki C,Weingarten SR. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics 2007;119: e77-85.
[22]Feinstein J,Dai DW,Zhong WJ,Freedman J,Feudtner C. Potential drug-drug interactions in infant,child,and adolescent patients in children's hospitals. Pediatrics 2015;135(1): e99-108.
[23]Ismail M,Iqbal Z,Khan MI,Javaid A,Arsalan H,Farhadullah,et al. Frequency,levels and predictors of potential drug-drug interactions in a pediatric ward of a teaching hospital in Pakistan. Trop J Pharm Res 2013;12(3): 401-6.
[24]Slight SP,Seger DL,Nanji KC,Cho I,Maniam N,Dykes PC,et al. Are we heeding the warning signs?Examining providers' overrides of computerized drug-drug interaction alerts in primary care. PLoS One 2013;8(12): e85071.
[25]Lopes P,Nunes T,Campos D,Furlong LI,Bauer-Mehren A,Sanz F,et al. Gathering and exploring scientific knowledge in pharmacovigilance. PLoS One 2013;8(12): e83016.
[26]Bates DE,Beaumont SJ,Baylis BW. Ototoxicity induced by gentamicin and furosemide. Ann Pharmacother 2002;36(3): 446-51.
[27]Rao SC,Ahmed M,Hagan R. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 2006;http://dx.doi.org/10.1002/14651858.CD005091.pub2.
[28]Liegler DG,Henderson ES,Hahn MA,Oliverio VT. The effect of organic acids on renal clearance of methotrexate in man. Clin Pharmacol Ther 1969;10: 849-57.
[29]Ferrazzini G,Klein J,Sulh H,Chung D,Griesbrecht E,Koren G. Interaction between trimethoprim-sulfamethoxazole and methotrexate in children with leukemia. J Pediatr 1990;117: 823-6.
[30]Yeh ML,Chang YJ,Yeh SJ,Huang LJ,Yen YT,Wang PY,et al. Potential drug-drug interactions in pediatric outpatient prescriptions for newborns and infants. Comput Methods Programs Biomed 2014;113(1): 15-22.
[31]Bjerrum L,Gonzalez Lopez-Valcarcel B,Petersen G. Risk factors for potential drug interactions in general practice. Eur J Gen Pract 2008;14(1): 23-9.
[32]Fadare J,Olatunya O,Oluwayemi O,Ogundare O. Drug prescribing pattern for under-fives in a paediatric clinic in South-Western Nigeria. Ethiop J Health Sci 2015;25(1): 73-8.
Objective:To determine the prevalence,level of severity of potential drug-drug interactions(PDDIs)and the associated factors for PDDIs in hospitalized pediatric patients of Gondar University Hospital.
Methods:A retrospective cross-sectional study was conducted for a period of 3 months from March to May 2014 in pediatric wards of Gondar University Hospital. Systematic random sampling technique was used to select charts from all pediatric patients' charts with every 7th interval to get sample size of 384. Univariate and multivariate analysis were performed to compute crude odds ratio and adjusted odds ratio respectively. Statistical significance was set at P value<0.05.
Results:A total of 176(45.8%)patients had at least one PDDI. A total of 393 PDDIs,which were comprised of 283 types of interacting combinations,were identified. Of the total of 393 PDDIs,most were of moderate severity[201(51%)]followed by minor[152 (39%)]and major severity[40(10%)]. The most common interacting pairs of major severity were gentamicin + furosemide(6),cotrimoxazole + methotrexate(4)and phenytoin + artemether(4). The occurrence of PDDIs was significantly associated with age and polypharmacy.
Conclusions:The study showed that most of the interactions had moderate severity followed by minor severity. Age and polypharmacy were found to show statistically significant association with the occurrence of PDDIs. Due to sensitive nature of pediatrics population,close monitoring is recommended for the detection and management of PDDIs to prevent its negative consequences.
*Corresponding author:Henok Getachew,Department of Clinical Pharmacy,School of Pharmacy,College of Medicine and Health Sciences,University of Gondar,Gondar,Ethiopia.
 Asian Pacific Journal of Tropical Biomedicine2016年6期
Asian Pacific Journal of Tropical Biomedicine2016年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Knowledge,attitude and practice of healthcare workers concerning Crimean-Congo hemorrhagic fever in Western Iran
- Epidemiological situation and molecular identification of cercarial stage in freshwater snails in Chao-Phraya Basin,Central Thailand
- Biofilm formation in clinical isolates of nosocomial Acinetobacter baumannii and its relationship with multidrug resistance
- Prevalence of latent eosinophilia among occupational gardeners at Babcock University,Nigeria
- Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii(Harms)J. Leonard stem barks(Caesalpiniaceae)in mice and rats
- Evaluation of the anticonvulsant activity of the essential oil of Myrothamnus moschatus in convulsion induced by pentylenetetrazole and picrotoxin
