Multiple linear equation of pore structure and coal–oxygen diffusion on low temperature oxidation process of lignite☆
Xianliang Meng ,Mingqiang Gao ,Ruizhi Chu ,*,Guoguang Wu ,Qiang Fang
1 School of Chemical Engineering and Technology,China University of Mining&Technology,Xuzhou 221116,China
2 Key Laboratory of Gas and Fire Control for Coal Mines,China University of Mining&Technology,Xuzhou 221116,China
1.Introduction
Lignite,as low rank coal,is characterized by high moisture content,high volatility and low calorific value[1].With the discovery of more lignite coal fields,lignite is playing an increasingly important role in supplying primary energy due to its large reserve,location in shallow depths and low price.However,low-level lignite,especially upgrading lignite through low temperature drying,is very active and has high spontaneous combustion tendency,which has been a disaster in the storage and transportation[2].The low temperature oxidation characteristics of upgraded lignite have become a concern[3],which greatly limits its application.
It is well known that the compounding of coal and oxygen at low temperature is the major reason responsible for coal spontaneous combustion,which has been studied by many investigators[4–6].Wang et al.[5]divided the process of low temperature oxidization into four phenomena:oxygen diffusion,chemical interaction between coal and oxygen,heat release and gas emission.The diffusion process of oxygen from environment to coal surface and pore is an essential premise for subsequent reactions,and plays a main control role in the development of coal spontaneous combustion.If the oxygen supply is blocked,the coal oxidization can be prevented[7].Therefore,it is an important job with practical significance to master the diffusion law of oxygen in coal.
In the field of coal spontaneous combustion,there have been some studies about oxygen diffusion.Brooks and David developed a simplified one-dimensional model,in which natural convection was taken into account as a mechanism for oxygen transport[8].Zhang and Yan investigated the impact of moisture content on the oxygen diffusion of brown coal[9].However,the diffusion of oxygen in loosen coalis a very complex mass transfer process,involving multiple influential factors such as temperature,pressure,water content,particle size,and air void.The perfect theory has not been established.
In view of the fact that there are very complex and a wide range of influence factors in the low temperature oxidation process of lignite[10],the author proposes that factors would be divided into external factors,including humidity[11],temperature[12]and air velocity[13,14],and internal factors,including particle size[15],pore structure[16,17]and surface chemical structure[18,19].All the influence factors will be studied separately in our present and future work.This paper focuses on the rule of the influence of pore structure of lignite on O2diffusion coefficient in low temperature oxidation process.
According to the theory of mass transfer in porous media,gas diffusion model is related to the pore characteristics[20,21].Fractal analysis is a powerful analytical tool that can define and describe the attributes of a material.For porous materials this includes description of pore irregularity.Using fractal analysis of porous materials,it has been shown that the irregularity of surfaces and pores is important for diffusion[20–24].The random distribution of pore in the coal implies that there is a pore fractal structure.Because the diffusion of gas in the coal are related to the random distribution pore of coal,a fractal method can be used to characterize and explain the relationship between the pore structures and the gas diffusion capacity.The study of relationship between fractal analysis and the effective diffusion coefficient of gas has received extensive attention[25–28].However,previous researches mainly focused on the numerical and theoretical analysis of oxygen diffusion model.The relationship between pore change law and coal–oxygen diffusion properties under different oxidation temperatures has not been reported.
Differing from previous researches,this study pays attention to the dynamic development of coal spontaneous combustion.Based on fractal theory and flow characteristics,the fractal dimension of gas diffusion in the pore ways was calculated under different temperature.Considering pore size distribution,connectivity distribution and Fick diffusion mechanisms,the relationship between the gas diffusivity increases with pore area fractal dimension and porosity was investigated,and multiple linear equation of the coal–oxygen diffusion coefficients and pore parameters was obtained.Comparison between the experimental data and model prediction verifies the validity of the model.It is important to reveal the mechanism of coal spontaneous combustion and to make corresponding prevention measures.
2.Experimental
2.1.Sample preparation
Lignite obtained from three representative lignite samples,those are Baiyinhua lignite(Inner Mongolia of China),Zhaotong lignite(Yunnan province of China),and Xiaolongtan lignite(Yunnan province of China)were used as raw coals in this study.They were numbered No.1,No.2,and No.3,respectively.The raw coals were crushed to the particle size between 1.0 mm and 1.5 mm,in order to ensure that the oxygen molecules escape mainly for the internal hole diffusion and migration process.The raw coals were dried at 25°C for48 h in vacuum under constant temperature to eliminate inner moisture,in order to eliminate the influence of moisture.Then the coal samples were accurately weighed,loaded into well closed sample tube and vacuumized.Table 1 summarizes the results of the proximate analysis,the ultimate analysis and determination of equivalent diameter(de)sphericity of coal samples before vacuum drying.The particle size range and the equivalent diameter are basically same,so it can be considered that the external diffusion resistance are equal.
For investigating the effect of the temperature,the coal samples were oxidized for 8 h in air ambient by a humidity drying oven under 25 °C,50 °C,90 °C,130 °C,and 170 °C.All the experimental coals were marked and hermetic loaded in the well closed sample tubes.
2.2.Determination of surface morphology and pore structure
The FEI Quanta 250 Scanning Electron Microscope(SEM)was used to analyze the surface morphology of oxidized coal samples.The magnification times were fixed at 10000.The pore structure parameters of the oxidized coal samples were tested by the AS-1 Automatic surface area and pore size analyzer(Quantachrome Instruments)made in United States of America.Nitrogen as the adsorbate,the adsorption progress was carried out at liquid nitrogen temperature(-196°C).The coal surface area was determined by Brunauer,Emmett and Teller(BET)equation.The pore volume,the average pore diameter and pore size distribution were obtained by Density Functional Theory(DFT).The fractal dimensions of pore structure were calculated according to the fractal Frenkel–Halsey–Hill(FHH)mode from the N2adsorption data[29].
2.3.Determination of coal-oxygen diffusion coefficient
In this section,coal–oxygen diffusion coefficients were determined according to static proliferation dual-volume method,which has been used in the field of coal–oxygen diffusion because of its good reliability[30].
2.3.1.Experimental device
Device of measuring coal–oxygen diffusion is shown in Fig.1.
Two airtight gas chambers(5 L capacity)are connected by a perspex tube(9 cm long and 1 cm in diameter)with two valves.Every experimental coal sample was filled in the test tube with the same filling method and voidage.The oxygen was introduced to chamber A and nitrogen to B.The pressures of the two chambers were adjusted to be equal.When the valves were opened,the oxygen began to diffuse through coals.The gas compositions of the two chambers were analyzed by gas chromatography.
2.3.2.Methods
Feng et al.[31] find that the pore resistance of the limiting particles is constant,while the pore resistance of large pores is much smaller than that of tiny pores.According of that,we assume that the external diffusion time is negligible compared to the diffusion time because the external diffusion coefficient is much greater than the internal diffusion coefficient after the coal is broken to a certain size(1 mm or more),the flow of gas in the loose coal pile is dominated by internal diffusion.Therefore,The model assumes that coal particles are spherical particles with the same diameter,O2diffusion resistance between the coal particle surface and the coal particle is negligible and the diffusion process of O2in coal grains obeys Fick's law.
Under experimental conditions,the gas diffusion through loose coal is assumed quasi-static.Eq.(1)can be obtained according to Fick's law and conservation of mass.

where JAis mass flow rate of oxygen diffusion,mol·s-1·m-2.S is the cross-sectional area of experimental tube,m2.DABis coal–oxygen diffusion coefficient,m2·s-1.CAand CBare respectively the oxygen concentration of gas chamber A and B,mol·L-1.L is the length of experimental tube,m.VBis the volume of gas chamber B,m3.

Table 1 Basic characteristics of lignites

Fig.1.Schematic diagram of coal-oxygen diffusion coef fi cient determination device.
At equilibrium,the average concentration can be determined according to the initial concentrationat any time by mass balance.

3.Results and Discussion
3.1.Effect of low temperature oxidation on surface morphology
From Fig.2,it can be seen that the particle surface of lignite is irregular with many pores and fractures.As the oxidation processed,coal particle surface morphology and pore structure have great changes.The coal particle oxidized at 50°C become smooth relatively.After being oxidized at 90 °C and 130 °C,phenomenon of pore growth can be observed obviously,and the edge of the pore becomes round and smooth.However,after being oxidized at 170°C,the irregular degree of the particle surface increased again.The collapse of coal can be observed.Meanwhile,the cracks become richer.This shows that the physical structures of coal change obviously because of the temperature effect,which is the reflection of the degree of oxidation.
3.2.Effect of low temperature oxidation on pore morphology
The low-temperature liquid nitrogen adsorption isotherms and the pore size distribution of lignite under different temperature are shown in Fig.3.
Obviously,low-temperature oxidation causes great change both in pore diameter and fractal dimension of lignite.When relative pressure is less than 0.3,the separation degree of the adsorption/desorption isotherms increases with temperature(Fig.3a to e).When the relative pressure ranges from 0.4 to 0.95,the adsorbed amount increases sharply and large hysteresis loops appear.It can be concluded that experimental coal samples have wide pore size distribution.Meanwhile,many interconnected pores with larger diameter exist in coal.However,Fig.3e separates most obviously,which indicates blind pores in raw coal gradually evolve to interconnected pores because of gas expansion with the rising of ambient temperature.
The pore size(Fig.3f)is divided into class 1 pore(<5 nm),class 2 pore(5 nm–15 nm),class 3 pore(>15 nm).It can be seen that the average pore diameter first increases and then decreases and reaches maximum at 90°C.The microspore content first decreases and then increases,which is contrary to macrospore.
3.3.Effect of low temperature oxidation on pore parameters
Average pore diameter and fractal dimension are given in Table 2.The fractal dimension,ranging from 2 and 3, first decreases and then increases with the minimum at 90 °C.From 25 °C to 90 °C,it is considered that average pore diameter become larger and pore topology becomes more uniform due to the evaporation and removal of the adsorbed gases.From 90 °C to 170 °C,water was removed from small capillaries.Similar to physical structure changes in the process of lignite drying[1],the shrinkage forces caused by emptying such small capillaries lead to the collapse of macrospore and generation of tiny pore.So the pore structure finally becomes more complicated.
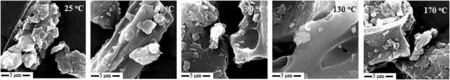
Fig.2.SEM images of No.1 lignite particles oxidized under different temperatures.

Fig.3.Adsorption isotherms of No.1 lignite oxidized under(a)25 °C;(b)50 °C;(c)90 °C;(d)130 °C;(e)170 °C;and(f)pore size distribution of lignite under different temperature.
3.4.Changing rule of coal-oxygen diffusion properties
Data collected by gas chromatography are shown in Table 3.The oxygen diffusion coefficients of coal samples oxidized at different temperature are calculated according to the methods of 2.3.2 and are shown in Table 4.
It can be seen from Table 4,with oxidation temperature increasing,the diffusion coefficient first increases and then decreases and reaches max at 90°C.
The coal–oxygen diffusion coefficients of all coal samples are determined at the room temperature and the void ratios are similar,but the different coal samples have different results.Therefore,the continuous evolution of pore structure in the oxidation process must influence the coal–oxygen diffusion properties.

Table 2 Average pore diameter and fractal dimension of No.1 lignite under different oxidation temperature
4.Effect of Pore Structure on Coal–Oxygen Diffusion Properties
The relationship among oxygen diffusion coefficient,average pore diameter and fractal dimension is shown in Fig.4.The coal–oxygen diffusion coefficient increases with the average pore diameter but decreases with the fractal dimension,and both of them have good linear relationship.
The interaction between oxygen diffusion and average pore diameter can be explained by the Knudsen number,which represents the relative size of pore diameter and average free path of molecule motion[32].The diffusion mode can be divided into Fick diffusion,Knudsen diffusion and Transition diffusion by the Knudsen number.Larger pore diameter is more advantageous to the occurrence of Fick diffusion which has larger diffusion rate.So the generation of microspore is unfavorable to the oxygen diffusion.This leads to the conclusion that the evolution process of microspore to macrospore is a boost for the oxygen diffusion in coal.
On the other hand,when the fractal dimension is smaller,the pore is more regular and uniform,which is beneficial to the diffusion of oxygen in coal.The effect of pore structure on carbon monoxide proliferation was reported by Guo et al.[33]Similar to the oxygen diffusion,the change of fractal dimension reflects the change of the surface morphology of diffusion channel.The difference is that fractal dimension and carbon monoxide proliferation has a quadratic curve relationship.Sothe certain influence relationship may be also related to the type of diffusion gas.

Table 3 Change of O2 concentration with time
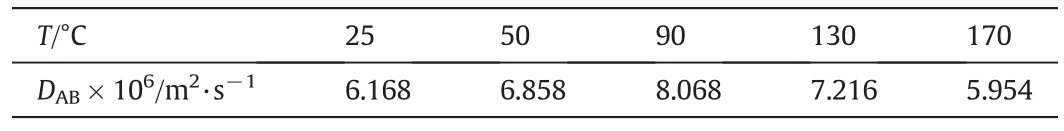
Table 4 Oxygen diffusion coefficients of No.1 lignite under different Oxidation temperature
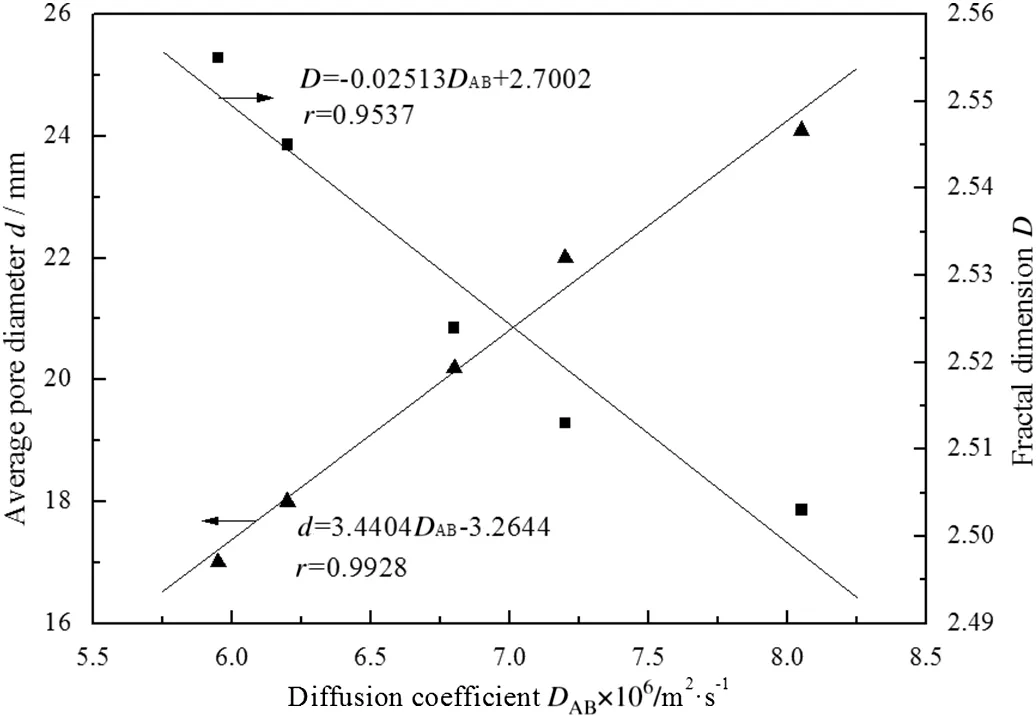
Fig.4.Relationship among oxygen diffusion coefficient,average pore diameter and fractal dimension of No.1 lignite.
The average pore diameter and fractal dimension are key parameters,which describe the classical and fractal pore structure respectively.The multiple linear regression of pore structure and coal–oxygen diffusion properties was made.

where d is average pore diameter,mm.D is fractal dimension.a,b1and b2are coefficients.
The results of multiple linear regression were obtained as following.

Correlation coefficient:r=0.998
Partial regression coefficients “t-Stat”:td=4.718,tD=1.327
A correlation coefficient of 0.998 was obtained which can pass the F inspection on a high level of significance.The established linear regression equation fits well with the experimental data.According to “t-Stat”,average pore diameter affects the coal-oxygen diffusion coefficients more significantly than fractal dimension.
5.Equation Verification
The experimental data of oxygen diffusion coefficient,average pore diameter and fractal dimension of No.1,No.2 and No.3 samples are given in Table 5.The 3D figure(Fig.5)was plotted with the experimental data and the calculated data of oxygen diffusion coefficient with multiple linear equation of pore structure and coal–oxygen diffusion.The relative error of calculated data are less than 5%,indicating that this multiple linear equation of pore structure and coal–oxygen diffusion can predict the oxygen diffusion of low temperature oxidation lignite.
6.Conclusions
(1)During low temperature oxidation processes,physical structure of lignite changed obviously with pore growth,cracks production.The physical structure changes reflected the degree of oxidation.
(2)Temperature had a significant impact on the pore evolution.The transition temperature was 90 °C.From 25 °C to 90 °C,the microspore became larger and the pore topology was more regular.From90 °C to 170 °C,the macrospore collapse and microspore developed again.The pore shape changed obviously and small permeable pores appeared gradually.
(3)The effect of pore structure changes on coal-oxygen diffusion properties was testified.Larger pore diameter and smaller fractal dimension are beneficial to the diffusion of oxygen.The relevant equation of the coal-oxygen diffusion coefficients and the pore parameters is.Average pore diameter is the main influencing factor.

Table 5 Data of lignite samples under different oxidation temperature
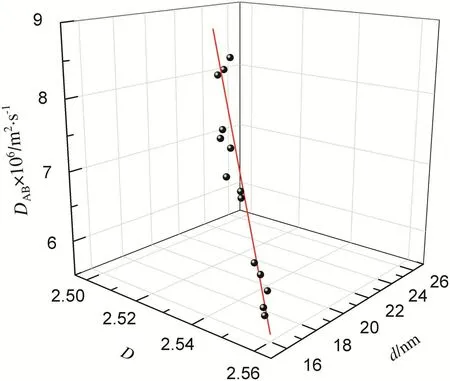
Fig.5.3D figure of oxygen diffusion coefficient between the experimental and calculated data.

[1]B.Sun,J.Yu,A.Tahmasebi,Y.Han,An experimental study on binderless briquetting of Chinese lignite:Effects of briquetting conditions,Fuel Process.Technol.124(10)(2014)243–248.
[2]Y.Fei,A.A.Aziz,S.Nasir,W.R.Jackson,M.Marshall,J.Hulston,A.L.Chaffee,The spontaneous combustion behavior of some low rank coals and a range of dried products,Fuel 88(9)(2009)1650–1655.
[3]H.Choi,C.Thiruppathiraja,S.Kim,Y.Rhim,J.Lim,S.Lee,Moisture readsorption and low temperature oxidation characteristics of upgraded low rank coal,Fuel Process.Technol.92(2011)2005–2010.
[4]S.Krishnaswamy,S.Bhat,R.D.Gunn,P.K.Agarwal,Low-temperature oxidation of coal.1.A single-particle reaction–diffusion model,Fuel 75(95)(1996)333–343.
[5]H.Wang,B.Z.Dlugogorski,E.M.Kennedy,Analysis of the mechanism of the lowtemperature oxidation of coal,Prog.Energy Combust.Sci.134(3)(2003)107–117.
[6]B.Taraba,R.Peter,V.Slovák,Calorimetric investigation of chemical additives affecting oxidation of coal at low temperatures,Fuel Process.Technol.92(3)(2011)712–715.
[7]X.M.Guo,J.C.Xu,S.E.Hui,Theoretical analysis on low of transporting oxygen in the loose coal,J.China Coal Soc.6(2001)643–648(in Chinese).
[8]K.Brooks,D.Glasser,A implified model of spontaneous combustion in coal stockpiles,Fuel 65(86)(1986)1035–1041.
[9]Z.Q.Zhang,K.F.Yan,Molecular dynamics simulation of oxygen diffusion in dry and water-containing brown coal,Mol.Phys.109(19)(2011)2367–2374.
[10]H.Wen,M.G.Xu,Z.P.Wang,A.P.Dai,Research of influence of ground temperature on coal spontaneous combustion,J.Xi'an Univ.Sci.Technol.21(1)(2001)1–3(in Chinese).
[11]Y.Xiao,Q.W.Li,J.H.Lu,Effects of air relative humidity on coal spontaneous combustion properties,China Saf.Sci.J.25(3)(2015)34–40(in Chinese).
[12]Y.N.Zhang,J.Deng,Z.M.Luo,H.Wen,W.Wang,Thermogravimetric analysis on influence factor of coal spontaneous combustion,J.Xi'an Univ.Sci.Technol.28(2)(2008)388–391(in Chinese).
[13]H.Wang,B.Z.Dlugogorski,E.M.Kennedy,Coal oxidation at low temperatures:oxygen consumption,oxidation products,reaction mechanism and kinetic modelling,Prog.Energy Combust.29(3)(2003)487–513.
[14]X.L.Meng,Y.F.Liu,R.Z.Chu,Q.Fang,Z.C.Zhang,Experiment study on the influence of oxygen diffusion and mass-transfer on the oxidation reaction of lignite in lowtemperament,China Coal 39(3)(2013)68–72(in Chinese).
[15]A.Küçük,Y.Kadıoğlu,M.Ş.Gülaboğlu,A study of spontaneous combustion characteristics of a turkish lignite:particle size,moisture of coal,humidity of air,Combust.Flame 133(3)(2003)255–261.
[16]Y.Wang,Y.S.Zhao,Z.Z.Feng,Evolution characteristics of pore structure during lignite seam spontaneous combustion developing,J.China Coal Soc.35(9)(2010)1490–1495.
[17]H.P.Corporation,A quaternized polysulfone membrane for zinc–bromine redox flow battery,J.Chem.15(1)(2014)1683–1685.
[18]W.Q.Zhang,S.G.Jiang,Z.Y.Wu,L.Y.Wang,X.R.Ju,Review of spontaneous combustion characteristic structures in coal surface,Saf.Coal Min.43(1)(2012)15–18.
[19]X.L.Meng,R.Z.Chu,G.G.Wu,J.M.Zhu,Z.H.Wang,J.Chen,Lab preparation and performance study on polyvinyl alcohol oxygen insulation gel to prevent coal spontaneous combustion,Coal Eng.9(2009)102–105(in Chinese).
[20]M.J.Watt-Smith,S.P.Rigby,T.R.Ralph,F.C.Walsh,Characterisation of porous carbon electrode materials used in proton exchange membrane fuel cells via gas adsorption,J.Power Sources 184(1)(2008)29–37.
[21]G.M.S.E.Shafei,C.A.Philip,N.A.Moussa,Fractal analysis of hydroxyapatite from nitrogen isotherms,J.Colloid Interface Sci.277(2)(2004)410–416.
[22]J.Guo,O.Posnansky,S.Hirsch,M.Scheel,M.Taupitz,J.Braun,I.Sack,Fractal network dimension and viscoelastic powerlaw behavior:II.An experimental study of structure-mimicking phantoms by magnetic resonance elastography,Phys.Med.Biol.57(12)(2012)4041–4053.
[23]S.I.Pyun,C.K.Rhee,An investigation of fractal characteristics of mesoporous carbon electrodes with various pore structures,Electrochim.Acta 49(24)(2004)4171–4180.
[24]Y.D.Cai,D.M.Liu,Z.J.Pan,Y.B.Yao,J.Q.Li,Y.K.Qiu,Pore structure and its impact on CH4adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China,Fuel 103(2013)258–268.
[25]G.S.Armatas,Determination of the effects of the pore size distribution and pore connectivity distribution on the pore tortuosity and diffusive transport in model porous networks,Chem.Eng.Sci.61(14)(2006)4662–4675.
[26]Y.Shi,J.Xiao,M.Pan,R.Yuan,A fractal permeability model for the gas diffusion layer of PEM fuel cells,J.Power Sources 160(1)(2006)277–283.
[27]Q.Zheng,B.Yu,S.Wang,L.Luo,A diffusivity model for gas diffusion through fractal porous media,Chem.Eng.Sci.68(1)(2012)650–655.
[28]Y.Shi,J.Xiao,S.Quan,M.Pan,L.Zhang,Fractal model for prediction of effective hydrogen diffusivity of gas diffusion layer in proton exchange membrane fuel cell,Int.J.Hydrogen Energy 35(7)(2010)2863–2867.
[29]A.L.Ahmad,N.N.N.Mustafa,Pore surface fractal analysis of palladium-alumina ceramic membrane using Frenkel–Halsey–Hill(FHH)model,J.Colloid Interface Sci.301(2)(2006)575–584.
[30]J.Deng,J.C.Xu,L.Li,Experimental research on diffusion coefficient of oxygen in crashed coal,J.China Univ.Min.Technol.32(2)(2003)145–147(in Chinese).
[31]B.Feng,S.K.Bhatia,Variation of the pore structure of coal chars during gasification,Carbon 41(3)(2003)507–523.
[32]X.C.Li,B.S.Nie,R.M.Zhang,L.L.Chi,Experiment of gas diffusion and its diffusion mechanism in coal,Int.J.Min.Sci.Technol.6(6)(2012)885–889.
[33]L.W.Guo,Z.Y.Xiao,Y.X.Liu,Effect of coal pore structure on the CO proliferation,J.China Univ.Min.Technol.36(5)(2007)636–640(in Chinese).
 Chinese Journal of Chemical Engineering2016年6期
Chinese Journal of Chemical Engineering2016年6期
- Chinese Journal of Chemical Engineering的其它文章
- A stepwise optimal design of water network☆
- The turbulent behavior of novel free triple-impinging jets with large jet spacing by means of particle image velocimetry☆
- Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange☆
- Online process monitoring for complex systems with dynamic weighted principal component analysis☆
- Evolvement behavior of microstructure and H2O adsorption of lignite pyrolysis☆
- Integration of coal pyrolysis process with iron ore reduction:Reduction behaviors of iron ore with benzene-containing coal pyrolysis gas as a reducing agent☆
