Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange☆
Yu Niu ,Fuying Li,Kai Yang ,Ting Qiu *,Renzhang Wang ,Cheng Lin
1 School of Chemical Engineering,Fuzhou University,Fuzhou 350002,China
2 Collaborative Innovation Center of Clean Coal Gasification Technology,Sanming University,Sanming 365004,China
3 College of Resources and Chemical Engineering,Sanming University,Sanming 365004,China
4 Research Institute of Photocatalysis,State Key Laboratory of Photocatalysis on Energy and Environment,Fuzhou University,Fuzhou 350002,China
1.Introduction
Titanium dioxide(TiO2)semiconductors have attracted considerable academic and industrial interest due to their promising applications in catalysis[1–3].TiO2-based catalysts have high activity and stability for commercial applications such as wastewater treatment[4–9].Furthermore,modified TiO2solid acid nanocomposites are reported to exhibit better catalytic activity than pure TiO2nanomaterials[10].This is becausemodified TiO2solid acid contains both Brønsted and Lewis acid sites and also has many other unique properties[11].In 1979,Hino first reported a halogen-free Ssolid super acid system which has since aroused great interest among chemists[12].Researchers are much interested in enhancing the acidic properties of solid acid catalysts as the efficiencies of some catalysts are strongly dependent on the crystal phase,size,and pore-wall structure of the catalyst as well as its surface chemistry,particularly surface acidity.Therefore,with an appropriate modification to increase the surface acidity of TiO2,the catalytic performance may be improved.Ion implantation technique[13]and addition of other semiconductors such as WO3,ZnO,Al2O3,and Fe2O3[14–17]are some of the modifications that have been previously reported.Typically,various cumbersome methods such as wet impregnation method,sol–gel and co-deposition techniques have been used to prepare these composites,and these preparation methods can considerably influence the surface properties of the resulting composites[18].The environmentally friendlyTiO2solid acid catalyst has been extensively used to catalyze many organic reactions,and it also exhibits a high reactivity for the decomposition of H2O2[19].It has been found that organics were more easily adsorbed on thecatalystthan on TiO2.Increasing the number of surface acid sites is a proven method to improve the photocatalytic oxidation property.In addition,the dependence on acid sites could further benefit the acid-sensitive propylene glycol methyl ether esterification reaction.
Based on the above considerations,in this study,we synthesized the rhodium(Rh)modified sulfated TiO2solid acid nanocomposite via a photo-deposition method[20–26],where the Rh nanoparticles deposited on the surface ofare uniformly dispersed.Rh can be used as a co-catalyst for isomerization and photocatalytic reactions,especially for the decomposition of methyl orange(MO),because the nanostructures as well as the composition of noble metal nanoparticles are known to enhance the catalytic activity[27–29].Such several studies with Rh as co-catalyst have been reported for esterification reactions as well.To the best of our knowledge,there are no previous reports in the literature of the synthesis and sulfate group modification of Rh/TiO2nanocomposites and their application as catalyst for the esterification reaction of propylene glycol methyl ether(PM)and acetic acid(HAc).Propylene glycol methyl ether acetate(PMA)is a low-toxic,colorless,moderately volatile liquid with very good solubility properties.PMA is used in paints,lacquers,stains,inks and surface coatings,silk-screen printing,photographic and photo lithographic processes[30,31].MO is a common organic dye that is widely used in textiles,food,paper,and leather industries.However,the by-products and waste discharge from MO applications can cause serious environmental pollution problems[32–34].
The above two reactions of esterification and MO decomposition are seemingly very different,however,both reactions employ similar catalysts and are widely used in various industries.Generally,TiO2-based solid acid catalysts have been used to catalyze esterification reactions with high activity[35,36].Furthermore,in recent years,various studies have reported the use of TiO2-based solid acid catalysts as photocatalysts for the degradation of organic dyes[37,38].Therefore,using these two very different types of reaction systems,the utility and versatility of thecatalyst can be verified,and also relevant common factors can be identified which could help the design and preparation of the catalyst materials.Thus,this study aims to develop and evaluate this new catalyst system.Towards this end,a novel nanostructured Rh modifiedsolid acid catalyst was synthesized and thoroughly characterized by means of XRD,TEM,XPS,FT-IR,BET analysis and ESR.The performance of this new catalyst was then evaluated in the esterification reaction of propylene glycol methyl ether and acetic acid,and also in the decomposition of organic dyes.
2.Experimental Section
2.1.Chemicals and materials
Anatase TiO2(99%,15 nm APS powder)and RhCl3·3H2O were acquired from Alfa Aesar(USA)and Aladdin ChemicalCo.(China),respectively.H2SO4,MO,PM and HAc were of analytical reagent grade(Sinopharm Chemical Reagent Co.Ltd.,China)and used without further purification.
2.2.Preparation of catalysts
The TiO2sample(1 g)was immersed in a 1 mol·L-1H2SO4solution(15 ml)at room temperature with stirring for 12 h.Then,the resulting solids were filtered and rinsed twice with deionized water.Finally,the obtained solids were dried and calcined in an air stream at 773 K for 2 h to obtain thesolid acid.
The Rh modifiedsolid acid catalyst was prepared by photo-deposition method.Thepowder(1.0 g)and RhCl3·3H2O(w(Rh)=0.5%)were dissolved in 100.0 ml deionized water and sonicated for 5 min.A 300 W Xenon lamp was used as the light source,and the solution was irradiated for 5 h in air,with 1.0 ml ethanol as the sacrificial agent.After that,the mixture was filtered,washed,and dried.Then,the light yellow colored Rh–TiO2solid was filtered and rinsed twice with deionized water.The solid product was then dried and calcined in an air stream at 773 K for 2 h to obtain thesolid acid.The sulfated modified Rh–TiO2solid acid catalyst changed to dark yellow color after drying and calcination.
2.3.Characterization of catalysts
Powder X-ray diffraction(XRD)patterns were recorded on an X'Pert X-ray diffractometer(Panalytical,Netherlands)equipped with graphite monochromatized Cu Kα radiation(λ =0.15406 nm)from 20°to 90°(2θ).The Brunauer–Emmett–Teller(BET)surface areas of the samples were obtained from N2adsorption/desorption isotherms determined at liquid nitrogen temperature(77 K)on an automatic analyzer—ASAP2020(Micromeritics,China).The samples were degassed for 2 h under vacuum at 350°C prior to adsorption.Transmission electron microscopy(TEM)and HRTEM images were obtained by JEM 2010 EX instrument(JEOL,Japan)at an accelerating voltage of 200 kV.The X-ray photoelectron spectra(XPS)were recorded on a VG ESCALAB 250 XPS System(Thermo Fisher Scientific,USA)with a monochromatized AlKαX-ray source(15 kV,200 W,500 mm pass energy=20 eV).Fourier transform infrared spectroscopy(FT-IR)was performed on a Nicolet 670 FTIR spectrometer(Nicolet,USA).Samples were pressed by a KBr disk(18 mm diameter,25–30 mg)preparation apparatus.The samples were dried at 250°C for 2 h prior to pressing.The infrared spectra were recorded regularly on the same Nicolet 670 FTIR spectrometer with a deuterated triglycine sulfate(DTGS)detector at a resolution of 8 cm-1and for 128 scans.
2.4.Acid density test
The total acid density of all these catalysts was determined as follows:the catalyst samples(0.1 g)were placed in an Erlenmeyer flask,and mixed with 15 ml of 2 mol·L-1NaCl solution[45].As H+ions existed in the–SO3H group of sulfonated catalyst,they could be exchanged with Na+ions by ultrasonication for 60 min.After filtration,a 0.02 mol·L-1NaOH solution was used to titrate the filtrate using phenolphthalein as the indicator.When the color of the filtrate changed from colorless to slightly red,the end point of the titration was reached.The accurate acid quantity was calculated as follows:

where c(H+)represents the acid quantity of the sulfated samples;c(OH-)represents the concentration of the NaOH solution;△V represents the volume of the NaOH solution consumed in titration;and m represents the quality of the catalyst samples used in ultrasonication.
2.5.Catalytic performances
The solid acid catalyzed esterification reaction of propylene glycol methyl ether(28.8 g)and acetic acid(10.8 g)was conducted in liquid phase to evaluate the catalytic activity of the different catalysts.The reaction was carried out in a well-stirred oil batch reactor.A predetermined amount of the reagent mixture was loaded into the reactor and heated to 391 K for 4 h.At the end of the reaction,the liquid products were analyzed by gas chromatography GC 7900 Techcomp,China.
The photocatalytic performances ofsamples were evaluated by their activity in the decomposition of MO in an aqueous solution under a 300 W halogen lamp irradiation.The photocatalyst(100 mg)was added to 100 ml of aqueous MO solution(20 mg·L-1)at room temperature under air.Before the light was turned on,the solution was continuously stirred for 30 min in the dark to ensure the establishment of an adsorption–desorption equilibrium.At specific time intervals of irradiation,2 ml aliquots were withdrawn,and then centrifuged to separate all the catalyst.The concentration of MO during the degradation was monitored by measuring its absorbance at 664 nm using a BK UV-1600 UV–Vis spectrometer(Biobase,China).The degradation rate(D)of acid orange II was calculated according to the equation:D=(A0-A)/A0×100%(A0represents initial absorbance;A represents final absorbance).
3.Results and Discussion
3.1.The physicochemical properties of the catalysts
3.1.1.Crystal sizes and surface areas
The phase structure,crystal size,surface area and pore distribution of the synthesized materials were examined using XRD and N2physical adsorption experiments.Fig.1 shows the XRD patterns of the TiO2,Rh–TiO2,andsamples.The peaks at 2θ=25.1,37.6,48.0,53.8,55.0 and 62.7°can all be attributed to the anatase phase of TiO2(JCPDS:21-1272)and the three additional peaks at 2θ=27.4,36.1 and 54.3°with lower intensity were assigned to the reflections of the rutile crystal structure[39].The XRD patterns show that thestill retained the original TiO2anatase crystal structures,suggesting that deposition of Rh nanoparticles did not alter the structure of

Fig.1.XRD patterns of TiO2,Rh–TiO2,sulfated TiO2 and sulfated Rh–TiO2.
N2adsorption–desorption at 77 K was used to study the microstructure of TiO2,Rh–TiO2,samples.It can be seen that they exhibit the typical type-IV adsorption curves with a hysteresis loop between the partial pressure P/P0=0.4-1.0,indicating the mesoporous structure of the samples.As shown in Fig.2 and Table 1,the sample pairs of TiO2vs.Rh–TiO2andTiO2vs.possess similar specific surface areas and crystal sizes.This indicates that Rh–TiO2,maintain the mesoporous structure of thesupport.The BET surface area of the TiO2without Rh metalis 168.69and thatof Rh–TiO2is 174.08 m2·g-1is 190.03 m2·g-1andis 196.08 m2·g-1).So,we can clearly see that Rh does not significantly change the pore volumes and pore sizes compared to the corresponding parent materials,confirming that modification with Rh noble metal does not alter the microstructure of TiO2.These above results also indicate that Rh nanoparticles are highly dispersed on the surface of TiO2.Compared with theand,the crystal sizes and pore volumes of thesamples were nearly the same as those of thesample.However,although the pore sizes increased,thesamples stillexhibited much higher BET surface areas than the TiO2and Rh–TiO2,which may be due to the reservation of the porous structure inside the particle under supercritical conditions.The S-modification further increased the crystal sizes since the O atom in the O–Ti–O network was replaced by the S atom with relatively larger atomic radius,corresponding to the further increase of BET surface areas though the increase of pore size[40].
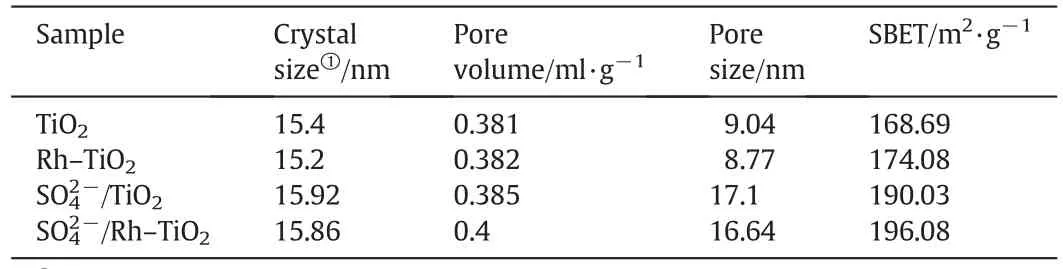
Table 1 Physicochemical properties of the catalyst samples
3.1.2.SEM and TEM
The morphologies of the sulfated TiO2and Rh–TiO2nanocomposite samples were analyzed by SEM,as shown in Fig.3(A)and(B).The sulfated TiO2is composed of a large quantity of relatively uniform powder-like particles,however there is no uniform morphology observed in this sample.In contrast,we found that the introduction of Rh results in a hollow structure,as seen in the Rh–TiO2sample.A recent investigation[41]suggests that the hollow spherical structures allow multiple reflections of irradiated light within their interior cavities,resulting in enhanced light-harvesting properties and thus improved photocatalytic activity.
TEM images ofsamples are presented in Fig.4.TEM provides a direct observation of the morphology and distribution of Rh nanoparticles within the solid sample.The two samples exhibit a broad particle size distribution with small Rh nanoparticles being observed in addition to the larger TiO2particles.The mean size of the Rh particles deposited on theis ca.2–4 nm(shown in HRTEM image in Fig.4(B.2)).In addition,Rh nanoparticles are homogeneously dispersed on the surface of the TiO2.TEM patterns ofshow that there are no obvious differences in the morphology of the TiO2compound itself,indicating that the dispersion of Rh nanoparticles does not influence the lattice spacing of the TiO2.

Fig.2.N2-sorption isotherms(A)and the BJH pore size distributions(B)for the TiO2,Rh–TiO2,sulfated TiO2 and sulfated Rh–TiO2 samples.

Fig.3.SEM images of(A)sulfated TiO2 and(B)sulfated Rh–TiO2.
3.1.3.XPS spectra
As the Rh0/Rh3+ratio in sulfated Rh–TiO2is likely to be a dominant factor in affecting the catalytic performance,XPS was employed to investigate the chemical states of the components in the samples.
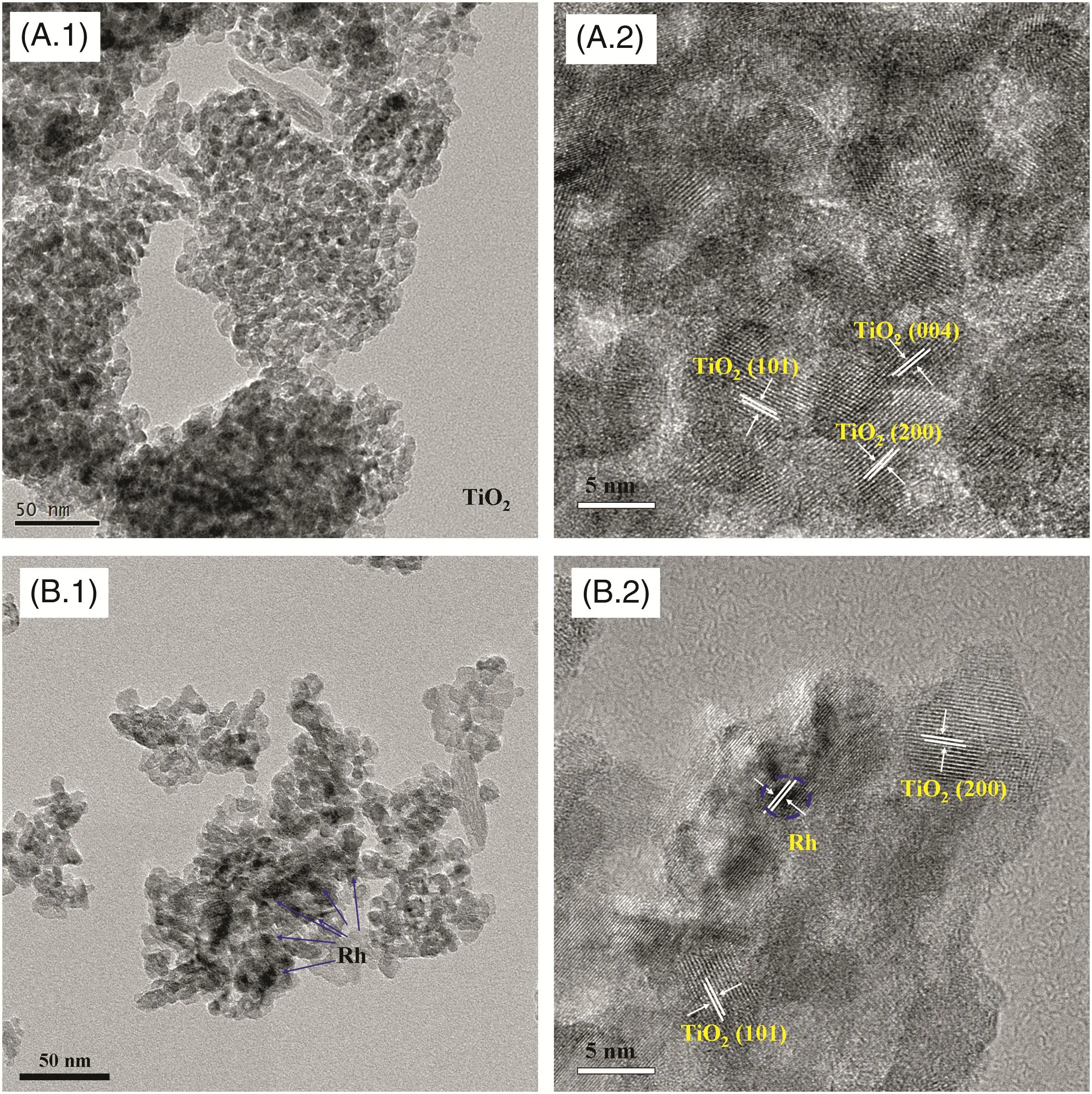
Fig.4.TEM and HRTEM images of(A.1,A.2)sulfated TiO2 and(B.1,B.2)sulfated Rh–TiO2.
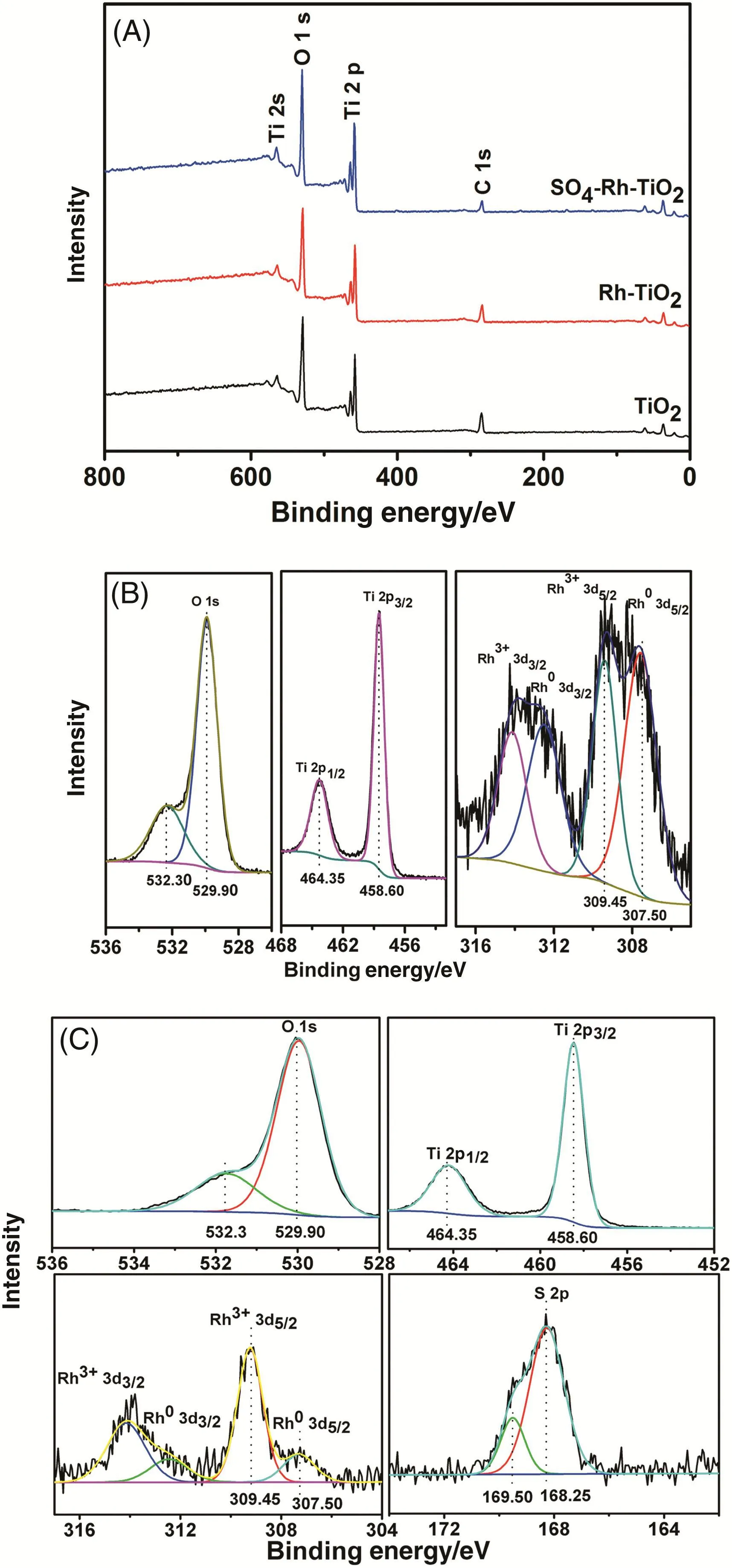
Fig.5.(A)Survey spectra of TiO2,Rh–TiO2 and sulfated Rh–TiO2(B)Rh 3d,Ti 2p and O 1s spectra of Rh–TiO2 powder(C)S 2p,Rh 3d,Ti 2p,and O 1s spectra of sulfated Rh–TiO2 powder.
Here,the reference values are obtained in situ by measuring the C 1s EB=284.6 eV,using the C 1s peak of aliphatic carbon as a reference.The survey spectra show that O,Ti and Rh are present in the samples.No differences occur in O and Ti atoms before and after the dispersion of Rh nanoparticles and the sulfate modification(Fig.5(A),(B)and(C)).The XPS data of Rh–TiO2and sulfated Rh–TiO2are shown in Fig.5(B)and(C),respectively.The binding energy(BE)of Rh 3d5/2at 307.0–307.1 eV is attributed to Rh0metal.The two peaks observed at ca.307.5 eV in Fig.5(B)and(C)can be attributed to metallic Rh0,which is 0.4 eV higher than the peaks for reduced Rh–TiO2[42].This indicates that the metallic Rh particles are electron-deficient,which may contribute to the catalytic activity in the esterification reaction and the oxidation of MO.The second pair of peaks with Bes of about 309.45 eV can be assigned to Rh3+valence state.Despite the fact that the photo-deposition procedure was performed in air,the Rh nanoparticles loaded sulfated TiO2shows the presence of both Rh0and Rh3+valence states.On the Rh/TiO2surface,sulfation has the effect of lowering the Rh0/Rh3+ratio,i.e.,the sulfated Rh–TiO2surface is located in a high oxidation state,which is likely responsible for the improved catalytic activity.Moreover,the S 2p peak was observed in Fig.5(C),confirming that sulfation has occurred.
3.1.4.FT-IR spectra
The infrared spectra of TiO2,are shown in Fig.6.The strong band at about 3422 cm-1is attributed to O–H bonds of surface hydroxyl groups on the solid surface and the band at 1637 cm-1corresponds to adsorption of water molecules from air onto the surface of the catalyst.The IR results confirm that these TiO2based nanocomposites have been successfully modified by sulfation,and two catalytically active sites are present on these nanocomposites.The surface sulfur complexes formed by the interaction of oxides with sulfate ions provide highly active sites for the catalyst.Compared with the TiO2and Rh–TiO2,the IR spectra of the sulfated catalysts,,show two new absorption bands at 1133 and 1062 cm-1.The absorption bands of thegroup in the region of 1200–900 cm-1are characteristic of inorganic chelating bidentate sulfate and the strong band observed at 1140–1030 cm-1can be assigned to S=O stretching frequency[43].Based on the previously reported S–O stretching frequencies[44–46],the bands at 1133 and 1062 cm-1can also be assigned to chelating bidentate.The intensity of both the 1133 and 1062 cm-1bands of the sulfated Rh–TiO2nanocomposite are stronger than those of,indicating that the introduction of Rh increases the concentration of surface-adsorbed sulfate groups.It is likely that the high valence state of Rh3+onchanges the surface properties of the catalyst,in accordance with the XPS data.

Fig.6.IR spectra of
Surface sulfate groups also play an important role in catalysis by offering active acid sites[10].From the acid density results shown in Table 2,it can be seen that the amount of sulfate groups on the newly prepared sulfated Rh–TiO2is about 542 μmol·g-1,which corresponds to 1.87groups per nm2on the surface of the nanocomposite.However,the amount of sulfate groups on sulfated TiO2is only 201 μmol·g-1,or about 0.71groups per nm2on the surface of TiO2.The surface coverage ofis calculated according to BET surface area.From the XPS spectra in Fig.5(B)and(C),it is evidentthat the Rh nanoparticles of/Rh–TiO2are mainly in+3 valence state.This allows the Rh nanoparticles to have abundant Lewis acid sites and combine easily with the negatively chargedgroups.Thus,it can be seen that loading Rh nanoparticles onTiO2enhances the acid density,which could be due to the positively charged Rh.

Table 2 The amount of sulfate groups on prepared catalysts
3.2.Catalytic activity
The catalytic activity of the sulfated TiO2and Rh–TiO2powders was investigated using the below PMA esterification reaction process as a model reaction(Fig.7).

Fig.7.PMA esterification reaction process.
PMA is a new but widely used solvent in the chemical industry.Therefore,we decided to test the performance of the catalysts in the esterification reaction of PM and HAc.The effects of different catalysts on the reaction rate and the conversion of PM are shown in Fig.8.

Fig.8.PM conversion with
Fig.8 shows the amount of PM conversion in the esterification reaction with TiO2,catalysts.Previous literature reports state that 70%PM conversion could be attained at~363–383 K,with a reaction time of more than 600 min[47].However,in this study,we could achieve a conversion rate of more than 70%at 391 K with thecatalysts,atless than one-third of the reaction time previously reported.These new catalysts show high specific catalytic activity for this esterification reaction,with a PM conversion ranging from 69.5%to 75.2%(Table 3)after the reaction reaches equilibrium.It can be seen that both the sulfated TiO2andshow high catalytic activity for PM conversion at 391 K,while blank experiments(without catalysts)show that the conversion of PM is less than 6%.After a reaction time of 220 min(Fig.8),almost 70%PM conversion is achieved with the Rh modified sulfated TiO2nanocomposite,while about65%PM conversion is observed for sulfated TiO2.With an increase in reaction time from 220 to 300 min,both catalysts show an increased conversion of PM.The results clearly show that the Rh modified sulfated TiO2nanomaterials exhibit better catalytic activity for esterification reaction compared to the sulfated TiO2nanomaterials.The higher conversion could be attributed to the stronger acidity ofwhich in turn can be attributed to the strong interaction between Rh andFurthermore,all the sulfated samples showed better stability over 300 min,which is in accordance with the previously reported esterification reaction results[48].
Table 3 PM conversion of esterification reaction catalyzed byRh–TiO2 solid acid catalysts①

Table 3 PM conversion of esterification reaction catalyzed byRh–TiO2 solid acid catalysts①
①Reaction conditions:T=391 K,P=0.1MPa,T=5 h.
Conversion/%TiO2 24.7 Sulfated TiO2 69.5 Sulfated Rh–TiO2(w(Rh)=0.5%) 75.2 Samples
The strong acidity ofwas also found to have a bene ficial effect on the decomposition of MO in an aqueous solution.The MO degradation reaction was used as a model to test the catalyst activity,as MO is a commonly used dye and is also known to have good resistance to light degradation.We investigated the effect of initial pH on the catalytic activity for this photodegradation reaction.Fig.8 shows a comparative performance of thecatalyst at different pH values of the solution.As shown,the decomposition efficiency is considerably affected by the initial pH.The final decomposition efficiency decreased from 100%to 53.2%when the initial pH increased from 1.0 to 7.0.At lower pH,˙OH radicals and ˙OOH radicals are the main oxidants present in large numbers with a relatively long residence time,thus increasing the MO decomposition rate[49,50].Based on the observed results,the lower pH is more favorable for the TiO2photocatalysis process(Fig.9).

Fig.9.Decomposition of MO at different pH.
The MO photocatalytic decomposition was also performed in the presence of H2O2and the results are displayed in Fig.10.The results clearly show that the sulfated Rh–TiO2nanocomposite materials exhibit an increased catalytic activity compared to the sulfated TiO2and unmodified TiO2nanomaterials.Noble metals such as Rh have a high work function and also have a Schottky-barrierbetween the semiconductor and Rh.This Schottky-barrier is able to trap the injected electrons of the conduction band in the TiO2,suppressing the recombination of photo-electrons and holes[14].After 2 h of light irradiation,the decomposition rates of MO are as follows:27.9%for TiO2;89.1%forand 100%for

Fig.10.Photocatalytic decomposition of
In the esterification reaction and the MO photocatalytic decomposition,the electron transfer efficiency and formation of active species induced by Rh modi fi cation and the enhanced surface acidity also play important roles.The corresponding photoelectrochemical and ESR measurements were conducted for the catalyst samples.Fig.11(A)shows that the transient photocurrent response foris as much as 8 times and 20 times higher than that ofand TiO2,respectively,under intermittent visible light illumination[51].This is due to the fact that the addition of Rh is able to enhance the photocurrent significantly,which indicates a more efficient separation of the photoexcited charge carriers from Rh–TiO2under visible light irradiation[52].In addition,whenand TiO2are evaluated by electrochemical impedance spectroscopy(EIS),a significantly decreased EIS radius(Fig.11(B))is revealed forThis indicates that Rh can reduce electronic impedance and improve charge mobility,as a result of the optimized electronic band structure and interface/surface properties induced by the modification[53].
The active species in the MO photocatalytic degradation were detected using[=dimethyl pyridine N-oxide(DMPO)]spin-trapping ESR spectroscopy of the samples in aqueous suspensions under band gap irradiation(λ =365 nm for,and the results are shown in Fig.12(A).Four characteristic peaks of the ESR signal of DMPO-·OH adduct can be detected in theandaqueous suspensions under UV light irradiation[54].The formation of the˙OH radicals can be attributed to the reaction between photoinduced holes(h+)and H2O molecules.On the other hand,when methanol is introduced into the system,six characteristic peaks of the·adduct are clearly observed in the ESR spectra for TiO2and Rh–TiO2(Fig.12(B))[55].Moreover,the signal intensities of the DMPO-·OH andadducts in the ESR spectra forare strongerthan those for.These results indicate that thecatalyst has higher photocatalytic activity thanfor the decomposition of MO.Thus,Rh modification of thecatalyst cannot only enhance the surface acidity for ester synthesis,but can also increase the activity in the MO photocatalytic decomposition,which will be of great practical value in industrial applications.
4.Conclusions
In conclusion,a novel Rh modified sulfated TiO2composite catalyst was effectively synthesized using the photo-deposition method and fully characterized.The Rh-modified catalyst showed improved activity in a model esterification reaction and the decomposition of MO dye.This enhanced catalytic activity may be attributed to the increased surface acidity and larger specific surface areas as a result of Rh incorporation.In addition,the stronger surface acidity ofhelped promote the decomposition of MO in an aqueous solution under visible light irradiation.Photoelectrochemical and ESR measurements also confirm the higher electron transfer efficiency and the formation of ˙OH active species for thesample.The present work thus leads to a better understanding of solid acid catalysts and may guide new approaches towards enhancing catalytic activity.

Fig.11.Photoelectrochemicalproperties of samples at0.4 Vbias potential vs.Ag/AgClin a 0.2 mol·L-1 Na2SO4 aqueous solution(pH=6.8).(A)Periodic on/off photocurrent response under visible light irradiation(k>420 nm)and(B)electrochemical impedance spectroscopy plots in the dark.
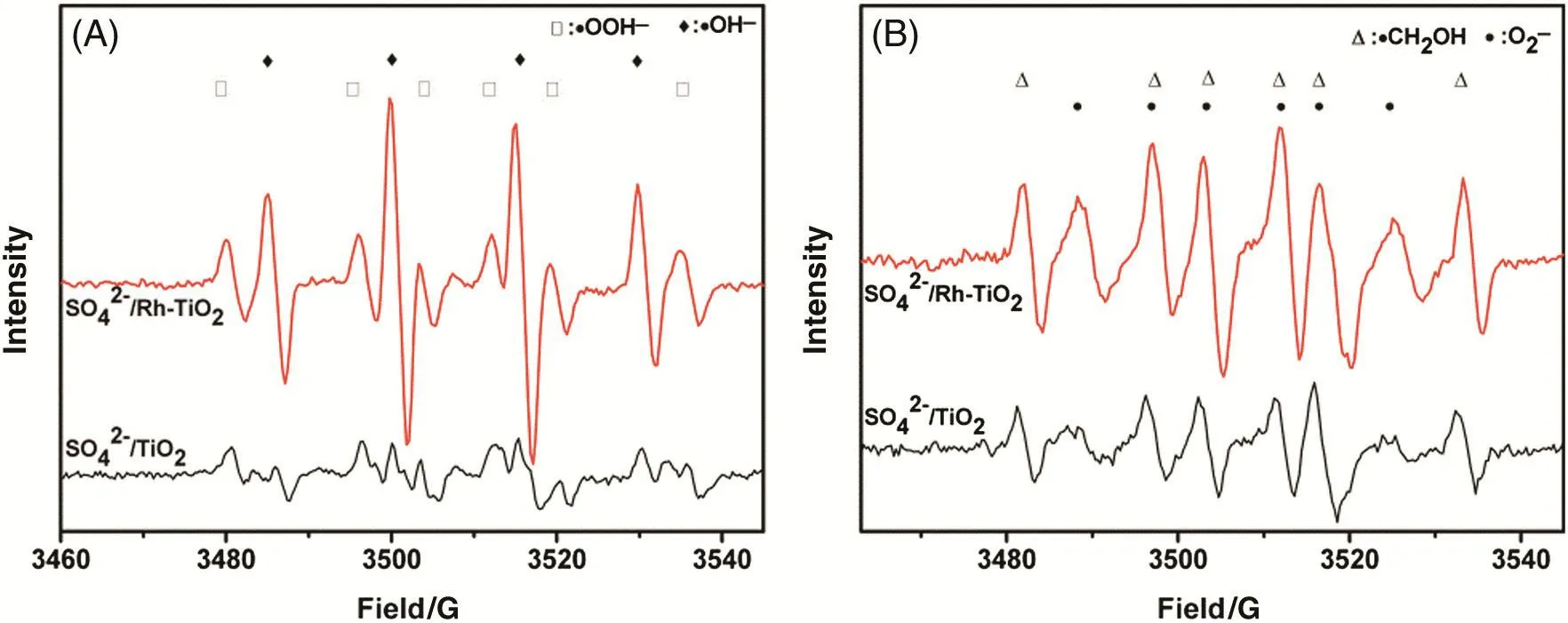
Fig.12.DMPO spin-trapping ESR spectra of samples recorded at ambient temperature:(A)in aqueous dispersion for DMPO-·OH and(B)in methanol dispersion for([DMPO]=0.05 mol·L-1,mass of samples=3 mg,volume of solvent=0.5 ml,wavelength of excitation=365 nm).
[1]A.Fujishima,X.Zhang,D.Tryk,TiO2photocatalysis and related surface phenomena,Surf.Sci.Rep.63(2008)515–582.
[2]Changlin Yu,Jimmy C.Yu,A simple way to prepare C–N-codoped TiO2photocatalyst with visible-light activity,Catal.Lett.129(2009)462–470.
[3]Michael Jean-Claude Nalbandian,Katherine E.Greenstein,Danmeng Shuai,Miluo Zhang,Yong-ho Choa,Gene F.Parkin,Nosang Vincent Myung,David M.Cwiertny,Tailored synthesis of photoactive TiO2nanofibers and Au/TiO2nanofiber composites:structure and reactivity optimization for water treatment applications,Environ.Sci.Technol.49(2015)1654–1663.
[4]Z.Zhang,C.C.Wang,R.Zakaria,J.Y.Ying,Role of particle size in nanocrystalline TiO2-based photocatalysts,J.Phys.Chem.B 102(1998)10871–10878.
[5]X.Li,H.Liu,L.Cheng,H.Tong,Photocatalytic oxidation using a new catalyst TiO2microsphere for water and wastewater treatment,Environ.Sci.Technol.37(2003)3989–3994.
[6]C.Yu,G.Li,S.Kumar,H.Kawasaki,R.Jin,Stable Au25(SR)18/TiO2composite nanostructure with enhanced visible light photocatalytic activity,J.Phys.Chem.Lett.4(2013)2847–2852.
[7]Changlin Yu,Qizhe Fan,Yu Xie,Jianchai Chen,shu Qing,Jimmy C.Yu,Sonochemical fabrication of novel square-shaped F doped TiO2nanocrystals with enhanced performance in photocatalytic degradation of phenol,J.Hazard.Mater.237-238(2012)38–45.
[8]Changlin Yu,Longfu Wei,Xin Li,Jianchai Chen,Qizhe Fan,Jimmy C.Yu,Synthesis and characterization of Ag/TiO2-B nanosquares with high photocatalytic activity under visible light irradiation,Mater.Sci.Eng.B 178(2013)344–348.
[9]Changlin Yu,Jimmy C.Yu,Wanqin Zhou,Kai Yang,WO3coupled P-TiO2photocatalysts with mesoporous structure,Catal.Lett.140(2010)172–183.
[10]S.Han,G.Zhang,H.Xi,D.Xu,X.Fu,X.Wang,Sulfated TiO2decontaminate 2-CEES and DMMP in vapor phase,Catal.Lett.122(2007)106–110.
[11]Pengfei Chen,Du.Mingxing,Lei He,Yan Wang,Guoliang Zhang,Fengbao Zhang,Xiaobin Fan,titania nanotubes as efficient solid superacid catalysts for selective mononitration of toluene,Catal.Commun.18(2012)47–50.
[12]H.Yan,Y.Yang,D.Tong,X.Xiang,C.Hu,Catalytic conversion of glucose to 5-hydroxymethylfurfural oversolid acid catalysts,Catal.Commun.10(2009)1558–1563.
[13]B.M.Reddy,V.R.Reddy,D.Giridhar,Eco-friendly Pt-Mo/ZrO2solid acid catalyst for selective protection of carbonyl compounds,Synth.Commun.31(12)(2006)1819–1823.
[14]V.Subramanian,E.E.Wolf,P.V.Kamat,Catalysis with TiO2/gold nanocomposites.Effect of metal particle size on the Fermi level equilibration,J.Am.Chem.Soc.126(2004)4943–4950.
[15]W.Zhaobin,X.Qin,G.Xiexian,E.Sham,P.Grange,B.Delmon,Titania-modified hydrodesulphurization catalysts:I.Effect of preparation techniques on morphology and properties of TiO2–Al2O3carrier,Appl.Catal.63(1990)305–317.
[16]Peng Sun,Ding Hua Yu,Yi Hu,Zhen Chen Tang,Jiao Jiao Xia,Heng Li,He Huang,H3PW12O40/SiO2for sorbitol dehydration to isosorbide:High efficient and reusable solid acid catalyst,Korean J.Chem.Eng.28(2011)99–105.
[17]J.R.Sohn,J.B.Park,H.W.Kim,Y.I.Pae,Infrared and raman characterization of V2O5on zirconia modified with WO3and activity for acid catalysis,Korean J.Chem.Eng.20(2003)48–57.
[18]R.Zanella,S.Giorgio,C.R.Henry,C.Louis,Alternative methods for the preparation of gold nanoparticles supported on TiO2,J.Phys.Chem.B 106(2002)7634–7642.
[19]J.Zou,J.Gao,Y.Wang,Synthesis of highly active H2O2-sensitized sulfated titania nanoparticles with a response to visible light,J.Photochem.Photobiol.A Chem.202(2009)128–135.
[20]T.Hirakawa,P.V.Kamat,Charge separation and catalytic activity of Ag@TiO2coreshell composite clusters under UV-irradiation,J.Am.Chem.Soc.127(2005)3928–3934.
[21]Dan I.Enache,Jennifer K.Edwards,Philip Landon,Benjamin Solsona-Espriu,Albert F.Carley,Andrew A.Herzing,Masashi Watanabe,Christopher J.Kiely,David W.Knight,Graham J.Hutchings,Solvent-free oxidation of primary alcohols to aldehydes using Au–Pd/TiO2catalysts,Science 311(2006)362–365.
[22]S.Tsubota,T.Nakamura,K.Tanaka,M.Haruta,Effect of calcination temperature on the catalytic activity of Au colloids mechanically mixed with TiO2powder for CO oxidation,Catal.Lett.56(1998)131–135.
[23]E.Formo,E.Lee,D.Campbell,Y.Xia,Functionalization of electrospun TiO2nano fibers with Pt nanoparticles and nanowires for catalytic applications,Nano Lett.8(2008)668–672.
[24]J.R.Sohn,D.G.Lee,Characterization of zirconium sulfate supported on TiO2and activity for acid catalysis,Korean J.Chem.Eng.20(2003)1030–1036.
[25]J.R.Sohn,J.H.Bae,Characterization of tungsten oxide supported on TiO2and activity for acid catalysis,Korean J.Chem.Eng.17(2000)86–92.
[26]M.Shyamsundar,S.Z.M.Shamshuddin,J.N.Sahu,Catalytic synthesis of biodiesel from pongamia glabra over zirconia and its modified forms,Korean J.Chem.Eng.30(2013)2186–2190.
[27]H.Song,P.F.Dong,X.Zhang,Effect of preparation conditions on the catalytic isomerization performance of,Acta Petrol.Sin.(Petroleum Processing Section)6(2010)877–882.
[28]A.Cornia,F.Melo,S.Mendioroz,J.Fierro,State of metals in the supported bimetallic Pt–Pd/system,12th International Congress on Catalysis:Proceedings of the 12th ICC,Granada,Spain,July 9–14,2000,Elsevier Science Ltd 2000,p.263.
[29]S.Tauster,S.Fung,R.Garten,Strong metal-support interactions.Group 8 noble metals supported on titanium dioxide,J.Am.Chem.Soc.100(1978)170–175.
[30]Z.S.Jin,X.M.Jiang,Y.F.Chen,A new technological development on the synthesis of propylene glycol ethers,Shanghai Chem.Ind.3(1997)6–11.
[31]Y.M.Cui,S.H.Fan,A study on the synthesis of amyl butyrate catalyzed by solid superacid,J.Funct.Mater.37(2006)452–455.
[32]S.Liu,J.H.Yang,J.H.Choy,Microporous SiO2–TiO2nanosols pillared montmorillonite for photocatalytic decomposition of methyl orange,J.Photochem.Photobiol.A Chem.179(2006)75–80.
[33]S.K.Kansal,M.Singh,D.Sud,Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts,J.Hazard.Mater.141(2007)581–590.
[34]Changlin Yu,Jimmy C.Yu,Mui Chan,Sonochemical fabrication of fluorinated mesoporous titanium dioxide microspheres,J.Solid State Chem.182(2009)1061–1069.[35]Zhonglai Li,Renata Wnetrzak,Witold Kwapinski,James J.Leahy,Synthesis and characterization of sulfated TiO2nanorods and ZrO2/TiO2nanocomposites for the esterification of biobased organic acid,ACS Appl.Mater.Interfaces 4(2012)4499–4505.
[36]Hui Zhao,Pingping Jiang,Yuming Dong,Min Huang,Boliang Liu,Effects of morphology and crystal phase of sulfated nano-titania solid acids on catalytic esterification,React.Kinet.Mech.Catal.113(2014)445–458.
[37]Pradeepan Periyat,Suresh C.Pillai,Declan E.McCormack,John Colreavy,Steven J.Hinder,Improved high-temperature stability and sun-light-driven photocatalytic activity of sulfur-doped anatase TiO2,J.Phys.Chem.C 112(2008)7644–7652.
[38]Darrin S Muggli,LefeiDing,Photocatalytic performance of sulfated TiO2and Degussa P-25 TiO2during oxidation of organics,Appl.Catal.B Environ.32(3)(2001)181–194.[39]S.M.Jung,P.Grange,Characterization and reactivity of pureSCR catalyst:influence ofcontent,Catal.Today 59(2000)305–312.
[40]Hexing Li,Xinyu Zhang,Yuning Huo,Jian Zhu,Supercritical preparation of a highly active S-doped TiO2photocatalyst for methylene blue mineralization,Environ.Sci.Technol.41(2007)4410–4414.
[41]X.P.Lin,D.M.Song,X.Q.Gu,Y.L.Zhao,Y.H.Qiang,Synthesis of hollow spherical TiO2for dye-sensitized solar cells with enhanced performance,Appl.Surf.Sci.(2012)816–820.
[42]Y.V.Larichev,O.V.Netskina,O.V.Komova,V.I.Simagina,Comparative XPS study of Rh/Al2O3and Rh/TiO2as catalysts for NaBH4hydrolysis,Int.J.Hydrog.Energy 35(2010)6501–6507.
[43]Y.Wu,L.Qin,G.Zhang,L.Chen,X.Guo,M.Liu,Porous solid superacidFenton catalyst for highly effective oxidation of X-3B under visible light,Ind.Eng.Chem.Res.52(2013)16698–16708.
[44]Y.H.Xu,L.Y.Wang,Q.Zhang,S.J.Zheng,X.J.Li,C.Huang,Correlation between photoreactivity and photophysics of sulfated TiO2photocatalyst,Mater.Chem.Phys.92(2005)470–474.
[45]T.Yamaguchi,Recent progress in solid superacid,Appl.Catal.6(1990)11–25.
[46]Y.M.Wang,C.Z.Chen,Z.H.Luo,D.S.Gao,D.Li,M.X.Wu,J.B.Ma,Studies on the acidity,structures and crystalline phases ofsolid acids,Chin.J.Struct.Chem.18(1999)175–181.
[47]Z.S.Jin,W.D.Zhang,L.Zhang,Y.F.Chen,Synthesis of propylene glycol methyl ether acetate,Fine Chem.18(2001)376–378.
[48]Pradeepan Periyat,Declan E.McCormack,Steven J.Hinder,Suresh C.Pillai,One-pot synthesis of anionic(Nitrogen)and cationic(Sulfur)codoped high-temperature stable,visible light active,anatase photocatalysts,J.Phys.Chem.C 113(8)(2009)3246–3253.
[49]B.Neppolian,H.Choi,S.Sakthivel,B.Arabindoo,V.Murugesan,Solar light induced and TiO2assisted degradation of textile dye reactive blue 4,Chemosphere 46(2002)1173–1181.
[50]M.R.Rojas,F.Pérez,D.Whitley,R.G.Arnold,A.E.Sáez,Modeling of advanced oxidation of trace organic contaminants by hydrogen peroxide photolysis and Fenton's reaction,Ind.Eng.Chem.Res.49(2010)11331–11343.
[51]G.Zhang,M.Zhang,X.Ye,X.Qiu,S.Lin,X.Wang,Iodine modified carbon nitride semiconductors as visible light photocatalysts for hydrogen evolution,Adv.Mater.26(2014)805–809.
[52]N.Zhang,M.-Q.Yang,Z.-R.Tang,Y.-J.Xu,CdS–graphene nanocomposites as visible light photocatalyst for redox reactions in water:A green route for selective transformation and environmental remediation,J.Catal.303(2013)60–69.
[53]G.Zhang,X.Wang,A facile synthesis of covalent carbon nitride photocatalysts by Co-polymerization of urea and phenylurea for hydrogen evolution,J.Catal.307(2013)246–253.
[54]W.Wu,L.Wen,L.Shen,R.Liang,R.Yuan,L.Wu,A new insight into the photocatalytic reduction of 4-nitroaniline to p-phenylenediamine in the presence of alcohols,Appl.Catal.B Environ.130–131(2013)163–167.
[55]W.Adam,M.A.Arnold,M.Grüne,W.M.Nau,U.Pischel,C.R.Saha-Möller,Spiroiminodihydantoin is a major product in the photooxidation of2′-deoxyguanosine by the triplet states and oxyl radicals generated from hydroxyacetophenone photolysis and dioxetane thermolysis,Org.Lett.5(2002)725–728.
 Chinese Journal of Chemical Engineering2016年6期
Chinese Journal of Chemical Engineering2016年6期
- Chinese Journal of Chemical Engineering的其它文章
- Mixture temperature prediction of waxy oil–water two-phase system flowing near wax appearance temperature☆
- The turbulent behavior of novel free triple-impinging jets with large jet spacing by means of particle image velocimetry☆
- Online process monitoring for complex systems with dynamic weighted principal component analysis☆
- A stepwise optimal design of water network☆
- Integration of coal pyrolysis process with iron ore reduction:Reduction behaviors of iron ore with benzene-containing coal pyrolysis gas as a reducing agent☆
- Multiple linear equation of pore structure and coal–oxygen diffusion on low temperature oxidation process of lignite☆
