Evolvement behavior of microstructure and H2O adsorption of lignite pyrolysis☆
Yingyue Teng,Shijun Lian,Quansheng Liu*,Yuzhe Liu,Yinmin Song,Runxia He,Keduan Zhi
College of Chemical Engineering,Inner Mongolia University of Technology,Inner Mongolia Key Laboratory of Industrial Catalysis,Hohhot 010051,China
1.Introduction
Lignite is characterized by low heat value but high transportation cost[1],which limits its wide application;these characteristics are attributed to its high porosity(similar to plastic material)and high moisture content(25%–60%).Heat treatment is one of the effective solutions to the limitations of lignite.Pyrolysis can reduce the moisture content and spontaneous combustion tendency of lignite,but increase the heat value.However,pores and cracks are abundant on the surface of heat-treated lignite,which re-absorb moisture in wet environment.Such re-absorption lowers the heat value of lignite and may increase spontaneous combustion[2].The pore specific surface area of lignite expands as temperature rises,accompanied with the production of microcracks in low-temperature phase[3,4].Numerous open holes within the lignite are developed after low-temperature dehydration,and the number of mesopore reaches the maximum at 140°C.Re-absorption is significantly influenced by specific surface area[5,6].–OH and C=O are the main oxygen-containing functional groups that influence reabsorption and have different moisture absorption performances[7–9].Chemical property changes of low-rank coal are mainly caused by damages in coal surface and oxygen-containing functional groups during drying[10].After absorbing a certain amount of moisture,low-rank coal becomes less sensitive to low-temperature oxidation and spontaneous combustion[11].Porous structure and oxygen-containing functional group are related to coal hydrophilicity[12].Existing state of moisture can be determined through diffuse reflectance infrared spectroscopy and low field nuclear magnetic resonance(1H NMR)[13–15].
Among the study methods of pore diameter and specific surface area,mercury porosimetry and gas adsorption require sample pre-processing,which destroys the cellular structure of samples,thereby making it difficult to determine the existing state of moisture.Nevertheless,a few investigations have been conducted on the porous structure and existing state of moisture in heat-treated lignite by1H NMR technology.This technology is a non-invasive measurement for moisture content and existing state in coal samples.Most existing studies focus on lowtemperature and middle-temperature zones(<600 °C),and only a few discuss coal treatment at higher temperature.Moreover,no uniform adsorption mechanism has been developed yet.A systematic comprehension on porous structure,functional group changes,and the moisture adsorption mechanism of lignite could be obtained through hightemperature heat treatment.Basing on previous research[16,17],this study conducted a systematic survey on porous structure and moisture adsorption characteristics of heat-treated lignite(<950 °C)by using1H NMR.Relationships of moisture adsorption with porous structure and functional groups,as well as the moisture adsorption pattern,were investigated.Moisture adsorption mechanism was further analyzed.Research results could provide theoretical bases for industrial use of lignite.
2.Materials and Methods
2.1.Coal sample
Lignite samples were collected from Shengli 2#Mine in Xilinguole League in Inner Mongolia,China.The samples were crushed into 2–4 mm and 0.037–0.075 mm particles.An industrial analyzer(5E-MAG6700),an element analyzer(5E-CHN2000),and an infrared sulfur meter(5E-IRS II)that were manufactured by Changsha Kaiyuan Company were employed for industrial and element analyses.Results are listed in Table 1.

Table 1 Proximate and ultimate analyses of trial coal samples
2.2.Heat treatment
Heat treatment of lignite was conducted in a drying oven(5EMIN6150)and then in a fixed-bed reactor(Fig.1)under N2atmosphere.
Drying temperatures were set at 25 °C,50 °C,75 °C,and 105 °C,and pyrolysis temperatures were set at 200 °C,300 °C,500 °C,700 °C,and 950 °C.Coal samples(8–10 g),with a particle size of 2–4 mm,were placed into a drying oven ora fixed-bed reactor.The rate of temperature was 25°C min-1,and the maximum temperature was maintained for 1 h.Heat-treated samples were called SLH.The samples were stored in a sealed bag and then placed in a drying vessel.
Thermogravimetric(TG)curve was analyzed with a Diamond TG/DTA 6300 thermogravimetric analyzer(Japan).Coal samples(15–20 mg),with a particle size of 0.0374–0.0750 mm,were placed into the crucible of a TG analyzer.Air in the instrument was replaced with carrier gas(argon)prior to the experiment.Temperature program was initialized as soon as the flow rate achieved stabilization.The in flow rate of carrier gas was 100 ml·min-1and the temperature increased from room temperature to 950 °C at a rate of 25 °C min-1.
When carbocoal was prepared in a fixed bed reactor,gas chromatography was performed with a TCD detector(Beijing Analyzer Plant)to analyze H2,CO2,CO,and CH4in pyrolysis products.The separation column was filled with TDX02.The column box and TCD temperatures were 160 °C and 180 °C,respectively.The carrier gas was Ar,with a flow rate of 34 ml·min-1.Sampling interval was 5 min.
2.3.Pore structure and existing state of water
The principle of1H NMR[18]shows that the chemical environment of the proton and the transverse relaxation time(T2i)are different.Shorter T2results in smaller degree of freedom(DOF)of protons,whereas longer T2iresults in greater DOF of protons.More products are likely to be released with a greater degree of binding of moisture in the sample.T2can reveal the occurrence state of moisture content in coal.Inversion of T2ispectrum in different waves represents the water state,and wave covered amplitude value represents the relative content of the moisture state;thus,samples after immersion in water by moisture measuring the relative amount of known pore distribution.Studies show that[14],due to the low field conditions,even if coal is present in trace amounts along the magnetic minerals nor to the measuring result caused by,the coal in solid-state13C nuclei and1H nuclear signal will be blocked,will not affect the detection results.
1H NMR(VTMR20-010V-T;Shanghai Niumag)was performed to test the water content and porous structure.Testing parameters include the following:resonance frequency,21.306 MHz;magnetic intensity,0.5 T;coil diameter,10 mm;and magnetic temperature,35.00°C.Sample signal values were collected using nuclear magnetic resonance analysis software and CPMG sequence.On this basis,T2ispectrum was obtained through inversion with SIRT algorithm.Coal samples(1–1.25 g)were placed into a detector oven.The detection limit of water was 10 mg.
2.4.Moisture adsorption
The moisture adsorption experiment was performed in the SHBY-40B standard curing box(Jiangsu Wuxi Southern China Experimental Instrument Inc.).SLH were paved on a 35 mm culture dish,which was then placed into a temperature-constant and humidity-constant box.Temperature was set at 30°C.The relative humidity(RH)was set at 85%and 95%.The first 6 h was divided into three phases,with 2 h for each phase.The interval time between phases was 30 min.Samples were weighed every 1 h or 2 h,and then every 12 h.Equilibrium moisture adsorption is reached if the difference between two adjacent weights is smaller than 0.01 g.The corresponding adsorption ratio is viewed as the balance adsorption ratio.Adsorption ratio is calculated as follows:

where m0is the mass of lignite sample and mtis the mass of lignite sample after water absorption.
2.5.Fourier transform infrared spectroscopy(FTIR)analysis
Oxygen-containing functional groups were tested using an FTIR infrared spectrometer(NEXUS670;American Nicolet Corporation).The KBr method was performed to prepare the samples.The samples and KBr were mixed at a ratio of 1:120.Infrared spectrum was obtained within the range of 400–4000 cm-1,and scanning time was set at 25 s.The resolving power and wavenumber accuracy were 0.125 and 0.001 cm-1,respectively.

Fig.1.Flow charts of a fixed-bed reactor.
3.Results and Discussions
3.1.Microstructural changes
3.1.1.Porous structure
Based on the principle of1H NMR technology[14,18],relaxation time is related to pore size to a certain extent[16,19,20].Let SLH adsorb sufficient moisture and maintain saturated at 25°C for 24 h[18].Pore diameter distribution could be determined based on T2idata(Fig.2)[16].Table 2 shows pore diameter distribution and amplitude intensity of T2i.Fig.3 shows the amplitude intensity curve of T2iof heat-treated lignite samples under different temperatures.IUPAC[16]defines pores with sizes <2 nm,2–50 nm,and 50 nm as micropore,mesoporous,and macropore,respectively.In raw coals(Fig.2(a)),hole sizes<2 nm are rare,and pore distribution>100 nm is complex.The IUPAC classification of aperture is not sufficient to reveal the distribution characteristics of lignite hole.Therefore,the apertures in Shengli lignite are divided into small pores(<20 nm),large pores(20–300 nm),and fracture(>300 nm),based on literature[16].The increase in heat-treatment temperature results in the initial increase and then decrease in water storage capacity of lignite samples,showing small fluctuations at 700°C and 950 °C.In raw lignite samples,93.06%had large(20–300 nm),accompanied with 2.75%small pores and 3.65%cracks(Fig.2).
Figs.2,3 and Table 2 show the changes of the hole,which can be divided into 3 stages.In the first stage(≤105 °C),the increase of total pore volume is mainly due to the increase in fracture(>300 nm);the number of small pores(<20 nm)and large pores(20–300 nm)decreased,but the data was abnormal at drying temperature 50°C,which may be due to the complex process of water escape.In the second stage(200–500 °C),the decrease of the total amount of pore volume mainly depends on the reduction of the large hole,and a large number of small pores(<20 nm)generated in this stage.In the third stage(700–950°C),the increase of total pore volume is mainly due to the increase in fracture(>300 nm),and the small pores(<20 nm)disappeared in this stage.
Analysis of Figs.4 and 5 shows that lignite samples heat treated in 25 °C–105 °C were only dehydrated and maintained the same chemical structure,but did not generate new materials[21,22].Therefore,abovementioned changes could be attributed to escaped moisture,which caused internal stress to lignite,thereby large pores deformed into cracks and small pores developed into large pores.
Lignite samples heat treated in 200–500 °C changed more greatly than those at105°C.Crack development continued.Proportion of cracks in lignite samples treated at 200°C even reached as high as 56.03%.Meanwhile,few micro-pores were developed,but small and large pores reduced gradually.Polarization of pore diameter distribution occurred.Given that this phenomenon belongs to the active thermal decomposition phase of lignite(Fig.4),degasification reaction occurred.Degasification mainly eliminated closed methane,CO2,and N2that are adsorbed on lignite and internal holes(Fig.5).Several new holes weredeveloped as degasification proceeded.Moreover,breakage of several branched chains produces gas products that cause micro-pores[23].Holes of lignite treated at 300 °C and 500 °C were fewer than those at 200 °C because holes developed at 200 °C cracked and pulverized.As a result,the total pore volume declined.

Table 2 Pore size distribution and amplitude of T2i spectrum of SLH

Fig.3.Amplitude of T2i spectrum of SLH.

Fig.4.TG and DTG curves during lignite pyrolysis.
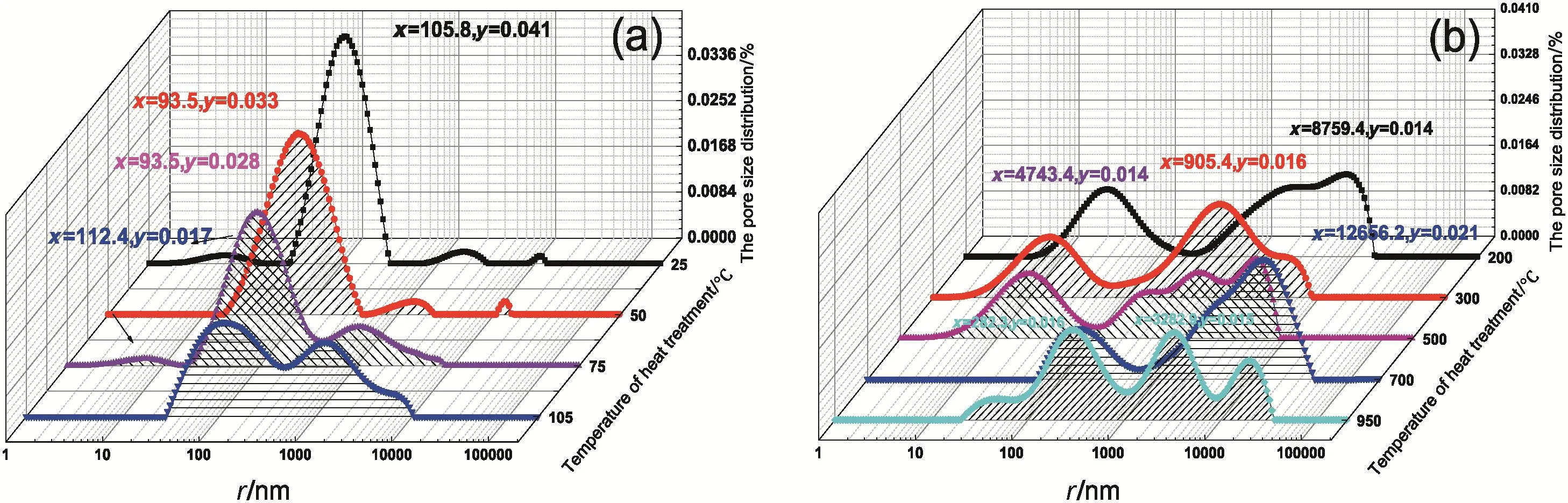
Fig.2.T2i distribution of moisture and pore of SLH.(a)25–105 °C(b)200–950 °C.

Fig.5.Cumulative amount of lignite pyrolysis gas.
When lignite samples were treated at 700°C,micro-pores disappeared and proportion of small and large ones decreased dramatically compared with that of previous phase.Cracks accounted for the largest proportion,reaching 75.97%of total pore diameter.Combined with previous studies,Figs.4 and 5 present that polycondensation occurred in lignite at 700°C.Micro-pores closed,and the number of large and small pores decreased because of volume shrinkage and production of abundant coal tars that filled the micro-pores and some large and small pores.Meanwhile,polycondensation at 700°C generated tremendous H2and CO(Fig.5).Similar to dehydration,abundant gas would cause numerous cracks in lignite,increasing pore volume compared with that at 500°C.

Fig.6.FTIR spectra of SLH.
When lignite samples were treated at 950°C,no micro-pores were observed and the number of cracks decreased,but the amount of small and large pores doubled compared with that at 700°C.Lignite sample would continue to produce more gases at 950°C;these gases,together with polycondensation,destroyed cracks that were developed at 700°C continuously.Hence,the pore volume of lignite samples at 950 °C is lower than that at 700 °C.
3.1.2.Oxygen-containing functional groups
In Fig.6,the FTIR spectra of lignite samples treated under different temperatures exhibit the same shape but show different intensities of absorption peak.The intensity of the-OH absorption peak at3400 cm-1declined significantly as temperature rose.Meanwhile,the spectrum ranged from 1000 cm-1–1800 cm-1.Tahmasebi et al.[24]defined spectrum from 1500 cm-1to 1800 cm-1as oxygen-containing functional groups.
In this study,the spectrum was further divided:the peak at 1700 cm-1is the stretching vibration peak of aromatic C=O,and the peak at 1600 cm-1is the stretching vibration peak of aromatic C=C.These two peaks overlap in Fig.6.The peak at 1383 cm-1is the vibration peak of-CH2/-CH3,and the peak at 1110 cm-1is the stretching vibration peak of–O–[25,26].
The integral area(Fig.7)was acquired through fitting integration to spectra 3000–3700 cm-1(-OH),1550–1800 cm-1(C=O),1300–1450 cm-1(-CH2/-CH3),and 1100–1200 cm-1(-O-)of the FTIR by using the curve fitting method(Fig.8).
The effect of heat treatment temperature on four functional groups can be analyzed based on Fig.7.–OH and C=O slightly changed and-O-increased before 105°C.This finding is mainly due to the small effect of-O-on moisture molecules[7].As temperature rises,adsorbed moisture on-O-is first eliminated to expose-O-,but the relative content of-O-is extremely small.-CH2/-CH3change is relatively complicated,which may be related to the existence of moisture.
After 105 °C,relative contents of–OH and C=O gradually reduced.Lignite samples began to decompose and depolymerize and produce a series of reactions.-OH and C=O are removed as H2O,CO2,and CO(Figs.4 and 5).The relative content of C=O reduced to 0 at500°C,indicating that C=O has been decomposed completely.About 30%of–OH remained in lignite samples at950°C.The overall relative content of-O-presents a reduction trend,but it increases at300 °C and 950 °C.This is mainly because the condensation reaction of hydroxy(-OH+-OH→-O-+H2O)in lignite samples at300°C produces some–O–,but they break as temperature rises continuously[9].Pyrolysis at950°C belongs to polycondensation reaction,which is a condensation of aromatic rings.Ether bond is the significant connection point of condensed aromatic rings,which produces new materials with-O-.The relative content of-CH2/-CH3begins to decrease at 200 °C and reaches the lowest at 500 °C.Small increases are observed at 700 °C and 950 °C.

Fig.7.Absorption peak area of–OH,C=O,-CH2/-CH3,and–O– of SLH.

Fig.8.Functional groups–OH,C=O,-CH2/-CH3,and–O–peak differentiation and imitation of SLH.
During heat treatment of Shengli lignite,production and escape of new materials change the porous structure and functional groups of residual matters,thus leading to different moisture adsorption characteristics.
3.2.Moisture adsorption characteristics
3.2.1.Adsorption capacity
The moisture adsorption and balance adsorption ratio of SLHat30°C and 85%humidity are presented in Fig.9.In the first 6 h,the adsorption ratio of heat-treated lignite quickly increased,reaching about 50%of the balance adsorption ratio.It increased continuously to approximately 80%after 1 day and reached the balance adsorption ratio after 5 days.Fig.10 shows balance adsorption ratio curves under two different RH(RH=85%and RH=95%).The continuous increase in temperature resultsin an“N-shaped”variation trend of balance adsorption ratio.When RH is 85%,the balance adsorption ratio gradually increases as temperature increases from 25 °C to 105 °C.The ratio increases from 11.97%at 25 °C to 22.8%at 105 °C.Moisture adsorption ratio decreases to 12.66%from 105 °C to 700 °C but increases again to 22.82%at 950 °C.
The moisture adsorption ratio of lignite that was pyrolyzed at different temperatures has a strong law(Fig.10).Before 105°C,the moisture adsorption ratio increased with increasing drying temperature.This result is mainly due to the higher temperature;original water absorbent functional groups are exposed with more discharged water,resulting in higher moisture absorption ratio.In 105 °C–700 °C,the oxygen containing functional groups decreased with increasing temperature(Fig.7);the oxygen is released in the form of pyrolysis products(Fig.5),which are less functional groups and absorb less water.In 700 °C–950 °C,C=O is decomposed completely,and 30% –OH is still present in lignite samples at 950 °C.Small increases are observed in –CH2/-CH3and –O– at 700 °C and 950 °C,but the increase in water adsorption ratio is due to the increase in cracks(Fig.2),changes in adsorption state,and decreases in adsorption capacity with extended relaxation(Fig.11).

Fig.9.Moisture adsorption plot of SLH(a)25–105 °C(b)200–950 °C.
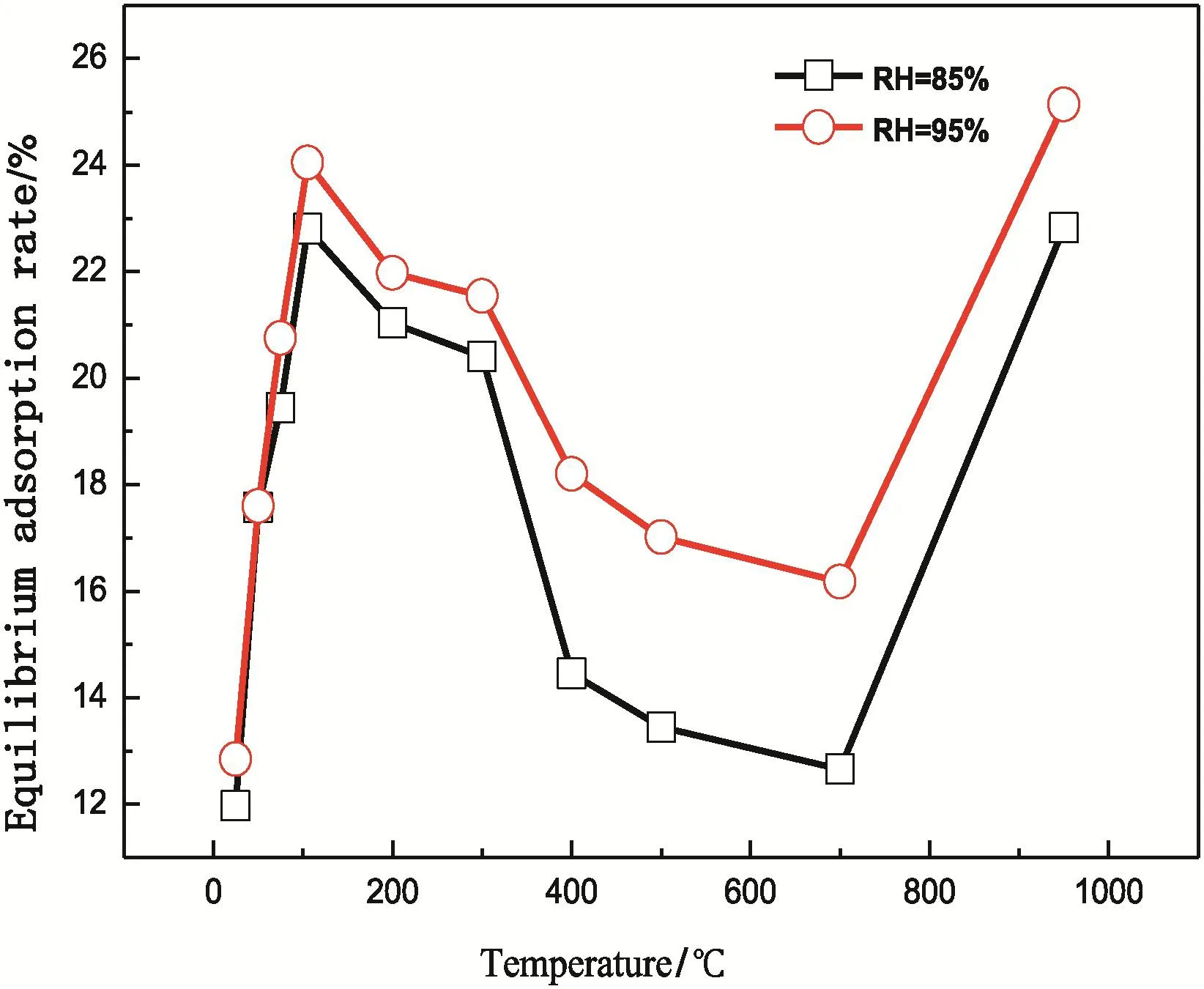
Fig.10.Equilibrium adsorption ratio plot of the SLH in different RH.
At the same temperature,the balance adsorption ratio under 95%RH is higher than that under 85%RH.A small difference(0.1%–0.8%)is observed in low-temperature phase,but it increases in middletemperature phase(3.56%at 500°C)and high-temperature phase(3.52%at 700°C).Higher RH results in larger balance adsorption ratio of heat-treated lignite samples.Humidity influences adsorption more significantly under high heat treatment temperature compared with low heat treatment temperature.This is because given fixed other conditions,higher humidity leads to stronger pressure and stronger physical adsorption,thereby resulting in large difference of balance adsorption ratio[27].
3.2.2.Effect of pore volume on adsorption ratio

Fig.11.T2i distribution of moisture balance adsorption of the SLH(RH=85%).(a)25–105 °C(b)200–950 °C.
Comparison of Figs.3 and 10 shows that semaphore of pore volume conforms well to the balance adsorption ratio of lignite samples before the first 500°C.Both volumes increase firstly and then decrease as pyrolysis temperature increases and reaches the maximum at 105°C.In particular,abnormalities of both semaphore of pore volume and the balance adsorption ratio were observed at 300°C.However,such consistency disappears after 500°C.Semaphore of pore volume increases at 700 °C and decreases at 950 °C,whereas the balance adsorption ratio,compared with that at 500 °C,still decreases at 700 °C and increases at 950 °C.This finding may be the consequence of–O– growth in oxygen-containing functional groups.
3.2.3.Effect of oxygen-containing functional groups on balance adsorption ratio
When T<105°C,lignite samples still possesses high moisture and maintain the same chemical structures[28](Fig.7).Investigating functionalgroups in this phase is meaningless.The linear fitting of functional groups through the balance adsorption ratio of heat-treated lignite samples after 150°C is shown in Fig.12.
The balance adsorption ratio of treated lignite samples under different temperatures has a linear relationship with relative contents of–OH,C=O,and–O–(except950°C).–OH and C=O have possess polarity and higher interaction activity with H in water molecules than–O–[7].Therefore,they show slightly higher degree of fitness than–O–.As temperature increases,the relative content of oxygen-containing functional groups reduces and the balance adsorption ratio declines accordingly,especially at 500°C.The correlation between the adsorption peak area of-CH2/-CH3and the balance adsorption ratio is not so significant,this is due to-CH2/-CH3is hydrophobic effect[29].In a word,oxygencontaining functional groups are the main influencing factor of balance adsorption ratio in this phase(200–700°C).In 700°C–950 °C,the adsorption state changed and the adsorption capacity decreased with the relaxation extended(Fig.11).The correlation between the equilibrium adsorption ratio and the oxygen containing functional groups is weak;thus,point of 950°C is out of the linear correlation.
3.2.4.Existing state of H2O
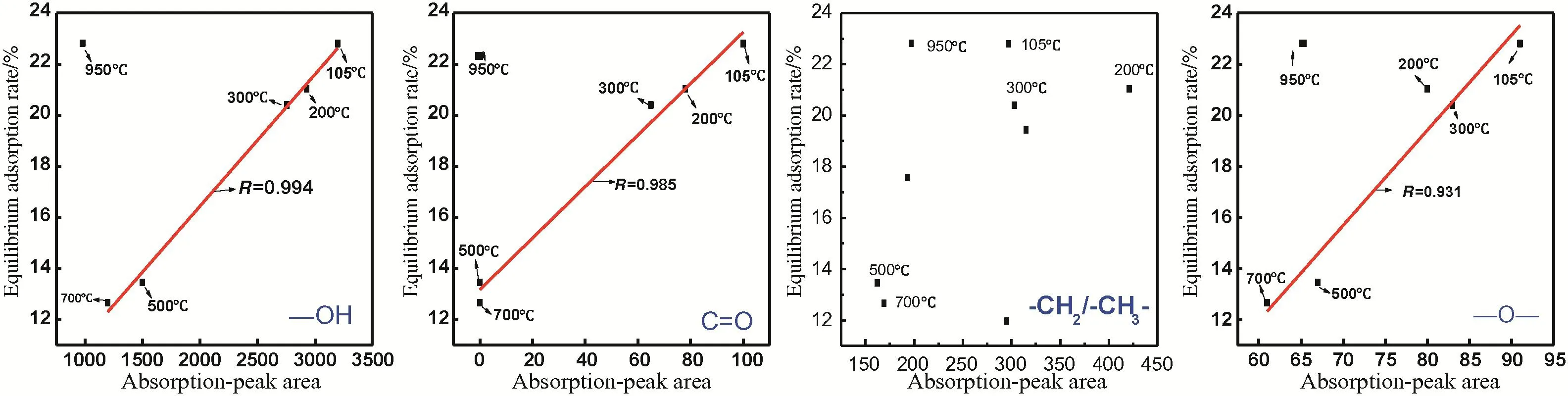
Fig.12.Relation between equilibrium adsorption ratio and oxygen-containing groups in SLH(RH=85%).

Table 3 Amplitude and distribution of T2i spectra of moisture adsorption of SLH

Fig.13.Amplitude of T2i spectra for moisture balance adsorption of SLH(RH=85%).
T2icharacteristic spectrum of adsorbed moisture by heat-treated lignite is displayed in Fig.11,and T2iamplitudes of adsorbed moisture are listed in Table 3.Adsorbed moisture by lignite samples treated under different temperatures exists in different states.When T < 500 °C,relaxation time(T2i)moves leftward,implying that it shortens gradually with increasing temperature.T2ireveals DOF of protons,and the leftward movement of T2iindicates that the interaction between lignite samples and water molecules intensifies gradually.T2iat 700 °C and 950 °C moves rightward[14],indicating that the binding force of lignite samples to the adsorbed moisture is weakened.As shown in Table 3 and Fig.13,the total moisture content(Atotal)reduces when temperature increases from 25 °C to 105 °C.Combined with the results presented in Fig.10,moisture adsorption ratio is speculated to increase.Based on the principle of1H NMR,lignite contains high moisture before 105°C.As temperature rises,moisture is lost more quickly than adsorption,which explains the decreasing total semaphore of moisture measured by1H-NMR.The original moisture in lignite samples before 105°C exists in different states.Some directly connect with the coal surface(monolayer moisture),and others connect with monolayer moisture(multilayer moisture)[17].Monolayer water molecules provide strong bonding,whereas multilayer water molecules provide weak bonding.Furthermore,the presence of multilayer water molecules weakens the bonding strength between the monolayer water molecules and coal surface.During heat treatment,multilayer moisture with poor bonding strength will spill over first.The preserved water molecules either adhere onto coalsurface strongly or interact with enhanced coal surface[16].Higher heat treatment temperature results in stronger bonding of preserved water molecules and shorter relaxation time(T2i).This phenomenon is reflected by the leftward movement of T2ibefore 105°C(Fig.11).
From 105 °C to 500 °C,lignite is dewatered nearly completely.Adsorbed moisture connects with oxygen-containing functional groups on coalsurface directly and occupies positions with strong hydrophilicity,forming hydrogen bonds with high bond energies[30].Moreover,oxygen-containing functional groups of lignite samples are exposed.With the increase in heat treatment temperature,oxygen-containing functional groups reduce,such as–OH,C=O,and–O–(Fig.7),resulting in decreased moisture adsorption(Fig.13).Meanwhile,oxygencontaining functional groups with weak bonding force are removed first and the rest strongly bond with skeleton.Such bonding force is strong once moisture is adsorbed,thereby causing the continuous leftward movement of T2i(Fig.11).
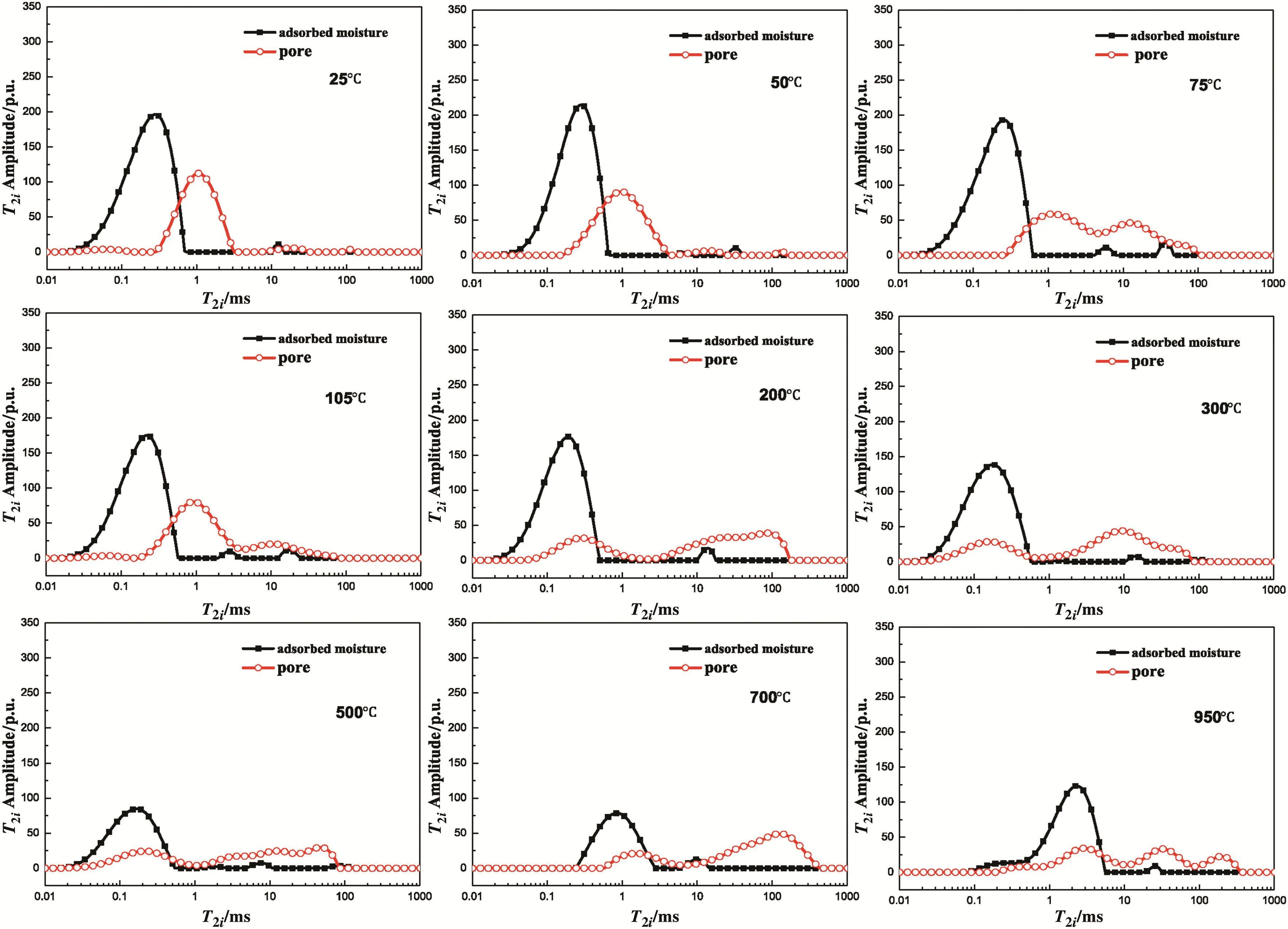
Fig.14.Distribution of pore and moisture in SLH.
Most undecomposed oxygen-containing functional groups are stable hydroxy and carbonylon the aromatic ring,which are less hydrophilic than free hydroxy and carbonyl groups[8].At this moment,the bonding force of adsorbed water molecules is far weaker than that before 700 °C.T2imoves rightward accordingly.At 950 °C,condensation reaction is initiated in lignite samples[26],producing plenty of aromatic ring compounds(C13).Coal rank of lignite is close to that of soft coal.Relative content of hydrophilic group in lignite samples is smaller.Given the constant saturated vapor pressure,multilayer moisture may be produced,which weakens the bonding force.Therefore,T2imoves rightward compared with that at 700°C.As shown in Figs.2 and 11,a small peak is observed at T2i=0.24 ms under 950°C,which indicates the strengthened bonding force of some water molecules.This finding is caused by the increase of relative content of ether bond at 950°C.
Remarkable difference between pore diameter distribution(Fig.2)and moisture adsorption distribution(Fig.11)of heat-treated lignite samples was discovered.This difference is attributed to porous structure and moisture content,which are measured according to transverse T2iof protons in1H NMR.However,the existing state of moisture in porous structure analysis is different from that in moisture adsorption analysis(Sections 2.3 and 2.4).Porous structure analysis requires immersion of lignite samples in moisture for 24 h until all pore spaces are filled with moisture(multilayer moisture),but moisture adsorption analysis only focuses on moisture adsorbed on the coal surface(monolayer moisture).Therefore,the bonding force between water molecules and coal surface in the two tests is different.Monolayer moisture possesses stronger bonding force,thus resulting in the rightward movement of transverse T2i(Fig.14).
4.Conclusions
Microstructure and moisture adsorption characteristics of lignite during heat treatment are investigated using1H NMR technology.
The pore diameter of raw lignite is generally about 100 nm.The increase in heat treatment temperature initially reduces the small and large pores,intensifying polarization of pore diameter.In high temperature,pore diameter tends to increase.The transition intervals lie in 105–200 °C and 500–700 °C,within which pore volume is relatively large.During the entire heat treatment,the highest pore volume of treated lignite samples is achieved at 105°C.With the increase of heat treatment temperature,the balance adsorption ratio of heat-treated lignite shows an “N-shaped”variation law.When T< 700 °C,pore volume and balance adsorption ratio are in good accordance.From 105°C to 700°C,oxygen-containing functional groups are highly correlated with the balance adsorption ratio.In 700–950 °C,the increase in water adsorption ratio is due to the increased cracks,changes in adsorption state,and decreased adsorption capacity with extended relaxation.Moisture adsorption by treating lignite samples under different temperature exists in different states.Before 500°C,T2imoves leftward as temperature rises,indicating the gradually enhanced interaction between lignite samples and water molecules.T2iat 700 °C and 950 °C moves rightward,implying that the bonding force of lignite samples to adsorbed moisture is weakening.
Acknowledgments
We greatly benefited from Shanghai Niumag Electronic Technology Co.,Ltd.
[1]C.Li,Advances in the science of Victorian brown coal,Chemical Industry Press,Beijing,2009 65–75.
[2]Muthusamy Karthikeyan,Wu Zhonghua,Arun S.Mujumdar,Low-Rank Coal Drying Technologies-Current Status and New Developments,Dry.Technol.27(3)(2009)403–415.
[3]Y.Yu,W.Liang,Y.Hu,Q.Meng,Study of micro-pores development in lean coal with temperature,Int.J.Rock Mech.Min.51(4)(2012)91–96.
[4]C.E.Salmas,A.H.Tsetsekou,K.S.Hatzilyberis,G.P.Androutsopoulos,Evolution lignite mesopore structure during drying,effect of temperature and heating time,Dry.Technol.19(1)(2001)35–64.
[5]Y.Yang,X.Jing,Z.Li,X.Liu,Y.Zhang,L.Chang,Effect of drying conditions on moisture re-adsorption performance of dewatered lignite,Dry.Technol.31(12)(2013)1430–1437.
[6]G.Zhou,J.Wu,Z.Miao,X.Hu,X.Li,X.Xin Shi,Z.Cai,Y.Shang,Effects of process parameters on pore structure of semi-coke prepared by solid heat carrier with dry distillation,Int.J.Min.Sci.Technol.23(3)(2013)423–427.
[7]M.Sakaguchi,K.Laursen,H.Nakagawa,K.Miura,Hydrothermal upgrading of Loy Yang brown coal-effect of upgrading conditions on the characteristics of the products,Fuel Process.Technol.89(4)(2008)391–396.
[8]Olayinka I.Ogunsola,Thermal upgrading effect on oxygen distribution in lignite,Fuel Process.Technol.34(1)(1993)73–81.
[9]H.N.S.Schafer,Pyrolysis of brown coals.2.Decomposition of acidic groups on heating in the range 100–900 °C,Fuel 58(9)(1979)673–679.
[10]J.Yu,A.Tahmasebi,Y.Han,F.Yin,X.Li,A review on water in low rank coals:The existence,interaction with coal structure and effects on coal utilization,Fuel Process.Technol.106(1)(2013)9–20.
[11]H.Choi,C.Thiruppathiraja,S.Kim,Moisture re-adsorption and low temperature oxidation characteristics of upgraded low rank coal,Fuel Process.Technol.92(10)(2011)2005–2010.
[12]Y.Zhang,X.Jing,K.Jing,Study on the pore structure and oxygen-containing functional groups devoting to the hydrophilic force of dewatered lignite,Appl.Surf.Sci.324(2014)90–98.
[13]D.Li,W.Li,B.Li,In situ diffuse reflectance FTIR study on water in lignite,Chem.Res.Chin.Univ.23(12)(2002)2325–2328.
[14]Y.Yao,D.Liu,S.Xie,Quantitative characterization of methane adsorption on coal using a low- field NMR relaxation method,Int.J.Coal Geol.131(2)(2014)32–40.
[15]H.S.Kim,Y.Nishiyama,K.Ideta,M.Jin,Y.Matsushita,J.I.Park,Analysis of water in Loy Yang brown coal using solid-state1H NMR,J.Ind.Eng.Chem.19(5)(2013)1673–1679.
[16]Y.Teng,S.Lian,Y.Song,Q.Liu,H.Yu,Y.Li,Relaxation time and pore structure evolution of Sheng Li lignite during in situ low temperature drying using1H NMR,J.China Coal Soc.39(12)(2014)2525–2530.
[17]Y.Teng,Q.Liu,K.Zhi,H.Yu,C.Chen,J.Zhao,Study of the water migration and drying kinetics of Sheng Li lignite in the upgrading process,Mater.Rev.28(4)(2014)145–148.
[18]W.Liu,L.Xing,Nuclear magnetic resonance logging,Petroleum Industry Press,Beijing,2011 86–88.
[19]Y.Yao,D.Liu,Y.Che,D.Tang,S.Tang,W.Huang,Petrophysical characterization of coals by low- field nuclear magnetic resonance(NMR),Fuel 89(7)(2010)1371–1380.
[20]Y.Yao,D.Liu,Advanced characterization of pores and fractures in coals by nuclear magnetic resonance and X-ray computed tomography,Sci.China Earth Sci.53(6)(2010)854–862.
[21]D.G.Evans,The brown-coal/water system:Parts 4.Shrinkage on drying,Fuel 52(3)(1973)186–190.
[22]X.Zhu,C.Sheng,Evolution of the char structure of lignite under heat treatment and its influences on combustion reactivity,Energy Fuel 24(1)(2010)152–159.
[23]M.Wang,Z.Li,W.Huang,J.Yang,H.Xue,Coal pyrolysis characteristics by TG–MS and its late gas generation potential,Fuel 156(15)(2015)243–253.
[24]A.Tahmasebi,J.Yu,Y.Han,F.Yin,S.Bhattacharya,D.Stokie,Study of chemical structure changes of Chinese lignite upon drying in superheated steam,microwave,and hot air,Energy Fuel 26(6)(2012)3651–3660.
[25]P.H.Giver,The distribution of hydroxyl in coal and its relation to coal structure,Fuel 39(1)(1960)147.
[26]K.Xie,Coal structure and its reactivity,Science Press,Beijing,2002 210–218.
[27]C.A.Tapas,J.C.Cjarles,R.V.Victor,Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass,Bioresour.Technol.102(7)(2011)4849–4854.
[28]D.Wang,X.Zhong,J.Gu,X.Qi,Changes in active functional groups during lowtemperature oxidation of coal,Min.Sci.Technol.20(1)(2010)35–40.
[29]C.Cao,The hydrophobic index of alkyl group,Chin.J.Org.Chem.16(2)(1996)133–138.
[30]H.H.Wang,Kinetic analysis of dehydration of a bituminous coal using the TGA technique,Energy Fuel 21(6)(2007)3070–3075.
 Chinese Journal of Chemical Engineering2016年6期
Chinese Journal of Chemical Engineering2016年6期
- Chinese Journal of Chemical Engineering的其它文章
- A stepwise optimal design of water network☆
- The turbulent behavior of novel free triple-impinging jets with large jet spacing by means of particle image velocimetry☆
- Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange☆
- Online process monitoring for complex systems with dynamic weighted principal component analysis☆
- Integration of coal pyrolysis process with iron ore reduction:Reduction behaviors of iron ore with benzene-containing coal pyrolysis gas as a reducing agent☆
- Multiple linear equation of pore structure and coal–oxygen diffusion on low temperature oxidation process of lignite☆
