A review of chemical absorption of carbon dioxide for biogas upgrading
Fouad R.H.Abdeen *,Maizirwan MelMohammed Saedi JamiSany Izan Ihsan ,Ahmad Faris Ismail
1 Department of Biotechnology Engineering,International Islamic University Malaysia,Kuala Lumpur 50728,Malaysia
2 Department of Mechanical Engineering,International Islamic University Malaysia,Kuala Lumpur 50728,Malaysia
1.Introduction
Fossil fuel depletion,rapidly growing energy demands,combined with greenhouse gas emissions encourage the development of renewable,sustainable,and environmentally friendly sources of energy[1].For this,the production of fuel methane from biogas produced via anaerobic digestion(AD)interests researchers and environmentalists[2].Biogas is a mixture of methane(CH4),carbon dioxide(CO2),and traces of hydrogen sulphide(H2S),ammonia(NH3),nitrogen(N2),hydrogen(H2),water vapourand other volatile compounds[3].The methane content in the produced biogas decides its heat value when converted to energy[4].The presence of H2S may damage the equipment and engines used in the conversion process due to its corrosive nature.Therefore,removing H2S is an important step for biogas cleaning prior to its utilisation[5].This process of purification has been discussed in several published works[5–9].
Since CO2is considered as an inert gas in terms of combustion,it should be removed from biogas to increase its heat value.Removing CO2from biogas,usually termed as biogas upgrading/enriching,is necessary when the targeted utilisation requires a high methane content[10].
The standard composition of purified and upgraded biogas depends on its target application and the country policy[11].The main biogas utilisation options include power generation in the combined heat and power(CHP)unit,injection to natural gas pipelines and converting it to vehicle fuel.When used as a vehicle fuel,biogas has a unique advantage of producing low greenhouse gas(GHG)emissions[12].
A major requirement for injecting biogas to natural gas grid or the transformation of biogas to a vehicle fuel is the upgrading to have more than 95%CH4content[13,14].Moreover,comparing the low heat value(LHV)between biogas and methane,biogas of 60 vol%CH4and 40 vol%CO2has a LHV of 17,717(kJ·kg-1),while a 100%CH4gas has a LHV of 50200(kJ·kg-1)[15].Hence,biogas upgrading is performed not only to enable its usage in wider applications but also to increase its heat value by the removal of non-combustible CO2.
CO2removal from gases has been performed for many years using techniques such as chemical and physical absorption,membrane separations,pressure swing adsorption(PSA),and cryogenic separation processes.Even though there are numerous experimental data available in literature discussing the CO2absorption,there is little published work discussing the implementation of these techniques for biogas upgrading[16].
This paper reviews the published works on CO2capture using chemical absorption processes with special attention given to data from biogas upgrading experiments.It highlights the potential chemical absorption of CO2in upgrading biogas.Data,important findings,and recommendations from each of the reviewed techniques are integrated,tabulated,and made easy for researchers to estimate the suitability of each technique.
2.Overview of CO2 Chemical Absorption
2.1.Background
Absorption is a process of transferring a component from its gas phase into a liquid provided that the gas is soluble in that liquid[17].In the case of CO2,the solubility of the gas is dependent on the solvent physical and chemical properties.When the gaseous molecules of CO2are attached to liquid molecules with weak intermolecular forces,the absorption is described as physical absorption.Therefore,the physical absorption process is usually operated at high pressure and low temperature to increase the CO2solubility in the absorbing liquid.The chemical absorption process is performed via absorbing CO2from biogas by covalently bonding it into the molecules of the absorbing liquid[18].The strong covalent bonds between the chemical solvent molecules and the CO2molecules make the chemical absorption process more efficient in absorbing CO2even at ambient temperature and pressure.
2.2.Process
The chemical absorption process for CO2removal from biogas,performed in a packed column like any other chemical scrubbing of any gas,can be optimised by selecting the best solvent,best contactor(tray or packing with respect to process conditions),best gas and liquid flow rates,and best stripping conditions[17,19].
The column in which chemical absorption process is performed can be represented with an ideal plug flow reactor where there is mixing only in the radial direction and not in the axial direction[20].Fig.1 shows the flow diagram of a typical gas absorption process.The detailed designs of the absorber,stripper,and solvent selection are governed by the composition of the feed gas and the required composition of the scrubbed(treated)gas.
When using a packed column for absorbing CO2from biogas,a water solution is usually prepared from the selected absorbent and is fed into the top of the packed column while the raw gas is fed from bottom in a counter current flow.The estimation of the column height and diameter are dependent on other factors such as gas and liquid flow[21].
2.3.Solvent selection
Solvent selection is the most important step in the biogas upgrading process.The suitability of a solvent to be used for absorbing CO2from biogas is decided by the difference in solubility between CO2and methane in that solvent.Water can be used to selectively absorb CO2from biogas by a pure physical process[22].The solubility of main biogas components NH3,H2S,CO2and CH4,in water at 25°C and 0.1 MPa partial pressure of diluted gas,is 280000,1020,340,and 13.2 mmol·kg-1·MPa-1,respectively,as reported by[10].In addition to solubility gradient between CO2and CH4,the solvent has to comply with other requirements of being available,cheap,environmental friendly,having a high CO2load,easy to regenerate,and having low viscosity[10].
To upgrade biogas to a vehicle fuel,several chemical absorption techniques have been used.Few liquids have been used for upgrading biogas including amines,caustic/alkaline solvents,and amino acid salts.
3.Chemical Absorption Techniques for Biogas Upgrading
Since solvent selection is the most important step in the chemical absorption of a gas,the techniques discussed in this section are categorised based on the solvent used.For each of the amine scrubbing,caustic solvent scrubbing,and amino acid salt scrubbing techniques,the theory,the early work,and the most recent improvements are discussed.The following review includes the process applied for CO2absorption with special attention to solvents and techniques performed for biogas upgrading.
3.1.Amine scrubbing
3.1.1.Theory and background
Amine scrubbing of CO2is where CO2is absorbed by an aminebased solution.The absorption is a chemical absorption process since covalent bonds are formed between the amine and CO2.The most common amines used for CO2removal are monoethanolamine(MEA),diglycolamine(DGA),diethanolamine(DEA),triethanolamine(TEA),methyldiethanolamine(MDEA),and piperazine(PZ)[10,18].MEA is the most used amine for absorbing CO2as a scrubbing agent.Eqs.(1)and(2)summarise the possible reactions using MEA mentioned by many researchers[23–25].
The amine solution absorbs CO2both physically to the liquid and chemically.However,the mass transfer of CO2from the gaseous phase to the liquid phase is increased by the chemical reaction between CO2and the amine.The chemical reaction consumes CO2in the liquid phase to maintain the concentration gradient of CO2in the two phases[26].One recommendation while performing amine scrubbing is to keep the molar flow of amine at a rate of at least four times of the molar flow rate of CO2[18].More about the mass transfer and the thermodynamic of the process is discussed by[23,27].
An early use of amine scrubbing for CO2removal was reported in 1930 by[28].The amines recommended for the CO2scrubbing were primary,secondary,and tertiary and may contain a carboxyl group[28].Other early studies examining amine-based CO2scrubbing used monoethanolamine,triethanolamine,and diaminoisopropanol[29],di-and tri-ethanol amine[30],ethylaminoethanol[31],methyldiethanolamine[32],piperidine methanol/ethanol/propanol/butanol or pentanol[33],and hydroxyethyl piperazine[34].One early study claimed that the effectiveness of monoethanolamine and diaminoisopropanol is twice that of triethanolamine[29].
More recent works emphasised the use of the sterically hindered amines for CO2removal from the mixture of gases[35–37].It was claimed that steric hindrance gives amines higher thermodynamic capacity and faster absorption rates when CO2concentration is high[37].Nevertheless,it was later claimed that hindrance of amines increases their absorption capacities but decreases the rate of absorption especially at low CO2concentrations[35].
Some studies used piperazine-based amine mixtures as CO2scrubbing solvents instead of MEA-based mixtures.One famous study used a mixture of PZ and MDEA[38].The mixture was claimed to be capable of removing other impurities present in gases such as H2S[38].The PZMDEA solution contains a tertiary amine(MDEA)and a secondary amine(PZ)and is proven to have an overall faster reaction with CO2when compared to MEAora blend of MEA and MDEA[39].The presence of a secondary amine increases the overall amine-CO2reaction rate while the presence of the tertiary amine reduces the energy of regeneration due to its low reaction energy[18].
3.1.2.Amine regeneration
The three most common challenges faced when performing amine scrubbing are reducing energy consumption(during regeneration),inhibiting corrosion,and preventing the amine degradation[40].Energy consumption and degradation of amines mainly occur in the regeneration step.
As indicated earlier,a small group of amines have the abilities of chemically bonding acidic gases at low pressure.These amines may later be regenerated by raising temperature[28,41]or reducing pressure[42]to free gases.In the case of CO2scrubbing,the reaction between CO2and amine in the scrubbing solution is regarded as exothermal.The regeneration of the scrubbing solution can be performed by stripping with water vapour or air after heating to 100–120 °C.Pure CO2can be produced after stripping if water is condensed from the stripper vapour leaving CO2in a gaseous phase[43].
Decreasing process energy consumption is the main challenge when producing or enriching an energy source.There are several comparisons between the various amines in terms of their regeneration abilities to reduce energy consumption of the process.Some studies claimed that it is easier to regenerate tertiary amines[18]and the satirically hindered amines[44]than regenerating primary and secondary amines.
Energy saving in the absorption process can be obtained by either increasing the absorption capacity of the absorbent,thus reducing the amount of liquid to be regenerated,or by lowering the regeneration process energy requirement.Options for lowering energy cost during regeneration were discussed by several researchers focusing on the type and design of stripper and regeneration conditions[26,45,46].Other studies emphasised the use of high volatility liquid as a stripping carrier to reduce energy use in the regeneration of the CO2rich amine[47].
3.1.3.Amine degradation
Degradation of amines refers to the action when amines bind with CO2[48],oxygen,or other gas components[10]to form a compound in an irreversible reaction.The degradation process causes solvent loss,decrease in the solvent absorption capacity,foaming,increased viscosity,increased disposal cost,and increased corrosion[49].Researchers have suggested different mechanisms explaining amine degradation pathways[49–53].Their focus was on identifying the degradation products with no clear recommendation on how to decrease the degradation process[49,51,53].
Some studies suggested few practises to be implemented for the sake of degradation hindrance such as lowering CO2loading,increasing amine concentration,and lowering temperature at reboiler to be below 110°C[50].Other studies recommended using amines with higher resistance to thermal degradation compared to MEA and DEA such as piperazine[52]and MDEA[54].The common recommendation is that the process of degradation of amines used to absorb CO2cannot be controlled by few factors of the process.Rather,the degradation is controlled by parameters interacting with each other including the type of amine,stripping temperature,pressure,CO2loading,and amine concentration.
The oxidative type of solvent degradation was discussed by[55].Oxidative degradation is believed to be more dominant than thermal degradation in the pilot scale in comparison to lab scale[56].A recent study claimed the ability of inhibiting MEA oxidation by compounds such as,2,5-dimercapto-1,3,4-thiadiazole(DMcT),diethylenetriamine pentaacetic acid(DTPA),hydroxyethylidenediphosphonic acid(HEDP),and methyldiethanolamine(MDEA)[57].The researchers tested these inhibitors on the MEA solution at low temperatures only,thus,they are yet to be confirmed at high temperatures.
Other than binding irreversibly with CO2,amines degrade when there are other impurities in the gas such as solid particles,SO2,NOx,and oxygen[10].The prior removal of these impurities from gas can halt solvent degradation.In the case of biogas upgrading,solvent degradation is considered an important factor due to the high CO2concentration and the presence of other impurities which may lead to unfavourable irreversible reactions.
3.1.4.Corrosion
Another important challenge when implementing amine scrubbing for CO2removalis corrosion.Some amines,such as MEA,have corrosive nature especially at high temperatures[40].Also,amines can degrade into corrosive products when reacting with CO2[58].The MEA corrosive power is estimated to be maximum at the inlet and outlet of the stripper at a rate of1 mm per year for carbon steel in a pilot plant with a capacity of removing not less than 1 t CO2per hour[58].It has been further claimed that highest corrosion occurs at a set of parameters combining high temperature with high CO2loading,high O2partial pressure,high amine concentration,and rough surface condition[58,59].
3.1.5.Recent improved processes
The recentmostamine scrubbing techniques focus on the possibility of lowering energy consumption,inhibiting corrosion,and preventing amine degradation.Many studies have sought to overcoming solvent degradation by using a mixture of amines as a solvent.In most of these studies,MEA was used as a benchmark.One study claimed the ability of preparing a new degradation-resistant amine mixture[60].It has been claimed that the new amine is sterically hindered and has a higher stability in terms of oxidative degradation since it absorbs CO2by forming the bicarbonate anion without forming the carabamate one[60].
Another study performed for the CO2capture from post-combustion of coal and natural gas verified the use of a mixture of 2-amino-2-methyl-propanol(AMP)and piperazine.The authors claimed that by using this mixture,compared to MEA,the overall process energy loss decreased significantly[61].
One novel method suggested for reducing the amine regeneration cost is the application of concentration swing absorption(CSA).The heat consumption for regenerating CO2rich solvent is reduced by swinging the amine and the CO2concentration in the solvent[62].The CSA is performed by implementing a conventional absorption process at the normal amine concentrations(up to 30%for MEA),then the rich solvent concentration of MEA is increased by removing water[62].A study applying the CSA using MEA for CO2absorption has shown that increasing the MEA from 30%to 60%has decreased the energy consumed for removal of 1 kg of CO2by 34%[62].
3.1.6.Cases of amine scrubbing for biogas upgrading
Most works on amine scrubbing for CO2capture were for postcombustion capture,natural gas processing,and coal gasification.However,there is a lack of published work on applying amine scrubbing for biogas upgrading.Many variables must be analysed to design an aminebased optimal biogas upgrading unit[63].Nevertheless,there are few published studies on biogas upgrading using amine scrubbing.These studies include both simulation based studies and experimental based studies.
Some studies concerning biogas upgrading involved the use of some software for simulating the whole process.A group of researchers compared DGA to five other amines and amine-blends(MEA,DEA,DGA,MDEA)and mixtures of(MDEA+MEA,MDEA+DEA),using the process simulator ProMax®.The study concluded that the most promising amine for CO2capture from biogas is DGA with the greatest advantages in terms of achieving better yields(97.3%CH4)with lower power consumption[63].
Other published works on performing amine scrubbing on biogas and land fill gas such as the works of[64,65],[66],[67],[68],[69]and[70]are reviewed in Table 1.
All cases reported in Table 1 showed thatamines in general have the ability to efficiently remove CO2from biogas.The amine scrubbing of CO2was performed in a counter current flow using column reactor packed with different types of packing in cases 1,2,3,5,and 6.However,case 4 verified the use of bubble column reactor for biogas upgrading.But as expected,this type of reactor cannot support the scrubbing of CO2at high flow rate.
The cases comparing the performance of different amines have all shown that using MEA has yielded the highest methane purity as compared to DEA[68]and MDEA[64,65,70].However,compared to MEA,a lower energy consumption was recorded for DEA[71]and MDEA[64,70].
Table 1 shows that few cases have examined the use of blends of different types of amines(primary,secondary,tertiary and sterically hindered)for the removal of CO2form biogas.Also,the cases reviewed in Table 1 have not presented enough details describing the stripping conditions of CO2or the energy cost associated with regenerating the different amines and blends of amines.Hence,future studies should analyse in more depth the difference in performance between various amines,blends of amines,and amines with special additives to optimise the absorption–stripping process.
Moreover,future studies should focus on the possibility of minimising amines degradation when used to upgrade biogas since biogas has the high potentialto degrade amines due to the high CO2partial pressure and the presence of other impurities in biogas that promote degradation.In conclusion,more research is required in the field of optimising the integrated process of scrubbing and stripping of CO2from biogas by different amines and amine mixtures.
3.2.Caustic solvent scrubbing
3.2.1.Theory and background
Caustic solvents(sodium hydroxide,potassium hydroxide,and calcium hydroxide)are the second most abundant solvents which can chemically absorb CO2from a mixture of gases after amine solutions.These alkaline solutions have been examined by several researchers for their ability to absorb CO2for more than 50 years.The early works on caustic solutions focused on measuring the solubility of CO2atdifferent concentrations as well as studying the kinetics and the thermodynamics of the absorption process.The early works were discussed,analysed,and reviewed by several researchers[31,72–74].
Many of the early published work on CO2absorption by caustic solutions focused on the use of sodium hydroxide[72,74–76].Other early works investigated the use of potassium hydroxide[77,78].However,less attention was given for the use of calcium hydroxide[73].This section reviews the theory involving the three caustic solvent reactions with CO2as well as the abundant,available,and potential techniques implementing caustic solutions for CO2absorption.More attention is given to the techniques performed for CO2absorption from biogas.
Sodium hydroxide,the most repeatedly used caustic solvent,is categorised as a strong alkaline;therefore it is completely ionised in water to Na+and OH-[79].Sodium hydroxide(NaOH)has been reviewed as an efficient CO2absorber by many researchers[79–81].Compared to MEA,NaOH has the advantage of being cheaper,more available and possessing a greater theoretical CO2capture capacity[79].
Similar to amines,other than being physically absorbed with the NaOH,CO2is chemically absorbed via the reaction given in Eq.(3).

CO2absorption with NaOH has been performed conventionally using a packed column by many researchers[76,79,82,83].However,column packing supplement method has been suggested by other researchers[84].The method is based on the idea of using spray nozzles with the ability of producing a fine spray of the solvent.The later method has been claimed to allow for the high rate capture of CO2using NaOHat low pressure drop,reduced capital cost and decreased water loss[84].

Table 1 Published cases implementing amine scrubbing for biogas upgrading
Potassium hydroxide(KOH)is the second most abundant caustic solvent used for CO2scrubbing.KOH reacts with CO2by the reaction given in Eq.(4).

The chemical absorption process of CO2using KOH is no different from the process applied using NaOH.KOH is more expensive than NaOH,however,it is reported that KOH is advantageous to NaOH due to the formation of K2CO3which has several industrial applications[85].Therefore,KOH cost can be reduced if the first product of Eq.(4)(K2CO3)is sold[85].
The third caustic solvent verified for its ability to absorb CO2is calcium hydroxide(Ca(OH)2).The reaction of Ca(OH)2with CO2is given by the reaction in Eq.(5).

A suspension of lime(Ca(OH)2solution)for the absorption of CO2has been performed by[73].In their study,the researchers used a bubble column with agitated contactors.In general,less attention was given to CO2absorption using Ca(OH)2due to the low solubility of the first in water which makes it not suitable to be used at higher CO2concentrations.
3.2.2.Solvent regeneration
A common challenge faced when implementing any technique for CO2absorption is the solvent regeneration in terms of possibility,efficiency,and cost.Regeneration of aqueous NaOH and KOH is considered very difficult,when compared to MEA regeneration.NaOH and KOH regeneration difficulty is due to the formation of thermally stable Na2CO3and K2CO3as the final products of CO2absorption.
Considering the regeneration of NaOH,a considerably low temperature of 160°C is needed to decompose NaHCO3into Na2CO3,H2O and CO2,whereas,a very high temperature of 800°C is required to form Na2O which is considered a suitable source of NaOH[79].
The conventional NaOH regeneration from Na2CO3is performed by treating the later with lime in a process usually called causticisation which is described by reactions in Eqs.(6),(7),and(8)[81].

The reactions in Eqs.(7)and(8)are also included in the regeneration process of KOH and Ca(OH)2.Therefore,the regeneration of the three caustic solutions is costly due to the high energy requirement explained by Eq.(7).
In addition to the high temperature demand,causticisation of sodium hydroxide using lime has intolerable drawbacks of limited efficiency and the production of low alkalinity solvent[81].Therefore,several researchers have studied the possibility of implementing a novel method for regenerating NaOH from Na2CO3[79].
Some researchers[81]suggested the use of sodium tri-titanate in a direct causticisation process to regenerate sodium hydroxide from sodium carbonate.They claimed that their novel technique requires half of the energy required by the conventional causticisation process using lime and the same with energy required to regenerate MEA after CO2absorption[-130 kJ·(mol CO2)-1].It was claimed that the regeneration process using sodium tri-titanate has produced a concentrated sodium hydroxide which will in turn reduce the water loss[81].
3.2.3.Mineral carbonation
The process by which CO2is stored in the form of CaCO3when reacting with calcium oxide(CaO)or calcium hydroxide(Ca(OH)2)is called mineral carbonation.The reaction between calcium oxide(CaO)and CO2is an exothermic reaction(the reverse reaction of Eq.(7))as shown in Eq.(9)[86].

In the presence of water,the aforementioned process is called aqueous mineral carbonation for which a standard detailed procedure has been recently explained by[87].The reaction of lime(Ca(OH)2)with CO2is given in Eq.(5)as an exothermic reaction with the heat of reaction(ΔH100°C)equals-109 kJ·(mol CO2)-1[88].
The use of a solution containing dissolved and suspended lime(Ca(OH)2)for the absorption of CO2is considered a slow process in the ambient temperature and pressure[87].The increase of the rate of reaction for the process may be obtained by increasing both temperature and pressure[89]and running the process at the best liquid-tosolid ratio[87,89].
The common term usually used in the field is accelerated carbonation which refers to the process ofCO2sequestration using calcium hydroxide at high rate of reaction.Research in this field has presented various ways for accelerating the absorption process.One method to accelerate the process is to use a gravity pressure vessel reactor to accelerate the carbonation process[89].
Another controversial issue when using mineral carbonation is raised regarding the source of lime used in the process.Due to the fact that synthetic calcium oxide or calcium hydroxide is usually produced via a reaction that produces CO2,there have been several trials to find a way of using calcium-containing waste as a source of CaO or Ca(OH)2.Cheap and environmentally friendly source of calcium oxide/hydroxide has been targeted by many researchers.Among the famous calcium-containing sources examined in the field ofCO2capture in general are;the fl y ash obtained from coal burning[90],slag from ground steel[91]and blast furnace[92],dust from cement kiln[93,94],waste combustion residues[95],bottom ash from municipalsolid waste incinerator[96],and paper mill effluent[97].Other calcium-rich waste to be considered for the CO2capture and storage are reviewed by[95,98,99].
3.2.4.CO2absorption using carbonate solutions
Sodium carbonate(Na2CO3)and potassium carbonate(K2CO3),the products of Eqs.(3)and(4)respectively,are known for their ability to chemically absorb CO2.
CO2is usually absorbed by the carbonate solutions at relatively high pressure values when compared to other methods.The process is operated at a pressure swing absorption–desorption cycle rather than at a temperature swing absorption process[100].When a carbonate solution is used to absorb CO2,the overall reaction can be written as Eq.(10).
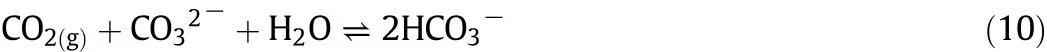
Several early works discussed the use of carbonate solutions in absorbing and capturing carbon dioxide[101–105].Some other more recent published works were focusing on the use of potassium carbonate solution for CO2absorption and storage[106–109].
The absorption of CO2using carbonate solutions has many advantages,such as the low volatility,low corrosion rate,and the possibility to absorb other impurity gases[110].Nevertheless,a major drawback associated with the process is the slow absorption rate of CO2compared to amines and caustic solvents[110].
Recent researches suggested improvements to speed up the process of CO2absorption using carbonates solutions.The recommended improvements included promoting the absorption process by adding piperazine[111],monoethanolamine[112],boric acid[113]and amino acids such as glycine,sarcosine and proline[114]to the carbonate solution.
The slow absorption rate of CO2makes the carbonate solutions less suitable for the removal of CO2from(40%CO2containing)biogas unless a tangible improvement is obtained.In fact,there is little published work discussing the use of carbonate solutions for the biogas upgrading process.Hence,there is a lack of experimental data to establish a fair and useful evaluation for the possibility of using the carbonate solutions for biogas upgrading.
3.2.5.Cases of caustic scrubbing and mineral carbonation for biogas upgrading
Chemical absorption of CO2using a caustic solvent for biogas upgrading can be classi fied into two types with respect to the regeneration procedure of the rich solvent.The first type is the one utilising a fresh caustic solution(NaOH or KOH)and the process is performed by the conventional regeneration method explained in the solvent regeneration section.Whereas the second and more novel type is performed using a waste source of calcium for the sake of solvent regeneration or environmentally friendly storage of CO2.
Several researchers have focused on the use of various types of waste residues as a source of lime to regenerate KOH or NaOH.The direct use of raw lime for the caucticisation of alkaline solutions such as NaOHand KOH is not favourable from the environmental point of view since the production of lime releases CO2.Therefore,using calcium-containing waste for the causticisation of alkaline solvent has several advantages in terms of reducing the environmental impact of the regeneration process[115].A group of cases reported using of caustic solutions for biogas upgrading are listed in Table 2 and Table 3.
Table 2 reviews the work of[85],[69]and[70].The cases reviewed in Table 2 prove the ability of the three caustic solutions in obtaining a biogas with 94%–97%methane content.The highest methane purity is obtained by case 1 when a 53%KOH solution is used;however,this is a very high concentration of the solvent which makes the process more costly.Ambient or near ambient temperature and pressure is maintained in the three cases reviewed.The absorption is performed using packed columns with different types of packing operating in a counter current flow.
The reviewed cases in Table 2 included a satisfactory discussion of absorption process and the results obtained;however,more justification should be given for the fast saturation of Ca(OH)2concluded in case 2.In addition,more information and discussion should be presented regarding the fate of the rich solvent used and the regeneration choices.
Table 3 reviews cases of biogas upgrading involving mineral carbonation performed by[116],[117,118]and[115].Case 1 is performed by the direct storage of CO2in the form of CaCO3.The authors have called this process bottom ash for biogas upgrading abbreviated as BABIU[116].
From Table 3,in Cases 2 and 3 CO2is absorbed by caustic solutions of NaOH and KOH while a waste source of calcium is used for the regeneration of the rich solvent.The authors of Cases 2 and 3 have called this process alkaline with regeneration abbreviated as AwR[115,118,119].
Alkaline with regeneration(AwR)and bottom ash for biogas upgrading(BABIU)both used mineral carbonation(as explained previously).BABIUis represented by the reaction in Eq.(9),while AwR is represented by the reactions in the Eqs.(6),(7),and(8).It is claimed that both AwR and BABIUuse waste as inputs to the process with the attractive advantage of enriching biogas and stabilising metals using waste[120].
Some studies highlighted the environmental impact of several biogas upgrading techniques including the two processes BABIU and alkaline with regeneration(AwR)[121–123].It was concluded that BABIU has the lowest environmental impact then alkaline scrubbing(scrubbing with a caustic solvent).On the other hand,AwR had the highest environmental impact in 11 of the 12 categories reviewed by[121].
In a nutshell,caustic solutions are promising solvents to be used for CO2removal from biogas.However,the process needs more attention focusing on the use of novel regeneration procedure to reduce energy consumption and additional solvent requirement.Likewise,techniques incorporating the use of waste source of calcium for the permanent storage of CO2(such as BABIU)are thought to be promising if more novel calcium-rich waste materials are tested.Nevertheless,the AwR process seems to have high environmental impact compared to other techniques and has to be optimised to be more environmentally friendly.
3.3.Amino acid salt solutions
3.3.1.Theory and background
Amino acids can replace the use of alkanolamines for CO2capture due to the presence of the amine group[124].When amino acids react with alkaline salts such as lithium,sodium or potassium hydroxide,the carboxylate group of the amino acid is neutralised forming amino acid salt(AAS)[124].The conventional amino acid neutralisation to AAS is performed by reacting the later with potassium hydroxide[125].In addition to reacting with potassium hydroxide,AAS can be prepared by reacting with amines to produce an amine-based AAS[126].

Table 2 Published cases implementing caustic scrubbing for biogas upgrading

Table 3 Published cases implementing BABIU and AwR
Even though AAS are considered expensive compared to other CO2absorbing solvents[125],they have the same or better advantages in absorbing CO2from biogas,than alkanolamines[127].Due to their ionic nature,amino acid salts perform similar to amines in absorbing CO2while having lower volatility[127].Holding a lower volatility reduces the expected absorbent loss during the regeneration of the solvent[124,127].
In addition,amino acid salts have the advantage of being naturally occurring,offering a higher resistance to oxidative degradation,and being biodegradable which enables the easy and environmentally safe disposal[124,125,127].Nevertheless,a key disadvantage in the use of amino acid salts is their high molecular weight which may lead to an increase in the absorber size especially when CO2fraction is high as in the case of biogas[127].
The reaction mechanism between amino acid salts and CO2is similar to the mechanism explained for the reaction of the later with alkanolamines.The kinetics of the reaction between amino acid salts and CO2are discussed by[124,125,128].
3.3.2.Amino acid salts for CO2absorption
Among the early studies on the potential that amino acid salts have in reactively absorbing CO2are[129–131].Other early works examining the kinetic of the reaction between CO2and AASs were reviewed by[125].More recent works about the use of AASs for CO2absorption has been discussed in many studies[124–126,132–134].
The recent studies have verified the use of various potassium-based AASs for the absorption of CO2.Among the recently recommended AASs are potassium salts of taurine and glycine[125,134],sarcosine[135,136],proline[124],alanine[137],serine,and α-aminobutyric acid[133].
Other recent studies recommended amine-based AASs as efficient absorbents of CO2.For instance,sarcosine neutralised with 3-(methylamino)propylamine(MAPA)was claimed to have a relatively good performance in absorbing CO2from a mixture of gases[126,138].In general,amine-based AASs have an additional advantage of lower energy requirement when compared to potassium-based AASs[126,138].
3.3.3.Amino acid salts for biogas upgrading
AASs are believed to have potential in the biogas upgrading process not only due to the high efficiency of CO2absorption but also due to their high selectivity between CO2and CH4.AAS selectivity between CO2and CH4was reported to be twice of the selectivity of MEA[139].There is little work published on the use of amino acid salts for biogas upgrading.However,some of the recent cases implementing amino acid salts are listed in Table 4.
Table 4 reviews the work of[127],[140]and[141].The cases listed in Table 4 have shown the ability of AAS to efficiently obtain a biogas of up to 99%methane content.The first two cases in Table 4 investigated the use of potassium-based AAS for biogas upgrading.However,the third case highlighted the use of amine-based amino acid salt as a CO2absorbent for the upgrading of biogas.
Case 1.verified the use of potassium glycinate(PG),potassium L-argininate(PA),potassium L-prolinate(PP),potassium L-ornithinate(PO)and potassium sarcosinate(PS)in a bubble column at near ambient temperature and pressure and concluded that PO and PG have the advantages of high absorption kinetics,very low absorbent loss,and high regeneration efficiency.In addition,increasing basicity increased CO2absorption rate but decreased CO2regeneration efficiency[127].
Case 2.investigated the use of 20%PA using a hollow fibre membrane contactor for biogas upgrading and compared it with MEA using the same apparatus.This study concluded that CO2absorption capacity of PA is four times that of MEA[140].Having high CO2absorption capacity is a key advantage which makes this solvent a promising solvent to replace alkanolamines if the whole absorption–desorption process is well optimised.
The third case listed in Table 4(Case 3)verified the use of two amine-based AASs(MEA-based L-ornithinate(MEAORN)and MEA-based glycinate(MEAGLY))and compared their performance to MEA and PZ.The study has concluded that MEAORN and MEAGLY both have higher CO2absorption capacity compared to MEA and PZ;and MEAORN had the highest value for CO2absorption capacity among all solvents[141].

Table 4 Published cases implementing AAS scrubbing for biogas upgrading
In brief,the process of applying AAS scrubbing for biogas upgrading is a promising technology due to its relatively high stability.The process can be further optimised if the effect of basicity is studied intensely.AAS scrubbing technology requires more comprehensive and detailed evaluation of the techno-economic feasibility with special concern given to the regeneration energy cost,environmental impact and efficiency.
4.Conclusions
Chemical absorption of CO2is a promising technology for biogas upgrading.Several cases implementing chemical absorption of CO2using amine scrubbing,caustic scrubbing,and AAS scrubbing have shown the ability to upgrade biogas to a methane-rich gas.The aforementioned techniques might be considered mature in the field of CO2capture and removal from post-combustion gases but they are still at the beginning of biogas upgrading.Among the three techniques reviewed in this study,amine scrubbing is the most mature.However,it requires more research to overcome the solvent degradation and energy loss when used for biogas upgrading in large scales.Future studies are required to evaluate the feasibility of using blends of amines(primary secondary,tertiary and sterically hindered)for biogas upgrading.The caustic solvent scrubbing is believed to have a higher CO2loading capacity than amine scrubbing but still requires improvements to lower the energy consumption during the regeneration step.Future works should focus on the possibility of converting the spent solutions into value added products rather than regenerating them.AASs are the least studied for their ability to upgrade biogas but they have attractive advantages of being resistant to oxidative degradation and having low volatility.However,AAS implementation for upgrading biogas in plant scale still requires extensive research due to the large absorber size required when using them.After all,the current data on chemical absorption for biogas upgrading is insufficient to use them at industrial plantscale with optimised performance.Therefore,chemical absorption techniques need more focused research to analyse the possibility of their optimised implementation in the field of biogas upgrading.Current efforts should be focused on the energy consumption and environmental impact of each technique when applied for biogas upgrading and the possibilities of reducing them by comprehensively analysing the different parameters of the absorption–regeneration process cycle.
[1]H.Oechsner,S.K.Khanal,M.Taherzadeh,Advances in biogas research and application,Bioresour.Technol.178(2015)177.
[2]D.Divya,L.R.Gopinath,P.Merlin Christy,A review on current aspects and diverse prospects for enhancing biogas production in sustainable means,Renew.Sust.Energ.Rev.42(2015)690–699.
[3]N.Korres,P.O'Kiely,J.A.H.Benzie,J.S.West,Bioenergy production by anaerobic digestion:using agricultural biomass and organic wastes,Routledge,2013.
[4]M.Persson,O.Jönsson,A.Wellinger,Biogas upgrading to vehicle fuel standards and grid injection,IEA bioenergy task,2006(http://www.businessregiongoteborg.com/download/18.37dc8386114072e4a2d80003405/1389235333763/upgrading_report_ fi nal.pdf(accessed March 5,2014)).
[5]F.Osorio,J.C.Torres,Biogas purification from anaerobic digestion in a wastewater treatment plant for biofuel production,Renew.Energy 34(2009)2164–2171.
[6]N.Abatzoglou,S.Boivin,A review of biogas purification processes,Biofuels Bioprod.Biorefin.3(2009)42–71.
[7]M.S.Horikawa,F.Rossi,M.L.Gimenes,C.M.M.Costa,M.da Silva,Chemical absorption of H2S for biogas purification,Braz.J.Chem.Eng.21(2004)415–422.
[8]W.-C.Lin,Y.-P.Chen,C.-P.Tseng,Pilot-scale chemical–biological system for efficient H2S removal from biogas,Bioresour.Technol.135(2013)283–291.
[9]D.Ramírez-Sáenz,P.B.Zarate-Segura,C.Guerrero-Barajas,E.I.García-Peña,H2S and volatile fatty acids elimination by biofiltration:Clean-up process for biogas potential use,J.Hazard.Mater.163(2009)1272–1281.
[10]D.Dieter,S.Angelika,Biogas from waste and renewable resources:An introduction,Wiley-VCH,Weiheim,Germany,2008.
[11]S.Rasi,J.Läntelä,J.Rintala,Trace compounds affecting biogas energy utilisation —A review,Energy Convers.Manag.52(2011)3369–3375.
[12]L.Yang,X.Ge,C.Wan,F.Yu,Y.Li,Progress and perspectives in converting biogas to transportation fuels,Renew.Sust.Energ.Rev.40(2014)1133–1152.
[13]P.Weiland,Biogas production:Current state and perspectives,Appl.Microbiol.Biotechnol.85(2010)849–860.
[14]Y.Xu,Y.Huang,B.Wu,X.Zhang,S.Zhang,Biogas upgrading technologies:Energetic analysis and environmental impact assessment,Chin.J.Chem.Eng.23(1)(2015)247–254.
[15]J.Arroyo,F.Moreno,M.Muñoz,C.Monné,N.Bernal,Combustion behavior of a spark ignition engine fueled with synthetic gases derived from biogas,Fuel 117,Part A(2014)50–58.
[16]E.Privalova,S.Rasi,P.Mäki-Arvela,et al.,CO2capture from biogas:Absorbent selection,RSC Adv.3(2013)2979–2994.
[17]A.L.Kohl,R.Nielsen,Gas purification,Gulf Professional Publishing,1997.
[18]F.Bauer,C.Hulteberg,T.Persson,D.Tamm,Biogas upgrading—Review of commercial technologies,SGC Rapp.,2013(http://lup.lub.lu.se/record/3512502(accessed October 16,2014)).
[19]R.H.Perry,D.W.Green,Perry's chemical engineers'handbook,McGraw-Hill,New York,2008.
[20]D.S.Clark,H.W.Blanch,Biochemical engineering,2 ed.CRC Press,New York,1997.
[21]J.Reynolds,J.S.Jeris,L.Theodore(Eds.),Handbook of chemical and environmental engineering calculations,1 ed.Wiley-Interscience,Hoboken,N.J.,2007.
[22]Y.Xiao,H.Yuan,Y.Pang,et al.,CO2removal from biogas by water washing system,Chin.J.Chem.Eng.22(2014)950–953.
[23]N.A.Al-Baghli,S.A.Pruess,V.F.Yesavage,M.S.Selim,A rate-based model for the design of gas absorbers for the removal of CO2and H2S using aqueous solutions of MEA and DEA,Fluid Phase Equilib.185(2001)31–43.
[24]N.Razi,H.F.Svendsen,O.Bolland,Validation of mass transfer correlations for CO2absorption with MEA using pilot data,Int.J.Greenhouse Gas Control 19(2013)478–491.
[25]J.T.Yeh,H.W.Pennline,K.P.Resnik,Study of CO2absorption and desorption in a packed column,Energy Fuel 15(2001)274–278.
[26]L.A.Pellegrini,S.Moioli,S.Gamba,Energy saving in a CO2capture plant by MEA scrubbing,Chem.Eng.Res.Des.89(2011)1676–1683.
[27]A.Aroonwilas,Mass-transfer with chemical reaction in structured packing for carbon dioxide absorption process(Ph.D.)The University of Regina,Canada,2001.
[28]R.B.Robert,Process for separating acidic gases,US1783901 A,1930.
[29]L.B.Gregory,W.G.Scharmann,Carbon dioxide scrubbing by amine solutions,Ind.Eng.Chem.29(1937)514–519.
[30]H.W.Wainwright,G.C.Egleson,C.M.Brock,J.Fisher,A.E.Sands,Removal of hydrogen sulfide and carbon dioxide from synthesis gas,using di-and tri-ethanolamine,US Department of the Interior,Bureau of Mines,1952.
[31]A.L.Shrier,P.V.Danckwerts,Carbon dioxide absorption into amine-promoted potash solutions,Ind.Eng.Chem.Fundam.8(1969)415–423.
[32]E.Bartholome,H.W.Schmidt,J.Friebe,Process for removing carbon dioxide from gas mixtures,3622267,1971.
[33]G.Sartori,F.Leder,Process and amine-solvent absorbent for removing acidic gases from gaseous mixtures,US4112051 A,1978.
[34]P.J.Birbara,T.P.Filburn,T.A.Nalette,Carbon dioxide,water,US5876488 A,1999.
[35]R.J.Hook,An investigation of some sterically hindered amines as potential carbon dioxide scrubbing compounds,Ind.Eng.Chem.Res.36(1997)1779–1790.
[36]M.T.Melchior,G.E.Milliman,G.Sartori,Method of removing carbon dioxide from gases utilizing an alkaline absorption solution containing a cyclic urea antifoaming agent,US4183903 A,1980.
[37]G.Sartori,D.W.Savage,Sterically hindered amines for carbon dioxide removal from gases,Ind.Eng.Chem.Fundam.22(1983)239–249.
[38]M.Appl,U.Wagner,H.J.Henrici,K.Kuessner,K.Volkamer,E.Fuerst,Removal of CO2and/or H2S and/or COS from gases containing these constituents,4336233,1982.
[39]S.Bishnoi,G.T.Rochelle,Absorption of carbon dioxide in aqueous piperazine/methyldiethanolamine,AIChE J.48(2002)2788–2799.
[40]R.Litonjua,I.Cvetkovski,Biogas:Production,consumption and applications,Nova Science Pub Inc.,Hauppauge,N.Y.,2012
[41]R.W.Millar,R.L.Maycock,Purification and separation of gaseous mixtures,2164194,1939.
[42]E.W.Zublin,Purification and separation of gaseous mixtures,2157879,1939.
[43]G.T.Rochelle,et al.,Amine scrubbing for CO2capture,Science 325(2009)1652–1654.
[44]P.Zhang,Y.Shi,J.Wei,W.Zhao,Q.Ye,Regeneration of 2-amino-2-methyl-1-propanol used for carbon dioxide absorption,J.Environ.Sci.20(2008)39–44.
[45]B.A.Oyenekan,Modeling of strippers for carbon dioxide capture by aqueous amines,ProQuest,2007.
[46]B.A.Oyenekan,G.T.Rochelle,Energy performance of stripper configurations for CO2capture by aqueous amines,Ind.Eng.Chem.Res.45(2006)2457–2464.
[47]R.A.Frimpong,J.E.Remias,J.K.Neathery,K.Liu,Solvent regeneration with a high volatility liquid as stripping carrier,Int.J.Greenhouse Gas Control 9(2012)124–129.
[48]M.Rashidian,Thermal degradation study by continuous thermal stability rig,http://www.diva-portal.org/smash/record.jsf?pid=diva2:6550632013(accessed November 8,2014).
[49]B.R.Strazisar,R.R.Anderson,C.M.White,Degradation pathways for monoethanolamine in a CO2capture facility,Energy Fuel 17(2003)1034–1039.
[50]J.Davis,G.Rochelle,Thermal degradation of monoethanolamine at stripper conditions,Energy Procedia 1(2009)327–333.
[51]I.Eide-Haugmo,O.G.Brakstad,K.A.Hoff,K.R.Sørheim,E.F.da Silva,H.F.Svendsen,Environmental impact of amines,Energy Procedia 1(2009)1297–1304.
[52]S.A.Freeman,J.Davis,G.T.Rochelle,Degradation of aqueous piperazine in carbon dioxide capture,Int.J.Greenhouse Gas Control 4(2010)756–761.
[53]V.P.Talzi,S.V.Ignashin,NMR study of decomposition of monoethanolamine under conditions of industrial gas treatment,Russ.J.Appl.Chem.75(2002)80–85.
[54]J.Reza,A.Trejo,Degradation of aqueous solutions of alkanolamine blends at high temperature,under the presence of CO2and H2S,Chem.Eng.Commun.193(2006)129–138.
[55]S.A.Bedell,Oxidative degradation mechanisms for amines in flue gas capture,Energy Procedia 1(2009)771–778.
[56]H.Lepaumier,E.F.da Silva,A.Einbu,et al.,Comparison of MEA degradation in pilotscale with lab-scale experiments,Energy Procedia 4(2011)1652–1659.
[57]A.K.Voice,G.T.Rochelle,Inhibitors of monoethanolamine oxidation in CO2capture processes,Ind.Eng.Chem.Res.53(2014)16222–16228.
[58]J.Kittel,R.Idem,D.Gelowitz,P.Tontiwachwuthikul,G.Parrain,A.Bonneau,Corrosion in MEA units for CO2capture:Pilot plant studies,Energy Procedia 1(2009)791–797.
[59]I.R.Soosaiprakasam,A.Veawab,Corrosion and polarization behavior of carbon steel in MEA-based CO2capture process,Int.J.Greenhouse Gas Control 2(2008)553–562.
[60]S.Saito,M.Udatsu,H.Kitamura,et al.,Development and evaluation of a new amine solvent at the Mikawa CO2capture pilot plant,Energy Procedia 51(2014)176–183.
[61]E.Sanchez Fernandez,E.L.V.Goetheer,G.Manzolini,E.Macchi,S.Rezvani,T.J.H.Vlugt,Thermodynamic assessment of amine based CO2capture technologies in power plants based on European benchmarking task force methodology,Fuel 129(2014)318–329.
[62]S.Yan,Q.He,P.Ai,Y.Wang,Y.Zhang,Regeneration performance of concentrated CO2-rich alkanolamine solvents:The first step study of a novel concept for reducing regeneration heat consumption by using concentration swing absorption technology,Chem.Eng.Process.Process Intensif.70(2013)86–94.
[63]B.Morero,E.A.Campanella,Simulación del Proceso de Absorción Química con Soluciones de Aminas Para la Purificación Biogás,Inf.Tecnol.24(2013)25–32.
[64]S.Gamba,L.A.Pellegrini,Biogas upgrading:Analysis and comparison between water and chemical scrubbings,Chem.Eng.32(2013)1273–1278.
[65]S.Gamba,L.A.Pellegrini,S.Langè,Energy analysis of different municipal sewage sludge-derived biogas upgrading techniques,Chem.Eng.37(2014)829–834.
[66]L.Günther,Method and device for separating methane and carbon dioxide from biogas,US20100024647 A1,2010.
[67]N.G.Peralta,Mass transfer in chemical engeering processes-Chapter 7-Removal of H2S and CO2from biogas by amine absorption,Ingeniería e Investigación 33(2013)75–76.
[68]A.Gaur,J.-W.Park,S.Maken,H.-J.Song,J.-J.Park,Land fill gas(LFG)processing via adsorption and alkanolamine absorption,Fuel Process.Technol.91(2010)635–640.
[69]N.Tippayawong,P.Thanompongchart,Biogas quality upgrade by simultaneous removal of CO2and H2S in a packed column reactor,Energy 35(2010)4531–4535.
[70]Q.Zhao,E.Leonhardt,C.MacConnell,C.Frear,S.Chen,Purification technologies for biogas generated by anaerobic digestion,Clim.Friendly Farming,http://csanr.wsu.edu/wp-content/uploads/2013/02/CSANR2010-001.Ch09.pdf 2010(accessed January 3,2015).
[71]L.Günther,Method and device for separating methane and carbon dioxide from biogas,US8231706 B2,2012.
[72]P.V.Danckwerts,A.M.Kennedy,The kinetics of absorption of carbon dioxide into neutral and alkaline solutions,Chem.Eng.Sci.8(1958)201–215.
[73]V.A.Juvekar,M.M.Sharma,Absorption of CO2in a suspension of lime,Chem.Eng.Sci.28(1973)825–837.
[74]D.Roberts,P.V.Danckwerts,Kinetics of CO2absorption in alkaline solutions—I transient absorption rates and catalysis by arsenite,Chem.Eng.Sci.17(1962)961–969.
[75]H.Hikita,S.Asai,T.Takatsuka,Absorption of carbon dioxide into aqueous sodium hydroxide and sodium carbonate–bicarbonate solutions,Chem.Eng.J.11(1976)131–141.
[76]J.B.Tepe,B.F.Dodge,Absorption of carbon dioxide by sodium hydroxide solutions in a packed column,Trans.Am.Inst.Chem.Eng.39(1943)255–276.
[77]L.B.Hitchcock,Mechanism of gas–liquid reaction batch absorption of carbon dioxide by stirred alkaline solutions,Ind.Eng.Chem.29(1937)302–308.
[78]F.Yoshida,Y.Miura,Effective interfacial area in packed columns for absorption with chemical reaction,AIChE J.9(1963)331–337.
[79]M.Yoo,S.-J.Han,J.-H.Wee,Carbon dioxide capture capacity of sodium hydroxide aqueous solution,J.Environ.Manag.114(2013)512–519.
[80]S.Gondal,N.Asif,H.F.Svendsen,H.Knuutila,Kinetics of the absorption of carbon dioxide into aqueous hydroxides of lithium,sodium and potassium and blends of hydroxides and carbonates,Chem.Eng.Sci.123(2015)487–499.
[81]M.Mahmoudkhani,D.W.Keith,Low-energy sodium hydroxide recovery for CO2capture from atmospheric air—thermodynamic analysis,Int.J.Greenhouse Gas Control 3(2009)376–384.
[82]A.Aroonwilas,P.Tontiwachwuthikul,A.Chakma,Effects of operating and design parameters on CO2absorption in columns with structured packings,Sep.Purif.Technol.24(2001)403–411.
[83]M.Mahmoudkhani,K.R.Heidel,J.C.Ferreira,D.W.Keith,R.S.Cherry,Low energy packed tower and caustic recovery for direct capture of CO2from air,Energy Procedia 1(2009)1535–1542.
[84]J.K.Stolaroff,D.W.Keith,G.V.Lowry,Carbon dioxide capture from atmospheric air using sodium hydroxide spray,Environ.Sci.Technol.42(2008)2728–2735.
[85]L.Lombardia,A.Corti,E.Carnevale,R.Baciocchi,D.Zingaretti,Carbon dioxide removal and capture for land fi ll gas up-grading,Energy Procedia 4(2011)465–472.
[86]J.Blamey,N.P.M.Paterson,D.R.Dugwell,P.Stevenson,P.S.Fennell,Reactivation of a CaO-based sorbent for CO2capture from stationary sources,Proc.Combust.Inst.33(2011)2673–2681.
[87]D.-R.Han,H.Namkung,H.-M.Lee,D.-G.Huh,H.-T.Kim,CO2sequestration by aqueous mineral carbonation of limestone in a supercritical reactor,J.Ind.Eng.Chem.21(2015)792–796.
[88]L.Reich,L.Yue,R.Bader,W.Lipiński,Towards solar thermochemical carbon dioxide capture via calcium oxide looping:a review,Aerosol Air Qual.Res.14(2014)500–514.
[89]R.M.Santos,W.Verbeeck,P.Knops,K.Rijnsburger,Y.Pontikes,T.Van Gerven,Integrated mineral carbonation reactor technology for sustainable carbon dioxide sequestration:“CO2energy reactor”,Energy Procedia 37(2013)5884–5891.
[90]J.-H.Wee,A review on carbon dioxide capture and storage technology using coal fly ash,Appl.Energy 106(2013)143–151.
[91]W.J.Huijgen,G.-J.Witkamp,R.N.Comans,Mineral CO2sequestration by steel slag carbonation,Environ.Sci.Technol.39(2005)9676–9682.
[92]S.Eloneva,S.Teir,J.Salminen,C.-J.Fogelholm,R.Zevenhoven,Fixation of CO2by carbonating calcium derived from blast furnace slag,Energy 33(2008)1461–1467.
[93]M.F.Bertos,S.J.R.Simons,C.D.Hills,P.J.Carey,A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2,J.Hazard.Mater.B 112(2004)193–205.
[94]D.N.Huntzinger,J.S.Gierke,S.K.Kawatra,T.C.Eisele,L.L.Sutter,Carbon dioxide sequestration in cement kiln dust through mineral carbonation,Environ.Sci.Technol.43(2009)1986–1992.
[95]G.Costa,R.Baciocchi,A.Polettini,R.Pomi,C.D.Hills,P.J.Carey,Current status and perspectives of accelerated carbonation processes on municipal waste combustion residues,Environ.Monit.Assess.135(2007)55–75.
[96]E.Rendek,G.Ducom,P.Germain,Carbon dioxide sequestration in municipal solid waste incinerator(MSWI)bottom ash,J.Hazard.Mater.128(2006)73–79.
[97]R.Pérez-López,G.Montes-Hernandez,J.M.Nieto,F.Renard,L.Charlet,Carbonation of alkaline paper mill waste to reduce CO2greenhouse gas emissions into the atmosphere,Appl.Geochem.23(2008)2292–2300.
[98]E.R.Bobicki,Q.Liu,Z.Xu,H.Zeng,Carbon capture and storage using alkaline industrial wastes,Prog.Energy Combust.Sci.38(2012)302–320.
[99]J.Sipilä,S.Teir,R.Zevenhoven,Carbon dioxide sequestration by mineral carbonation literature review update 2005–2007,Rep.VT 1(2008)2008.
[100]A.Kothandaraman,Carbon dioxide capture by chemical absorption:A solvent comparison study,Massachusetts Institute of Technology,2010.
[101]H.E.Benson,J.H.Field,R.M.Jimeson,CO2absorption:employing hot potassium carbonate solutions,Chem Eng Prog U.S.50(1954)7.
[102]H.A.Blum,L.F.Stutzman,W.S.Dodds,Gas absorption—Absorption of carbon dioxide from air by sodium and potassium hydroxides,Ind.Eng.Chem.44(1952)2969–2974.
[103]C.S.Comstock,B.F.Dodge,Rate of carbon dioxide absorption by carbonate solutions in a packed tower,Ind.Eng.Chem.29(1937)520–529.
[104]C.R.Harte,E.M.Baker,H.H.Purcell,Absorption of carbon dioxide in sodium carbonate–bicarbonate solutions,Ind.Eng.Chem.25(1933)528–531.
[105]D.W.Savage,G.Astarita,S.Joshi,Chemical absorption and desorption of carbon dioxide from hot carbonate solutions,Chem.Eng.Sci.35(1980)1513–1522.
[106]M.R.Bohloul,S.M.Peyghambarzadeh,A.Lee,A.Vatani,Experimental and analytical study of solubility of carbon dioxide in aqueous solutions of potassium carbonate,Int.J.Greenhouse Gas Control 29(2014)169–175.
[107]T.N.G.Borhani,A.Azarpour,V.Akbari,S.R.Wan Alwi,Z.A.Manan,CO2capture with potassium carbonate solutions:A state-of-the-art review,Int.J.Greenhouse Gas Control 41(2015)142–162.
[108]S.Li,H.Jin,L.Gao,K.A.Mumford,K.Smith,G.Stevens,Energy and exergy analyses of an integrated gasi fi cation combined cycle power plant with CO2capture using hot potassium carbonate solvent,Environ.Sci.Technol.48(2014)14814–14821.
[109]Z.Tang,W.Fei,Y.Oli,CO2capture by improved hot potash process,Energy Procedia 4(2011)307–317.
[110]B.P.Spigarelli,S.K.Kawatra,Opportunities and challenges in carbon dioxide capture,J.CO2Util.1(2013)69–87.
[111]Y.E.Kim,J.H.Choi,S.C.Nam,Y.I.Yoon,CO2absorption capacity using aqueous potassium carbonate with 2-methylpiperazine and piperazine,J.Ind.Eng.Chem.18(2012)105–110.
[112]H.Thee,Y.A.Suryaputradinata,K.A.Mumford,K.H.Smith,G.da Silva,S.E.Kentish,et al.,A kinetic and process modeling study of CO2capture with MEA-promoted potassium carbonate solutions,Chem.Eng.J.210(2012)271–279.
[113]K.H.Smith,C.J.Anderson,W.Tao,et al.,Pre-combustion capture of CO2—Results from solvent absorption pilot plant trials using 30 wt%potassium carbonate and boric acid promoted potassium carbonate solvent,Int.J.Greenhouse Gas Control 10(2012)64–73.
[114]H.Thee,N.J.Nicholas,K.H.Smith,G.da Silva,S.E.Kentish,G.W.Stevens,A kinetic study of CO2capture with potassium carbonate solutions promoted with various amino acids:Glycine,sarcosine and proline,Int.J.Greenhouse Gas Control 20(2014)212–222.
[115]R.Baciocchi,A.Corti,G.Costa,L.Lombardi,D.Zingaretti,Storage of carbon dioxide captured in a pilot-scale biogas upgrading plant by accelerated carbonation of industrial residues,Energy Procedia 4(2011)4985–4992.
[116]P.Mostbauer,L.Lombardi,T.Olivieri,S.Lenz,Pilot scale evaluation of the BABIU process—Upgrading of land fill gas or biogas with the use of MSWI bottom ash,Waste Manag.34(2014)125–133.
[117]R.Baciocchi,E.Carnevale,A.Corti,G.Costa,L.Lombardi,T.Olivieri,et al.,Innovative process for biogas upgrading with CO2storage:Results from pilot plant operation,Biomass Bioenergy 53(2013)128–137.
[118]R.Baciocchi,G.Costa,R.Gavasci,L.Lombardi,D.Zingaretti,Regeneration of a spent alkaline solution from a biogas upgrading unit by carbonation of APC residues,Chem.Eng.J.179(2012)63–71.
[119]R.Baciocchi,E.Carnevale,G.Costa,et al.,Performance of a biogas upgrading process based on alkali absorption with regeneration using air pollution control residues,Waste Manag.33(2013)2694–2705.
[120]P.Mostbauer,S.Lenz,Upgrading of lean land fill gas using MSWI bottom ash,Proc.Sard,2007.
[121]K.Starr,X.Gabarrell,G.Villalba,L.Talens,L.Lombardi,Life cycle assessment of biogas upgrading technologies,Waste Manag.32(2012)991–999.
[122]K.Starr,X.Gabarrell,G.Villalba,L.Talens Peiro,L.Lombardi,Potential CO2savings through biomethane generation from municipal waste biogas,Biomass Bioenergy 62(2014)8–16.
[123]K.Starr,L.Talens Peiro,L.Lombardi,X.Gabarrell,G.Villalba,Optimization of environmental benefits of carbon mineralization technologies for biogas upgrading,J.Clean.Prod.76(2014)32–41.
[124]J.van Holst,G.F.Versteeg,D.W.F.Brilman,J.A.Hogendoorn,Kinetic study of CO2with various amino acid salts in aqueous solution,Chem.Eng.Sci.64(2009)59–68.
[125]P.S.Kumar,J.A.Hogendoorn,G.F.Versteeg,P.H.M.Feron,Kinetics of the reaction of CO2with aqueous potassium salt of taurine and glycine,AIChE J.49(2003)203–213.
[126]U.E.Aronu,H.F.Svendsen,K.A.Hoff,Investigation of amine amino acid salts for carbon dioxide absorption,Int.J.Greenhouse Gas Control 4(2010)771–775.
[127]S.Yan,Q.He,S.Zhao,H.Zhai,M.Cao,P.Ai,CO2removal from biogas by using green amino acid salts:Performance evaluation,Fuel Process.Technol.129(2015)203–212.
[128]J.P.Brouwer,P.H.M.Feron,N.A.M.Ten Asbroek,Amino-acid salts for CO2capture from flue gases,Proc.4th Annu.Conf.Carbon capture sequestration Alex.Va.USA,2005.
[129]A.Jensen,J.B.Jensen,C.Faurholt,Studies on carbamates.6.The carbamate of glycine,Acta Chem.Scand.6(1952)395–397.
[130]A.Jensen,C.Faurholt,Studies on carbamates.5.The carbamates of alpha-alanine and beta-alanine,Acta Chem.Scand.6(1952)385–394.
[131]D.E.Penny,T.J.Ritter,Kinetic study of the reaction between carbon dioxide and primary amines,J.Chem.Soc.Faraday Trans.1 Phys.Chem.Condens.Phases 79(1983)2103–2109.
[132]U.E.Aronu,E.T.Hessen,T.Haug-Warberg,K.A.Hoff,H.F.Svendsen,Equilibrium absorption of carbon dioxide by amino acid salt and amine amino acid salt solutions,Energy Procedia 4(2011)109–116.
[133]H.-J.Song,S.Park,H.Kim,A.Gaur,J.-W.Park,S.-J.Lee,Carbon dioxide absorption characteristics of aqueous amino acid salt solutions,Int.J.Greenhouse Gas Control 11(2012)64–72.
[134]P.D.Vaidya,P.Konduru,M.Vaidyanathan,E.Y.Kenig,Kinetics of carbon dioxide removal by aqueous alkaline amino acid salts,Ind.Eng.Chem.Res.49(2010)11067–11072.
[135]U.E.Aronu,A.Hartono,K.A.Hoff,H.F.Svendsen,Kinetics of carbon dioxide absorption into aqueous amino acid salt:Potassium salt of sarcosine solution,Ind.Eng.Chem.Res.50(2011)10465–10475.
[136]H.Knuutila,U.E.Aronu,H.M.Kvamsdal,A.Chikukwa,Post combustion CO2capture with an amino acid salt,Energy Procedia 4(2011)1550–1557.
[137]S.Park,H.-J.Song,J.Park,Selection of suitable aqueous potassium amino acid salts:CH4recovery in coal bed methane via CO2removal,Fuel Process.Technol.120(2014)48–53.
[138]A.Hartono,U.E.Aronu,H.F.Svendsen,Liquid speciation study in amine amino acid salts for CO2absorbent with13C-NMR,Energy Procedia 4(2011)209–215.
[139]K.Simons,K.Nijmeijer,H.Mengers,W.Brilman,M.Wessling,Highly selective amino acid salt solutions as absorption liquid for CO2capture in gas–liquid membrane contactors,Chem.Sus.Chem.3(2010)939–947.
[140]S.Yan,Q.He,S.Zhao,Y.Wang,P.Ai,Biogas upgrading by CO2removal with a highly selective natural amino acid salt in gas–liquid membrane contactor,Chem.Eng.Process.Process Intensif.85(2014)125–135.
[141]Y.ShuiPing,Q.Y.He,C.Kai,A.Ping,Y.Y.Wang,Z.YanLin,Performance of CO2removal from biogas by using amine-based amino acid salts,Nongye Jixie Xuebao Trans.Chin.Soc.Agric.Mach.45(2014)199–205.
 Chinese Journal of Chemical Engineering2016年6期
Chinese Journal of Chemical Engineering2016年6期
- Chinese Journal of Chemical Engineering的其它文章
- Mixture temperature prediction of waxy oil–water two-phase system flowing near wax appearance temperature☆
- Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange☆
- Online process monitoring for complex systems with dynamic weighted principal component analysis☆
- A stepwise optimal design of water network☆
- Integration of coal pyrolysis process with iron ore reduction:Reduction behaviors of iron ore with benzene-containing coal pyrolysis gas as a reducing agent☆
- Multiple linear equation of pore structure and coal–oxygen diffusion on low temperature oxidation process of lignite☆
