Micro fluidic technology for multiphase emulsions morphology adjustment and functional materials preparation☆
Xuehui Ge,Hong Zhao,Tao Wang,Jian Chen,Jianhong Xu*,Guangsheng Luo
The State Key Lab of Chemical Engineering,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
Multiphase emulsions with the advantages of diversity in structures and components,have been broadly utilized as templates for producing materials with their high potential for applications in the fields of controlled substance release[1],separation[2],food science[3],cosmetics[4],pharmaceuticals[5],bio-compatibility[6]and the textile industry[7].For example,the multiphase emulsions could be used as microreservoir by preserving active substance in their cores and protecting them by the shell.After carrying them into desired place,the shell breaks to release the inner substance in a controlled strength and direction.This ability of preservation,transport and controlled release is essential for the drug release in biomedicine field.Their formation methods also have been researched for many decades.However,previous researches using traditional methods,for example,a two-step procedure of emulsification[8],have many disadvantages such as polydispersity,uncontrollable in emulsion size and distribution,and deficiency in regulation of the inner droplets.These disadvantages were resolved in the past decade when microfluidic technology was brought in this field as a novel method in the preparation of emulsions.This has enabled the formation of multiphase emulsions with controlled droplet sizes,structures,and compositions[9].Thus,the monodispersed and operability of multiphase emulsions have been highly improved,which are beneficial to prepare novel functional materials.
Moreover,the morphology of multiphase emulsions has attracted considerable interests because of their significant potential in application,including functional materials[10],medicine[11],ultrasonic imaging[12],and microcrawls[13].Forinstance,double emulsions have two morphologies which are the core–shell structure and Janus structure.The former structure might be used in drug-release while the latter could be emulsion templates for non-sphere materials.Multiphase emulsions could be good templates for preparing particles with different structures by polymerizing droplets in a chosen level[14].Researchers have obtained different morphologies with the same liquid and they could draw a phase diagram to describe that[15].Nicolas et al.[16]have furthered this work by solidifying the emulsions composed of the same liquids to different morphologies when they are in different states,i.e.equilibrium and non-equilibrium states.The equilibrium state is determined by the minimum interfacial energy of the system.And there are three possible morphologies depending on the spreading coefficients related to the interfacial tension between every couple phases in a three-phase system,for which the mathematic relationship was derived by Torza et al.[17].
Thus in the past decades,the research on the multiphase emulsions mainly focuses on their formation,the adjustment in the size and the number,the morphologies and the applications.In this review,we would depict the development of multiphase emulsions made by microfluidic technology about the factors above.We would introduce the typical micro fluidic devices used and describe the formation of multiphase emulsions with accurate control in the size,number and components.The controlled multiphase flow with different flow patterns by micro fluidic devices,as well as the advanced application in fields such as optics,biomedicine,controlled porous material and drug release,will be introduced.The systematic review will be useful to technologists and scientists with various backgrounds and occupations.And we believe that the review will contribute to the readers in this prospective area very well.
2.The Micro fluidic Devices
In the past decades,the application of micro fluidic technology has grown rapidly in various fields,particularly because of the new methods it has provided for the controllable preparation of monodispersed droplets.Generally,monodispersed emulsion droplets can be generated in flow-focusing,T-junction,and coaxial microchannel.Multiphase emulsions have more phases thus the micro fluidic devices have more channels and higher restriction in the interfacial ability of the channels.Because the coaxial microchannel could be easily scaling up by making parallel ones and has lower influence in the interface ability,thus we use coaxial microchannel as our main micro fluidic device.
The typical micro fluidic device is made with a polymethyl methacrylate(PMMA)chip using an end mill.There are several crosses that are craved with specific diameters.Usually,the glass capillaries are inserted into the crosses to work as liquid channels.The outer diameter of the glass capillaries is the same with that of the crosses to guarantee the coaxiality.According to the number of active glass capillaries,the connecting methods,and the fluids used,the typical devices are shown in Fig.1.The capillaries we used are classified as single bore and double bore capillaries.The former is used more generally while the latter is used to form water–oil Janus emulsions with the advantage to inject two liquid phases at the same time without contacting each other.Fig.1(a)shows the double-bore capillary micro fluidic devices.The double-bore capillary is injected into the cross junction to allow the flow of the inner water and inner oil phases.The continuous oil phase flows through the bilateral sides using polytetra fluoroethylene plastic(PTFE)pipes.And another single-pore glass capillary is inserted as the collecting tube of allliquids.One side of the double-bore capillary is pulled to a tip with an inner diameter of 50μm where the Janus droplets are formed.The diameter of the cross channel is 1.50 mm wide×1.50 mm high and the outer diameter of the glass capillary is 1.50 mm,that is how the glass capillary could maintain coaxiality with the channels.The joint of the tubes,capillaries and chips is sealed by glues.Fig.1(b)is the parallel coaxialdevice.The inner liquid phase is injected into the leftmost capillary,and then the middle liquid phase flows through the first cross and shears the inner phase as droplets.At the second cross,the outer phase shears the middle phase to form emulsion droplets with a core inside.The structures of these two crosses are the same.The single-bore capillaries are pulled to tips about 10–100 μm according to the droplet sizes we need.When forming fourphase emulsions,we combine the parallel coaxial device with double syringe needles(could be replaced with double-bore capillaries)in the first capillary as shown in Fig.1(c).The two inner phase fluids are introduced through the parallel micro-needles with inner-diameter of 160 μm and outer-diameter of 300 μm.The diameter of the tapered orifice is approximately 340 μm.The first tapered capillary is inserted into a second capillary for the outer phase and coaxiality is guaranteed by matching the outer diameter of the capillary with the inner dimension of the channel.The second capillary is not tapered.Four PTFE pipes are inserted into the sides of the chip channel for carrying middle and outer phase fluids.All liquids are injected into the micro fluidic device by syringe pumps.
Based on these basic devices,we use them for a specific application.For example,when forming G/W/O(gas in water in oil)or G/O/W(gas in oil in water)emulsions,a one-step micro fluidic device is applied.By making a little adjustment to the parallel coaxial capillary device:injecting a smaller capillary into another capillary,the distance of the two dispersion stage is reduced to a degree that the interference between their flowing conditions can be eliminated.Thus that is much easier to achieve the steady formation of gas/liquid/liquid double emulsions.This is shown in Fig.2.
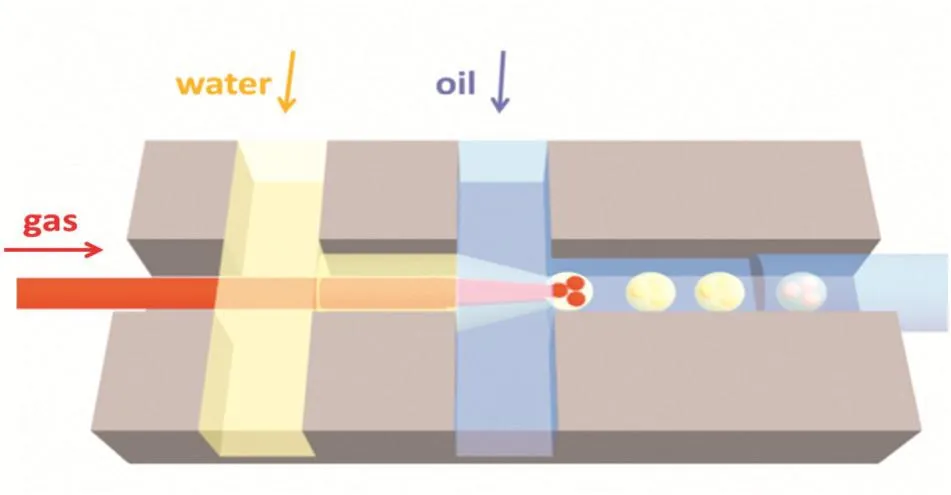
Fig.2.The sketch of the one-step micro fluidic capillary device.
3.The Formation of Multiphase Emulsions
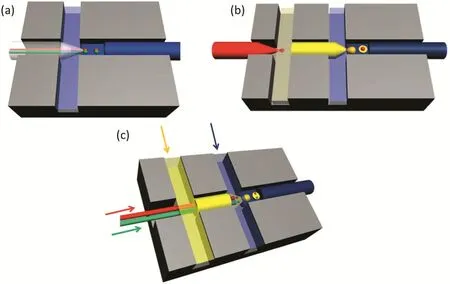
Fig.1.The microfluidic devices used in the multiphase emulsions.(a)The double-bore capillary microfluidic for water–oil Janus formation.(b)The parallel coaxial capillary microfluidic device for formation of the core–shell emulsions.(c)The microfluidic device used for formation of the four-phase emulsions.
The formation of the emulsions is the result of dynamic interface break-up and reconstruction.It is decided by the equilibrium between cross- flow shear force and interfacial force.A dimensionless number,Cacis used to describe the competition between the two forces.

where ucand μcare the velocity and viscosity of the continuous phase respectively,and γ is the surface/interfacial tension.
There are three regimes which distinguish the mechanisms of droplet formation under the different ranges of Cac,which are the squeezing regime(Cac<0.002),the dripping regime(0.01<Cac<0.3),and the transient regime(0.002<Cac<0.01)[18].Three corresponding correlations have been suggested in the different ranges of Cac.As for application,the dripping regime is always used because its stable range is wider.This makes the adjustment for the size and the structure of emulsions easier compared to other regimes.In this regime the droplet size and microbubble size are primarily determined by the balance of viscous shear force caused by the continuous phase and the surface tension/interfacial tension.Thus Caccan be used to predict the dimension of the microbubbles/microdroplets or plugs as Eq.(2)depicts.

ddand diare the dimensions of the dispersed droplets and inner part of the collecting capillary.When the emulsions increased,the analysis method is used to measure each droplet formed in the tips step by step.The velocity of each phase could be adjusted by the syringe pumps through changing the flow rate.
With the direction of Eq.(2)in the dripping regime,we make emulsions with controlled sizes,structures with the adjusted number and size of the inner cores,and various phase combinations by introducing gas and more immiscible phases.
The surfactants should also be introduced here.They are widely used in the drop emulsification process to adjust the interfacial tension and stabilize the emulsion droplets against coalescence[19].The emulsion formation process is the result of dynamic interface break-up and reconstruction of fresh interface in the micro fluidic device.It is unlike other traditional emulsification methods.It involves the sustainable formation of fresh interface between the immiscible fluids while traditional emulsification only forms fresh interface for once.The rate of fresh interface formation in microfluidic is proportionalto formation frequency of the droplets and their surface area.When the surfactants are dissolved in the liquids at sufficient concentrations,the transportation process of surfactants from the bulk onto the freshly created interface requires a finite amountoftime to complete.If the frequency of fresh interface is too high to allow the sufficient surfactant transportation,a surfactant concentration gradient and an interfacial tension gradient would be created at the interface.This gradient creates tangential stresses or“Marangoni stresses”,which may affect the flow dynamics around the interface[20].The interfacialtension involved is called dynamic interfacial tension.
Dong et al.[21]systematically investigated the effects of surfactant concentration and the dynamic interfacial tension of a liquid–liquid system.A novel method by applying dynamic interfacial tension in a coaxial micro fl uidic device to rapidly produce relatively small droplets is proposed.The results presented in this work provide a more in-depth understanding of the dynamic effects of surfactant adsorption on droplet formation and the precisely controllable preparation of monodispersed droplets in microfluidic devices.Fig.3(a)shows typical droplet formation processes in co- flowing microfluidic devices.It can be seen that monodispersed droplets with CV≤2.0%are successfully formed at the tips of the dispersed phase fluid capillary.As shown in Fig.3(b),the drop size decreases from180 to 80μm with the increase of shearing force in the micro fluidic device with a tapered orifice of 10.0 μm.And the droplet size changes little with the increase of the surfactant concentration,while the concentration of SDS is above 5 times the CMC(1.0 wt.%).As a whole,the decrease of surface tension with higher SDS concentration would lead to a smaller droplet size.Butthe in fluence of SDS has a relationship with its concentration itself.At low concentrations of SDS,droplet size decreased with the increase of surfactant concentration under identical shear because of the increase in the adsorption rate of the surfactant on the forming interface.At high concentrations of SDS,the adsorption process is sufficiently fast such that dynamic interfacial tension changes little with the increase of surfactant concentration.The concentration at which interfacial tension ceases to change is assumed to be the saturated adsorption concentration.From Fig.3(c),the droplet size decreases with the increase of shear and surfactant concentration until the concentration of SDS is above 30 times the CMC(8.0 wt.%)in the microfluidic device with a tapered orifice of 3.2 μm.Compared with larger sized droplets,the formation time of droplets with tens of micrometres in diameter is much shorter(several milliseconds),and higher surfactant concentrations are required to reach saturated adsorption of the surfactant at the liquid–liquid interface.
Then,the application of dynamic interfacial tension in enhancing the mass transfer coefficients inside and outside the droplet was evaluated during its formation process by Chen et al.[22].Combining the dynamic adsorption of the surfactantand the scaling law in a microfluidic device,the mass transfer coefficient in one phase was measured.Semiempirical equations with modified parameters were used to evaluate the mass transfer coefficient during the droplet formation process in experimental conditions.The mass transfer inside the droplet is faster than that outside the droplet during droplet formation.It can be concluded that the mass transfer is strengthened by the internal convection of liquid inside the droplet,and the enhancement factor is approximately compared with the diffusion process in our experiments.Therefore,compared with the surfactant in the dispersed phase,more surfactant is needed in the continuous phase to attain the same dynamic interfacial tension during droplet formation,which means that a lower dynamic interfacial tension can be attained by changing the method of surfactant addition instead of increasing the concentration.This work replenishes and puts forward the related theory and the surfactant application in the microfluidic devices.
Based on the dynamic interfacial tension research,it can be seen that in coaxial flow quite stable droplet formation can be achieved in a relatively wide range of conditions.Fig.4(a)and(b)shows how the droplets are made and flowed into the collecting tube.Fig.4(c)and(d)shows the microscopic images of the monodispersed single droplets.Fig.4(e)and(f)shows the SEM images of various particles.These pictures show that the monodispersed single droplets and particles could be easily made by a microfluidic device.
In addition to the single droplet formation process,the multiphase emulsions could be synthesized with accurate control in the size and the number.The emulsions with two and three inner droplets have been made.In Dong's work[22],Ethoxylated trimethylolpropane triacrylate(ETPTA)solutions consist of QDs are injected as inner phase fluid.The middle phase fluid is a poly-(ethylene glycol)diacrylate(PEG-DA)solution with surfactants and a photo-initiator.The outer phase fluid is made up of hexadecane and ABIL EM90 surfactant.By adjusting the flow rate,double emulsion diameter and shell thickness can be adjusted from 100 μm to 200 μmand 20 μm to 80 μm,respectively.By photo polymerizing these double-emulsion droplets in situ with UV light,monodispersed fluorescent microparticles with QD-trapped ETPTA cores and PEG hydrogel shells are produced controllably,as shown in Fig.5(a).In addition,by controlling on the formation frequency of the inner droplets,the inner droplet number could be adjusted.From Fig.5(b)and(c),it could be concluded that the multiphase emulsions composed of different numbers of inner droplets are monodispersed.The size of the shells and the cores,as well as the core number could be adjusted.
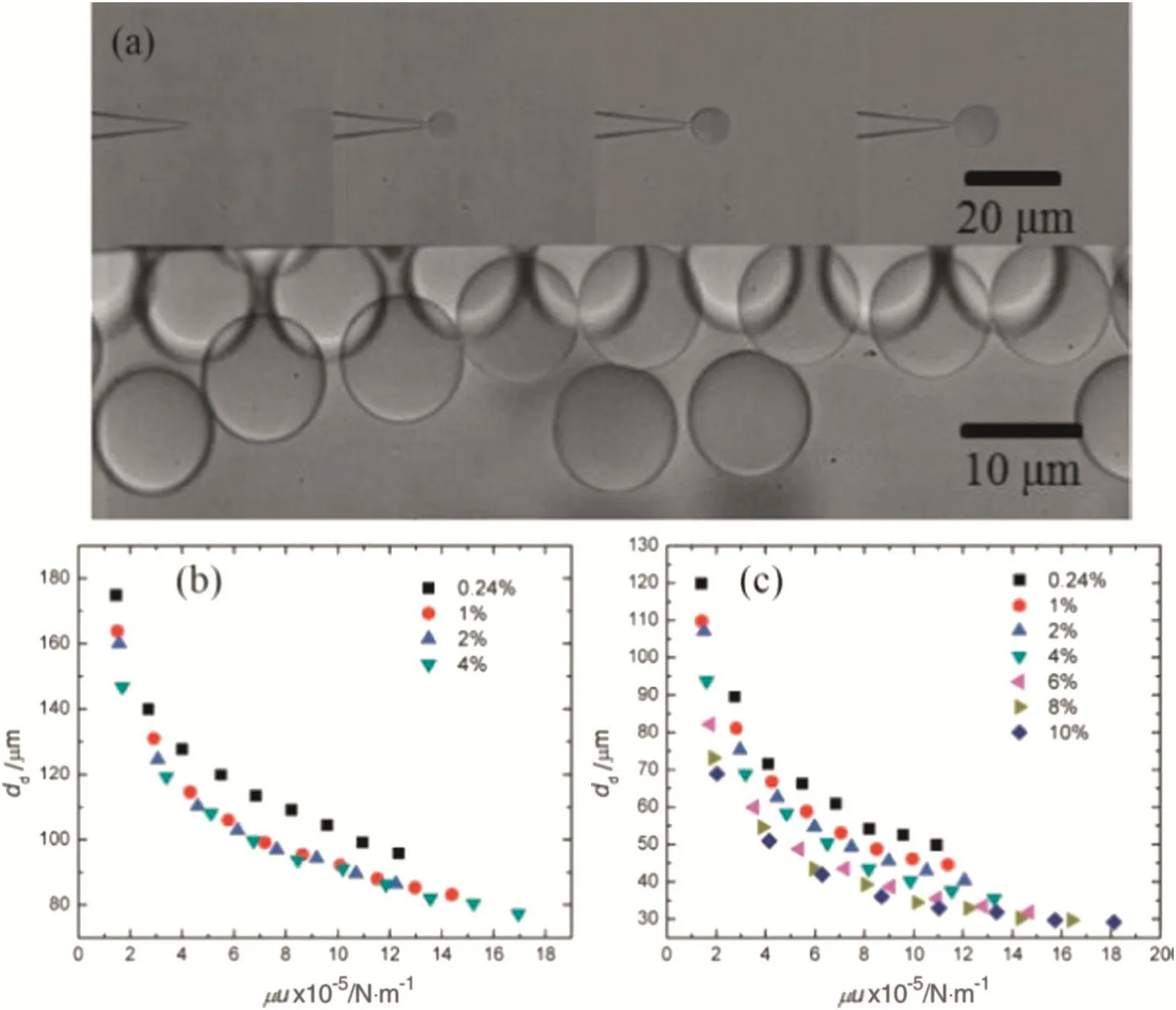
Fig.3.(a)The typical droplet formation process in coflowing microfluidic devices and monodisperse droplets in the collecting area(d n=3.2 μm,Q d=1 μl·min-1,Q c=1000 μl·min-1,CSDS=4.0 wt.%).The droplet size at different tip sizes and operating conditions with SDS as surfactant:(b)tip size of 10.0 μm;(c)tip size of 3.2 μm[21].
The images above show that the liquid–liquid–liquid multiphase emulsions could be synthesized with high monodispersity and controllability.Actually,the multiphase emulsions including the gas phase could also be formed.Chen etal.[23,24]have made great progress in this part of work.Fig.6 shows typical microscope images of the G/W/O double emulsions with different structures.We could observe that the size and number of the encapsulated microbubbles and the thickness of the aqueous phase layer can also be easily controlled by manipulating the three phase flow rates on the formation of G/W/O double emulsions.Even relatively higher volume fractions of gas microbubbles can be achieved inside the water droplets.The multiphase emulsions with gas phase build the foundation for preparing hollow and porous microspheres with microbubbles as the directcore/pore templates.The size of the microbubbles and water plugs can be adjusted by tuning the flow rates of the middle and outer phase fluids.The number of the inner microbubbles can be adjusted by tuning the relative size of the three phase flow rates.
Moreover,other than the three-phase emulsions,the four-phase emulsions have been formed and the evolution process has been observed.Ge etal.[25]have used different four-phase systems to form various emulsions.Similarly,the size and the number of the inner droplets can be adjusted by changing the flow rates of each phase.Fig.7 shows the microscopic pictures of emulsions with two immiscible phases inside and the number ratio of them could be made from 1:1 to 4:1.
4.The Morphology Control of the Multiphase Emulsions
The morphologies are also important for the emulsions because they strongly influence the emulsion abilities and the corresponding application fields.
Three-phase emulsions have two types of structures which are core–shell structure and Janus structure.Core–shell double emulsions are been used in drug delivery because of their ability to load actives in the core and protect them by the shell.Janus emulsions,with natural asymmetry,are used to produce compound functional microparticles with multiphase components and non-spherical shapes.The adjustment in morphology of the former includes the size of the core and the shell and the number of the inner cores.This has been depicted above.The control in the Janus morphology would be explained in this paragraph.It includes the adjustment in the interfacial tensions between immiscible phases and the volume ratio of two Janus lobes.
Four-phase emulsions,compared with three-phase emulsions,introduce another immiscible phase and have more complex structures.The structures they might form and the evolution process would also be shown in this paragraph.Moreover,the research on the four-phase system and the morphology evolution law are presented in this work.Aiming to control the preparation of multiphase emulsions,the researchers[25]have systematically studied the preparation of multiphase emulsions including the core–shell structure,Janus structure and other structures composing of four-phase systems by using a coaxial capillary microfluidic device.Based on that,different types of functional polymer microparticles have been fabricated.
To begin with,the fundament theory to determine the emulsion morphology would be depicted.It is also called the minimum interfacial energy theory.All emulsions tend to reach an equilibrium state of minimum interfacial energy of emulsion system.Researches have been devoted to using mathematic equations to describe that.The basic equations discovered by Torza and Mason[17]used spreading coefficient(Si)to predict the morphology of three fluids.It is as follows,Si=σjk-(σij+σik)(i≠j≠k=1,2,3),σjkrepresents the interfacial tension between j and k fluids.The equations and the corresponding structures are:



Fig.4.The typical droplet formation process in coaxial microfluidic devices(the diameter of the tapered orifice the tapered orifice was 5.0 μm,the collecting tube was 200 μm).(a)Q d=0.1 μl·min-1,Q c=40 μl·min-1;(b)Q d=0.1 μl·min-1,Q c=100 μl·min-1)and(c)and(d)the monodispersed droplets collected on glass slide.(e)and(f)are SEM images of polymer microparticles.(e)Polyhedral particles and(f)PMMA particles[21].
To research on the Janus morphology,Xu and Ge[26]chose the liquid–liquid system whose spreading coefficient meets the needs of Eq.(4).The subtle regulation in Janus morphology could be changed by adjusting the interfacial tensions and the flow rate ratio of two Janus-forming phases.The interfacial tension between each phase can be changed by the mass fraction of surfactant in each phase.They used the mass fraction of Span 80(surfactant)in the liquid paraffin to fulfill that.The interfacial tension between water and liquid paraffin would decrease with the increasing surfactant mass fraction.Fig.8 shows the Janus droplets and the controllable Janus morphologies.Fig.8(a)shows the microscopic image of the formation process of Janus droplets and the monodispersed droplets in a Petri dish.Fig.8(b)shows different Janus morphologies with different flow rate ratios of water to ETPTA.When the flow ratio of water to ETPTA is 1:10,the ETPTA(red)is much bigger than the water phase.When the flow ratio is 1:1,the two lobes are almost the same.When it is 10:1,the water phase is much bigger.This adjustment method is very simple and direct to change the Janus morphology.Fig.8(c)shows the various morphologies with increasing Span 80 mass fraction(Span 80 is surfactant in the liquid paraffin).Remarkably,when there is no Span 80 in the liquid paraffin,the water is engulfed in the ETPTA to form a core–shell structure.As long as a little amount of Span 80 is added into liquid paraffin,it decreases the interfacial tension between water and liquid paraffin heavily.Thus a Janus morphology is formed.When the mass fraction of Span 80 is 0.4 wt.%,a large part of water is engulfed in the ETPTA,while when the mass fraction increases to 0.8 wt.%,more part of water separates from the ETPTA.This could be easily explained by the minimum energy theory.The interfacial tension between water and liquid paraffin without surfactant is~10 mN·m-1while that between ETPTA and liquid paraffin is~4 mN·m-1.Thus water would tend to be engulfed by ETPTA to decrease the interfacial energy of the system.When the mass fraction of Span 80 in the liquid paraffin increases,the interfacial tension between water and liquid paraffin decreases while that between ETPTA and liquid paraffin remains almost the same.In this case,the water separates from the ETPTA to form Janus and the separating part of water increases as the interfacial tension decreases.
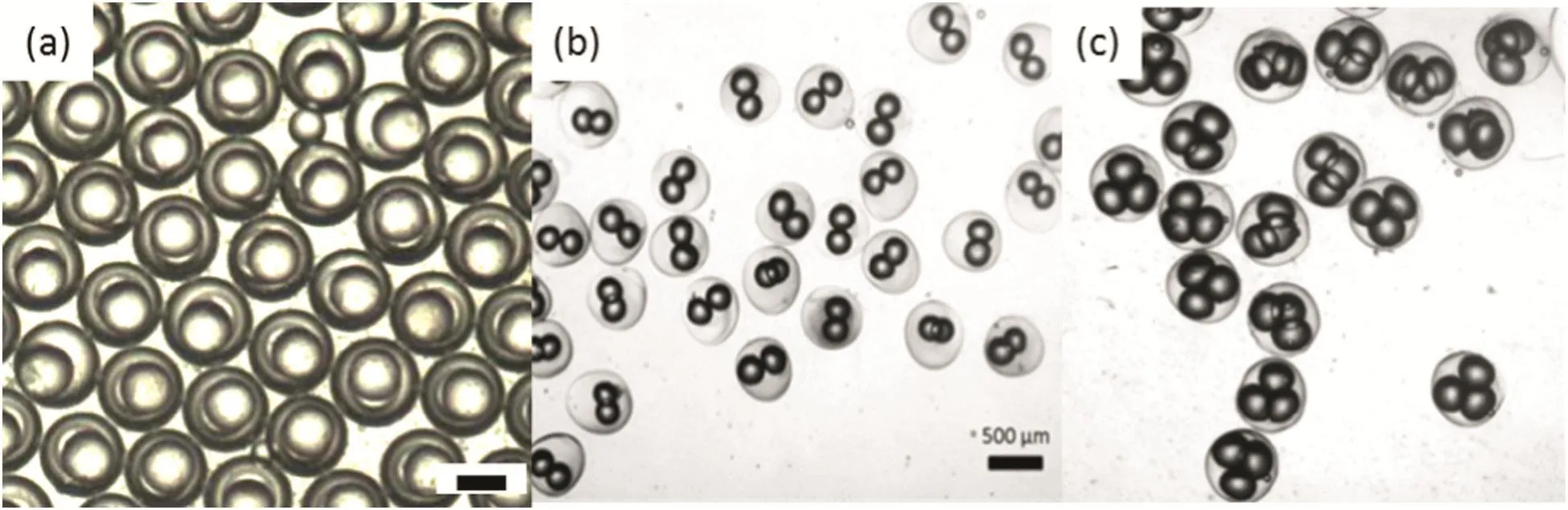
Fig.5.The double emulsions with controlled number cores.(a)Optical micrograph of the core–shell microparticles with QD-tagged polymer cores and PEG hydrogel shells.The scale bar is 200 μm.(b)Optical micrograph of the core–shell microparticles with bi-cores.(c)Optical micrograph of the tri-colour core–shell microparticles[22].
Based on the minimum interfacial energy theory,the four-phase emulsions are synthesized and their morphology evolution processes are observed and analysed.Ge et al.[25]developed a simple capillary microfluidic device to prepare double emulsions with two-phase cores.They can control the structure and size of double emulsions by changing the four-phase flow rates.Based on the formation of these four-phase double emulsions,they investigated the structure evolution of the double emulsions for different systems,and successfully obtained different multiphase emulsions with four typical inner structures.They are separately-engulfing structure,triple emulsion structure,Janus-engulfing structure,and inner-Janus structure.Fig.9 shows the microscopic images of the evolution process and equilibrium state of the four-phase system.Fig.9(a)shows the evolution process from the core–shell structure to various morphologies according their interfacial tension relationship.These images are taken through the high-speed camera.It could be observed that the evolution speed is very fast that emulsions would reach their equilibrium states less than 1 s.Fig.9(b)shows the equilibrium morphologies.They are separately-engulfing structure(Fig.9(b)1),triple emulsion structure(Fig.9(b)2),inner-Janus structure(Fig.9(b)3)and Janus-engulfing structure(Fig.9(b)4).The separately-engulfing structure is formed when two inner droplets separate from each other completely,and they tend to remain apart in the equilibrium state.The triple emulsion structure means that the inner two droplets get coalescence when they come in contact and then one phase surrounds the other phase to finally form a triple emulsion.The inner-Janus structure is a novel structure with two inner droplets forming a Janus structure within the middle phase droplet.The Janus-engulfing structure is formed when the two inner droplets keep apart with each other and one forms a Janus structure with the middle phase droplet while the other one is surrounded inside it.The equilibrium morphologies of the double emulsions could be estimated by using the relationship of interfacial tension between different phase fluids.Remarkably,the physics of evolution of double emulsions with two-phase cores in microfluidic device allows the synthesis of microparticles with new morphologies and more applications in encapsulation of multiple active substances.

Fig.6.Microscope photographs of the different structures of the G/W/Odouble emulsions.(a)Droplets containing different numbers of bubbles of the same size in the observation channel.(b)The G/W/O emulsions with one,three and four microbubbles per droplets collected outside in a glass culture plate[23,24].

Fig.7.The typical micrographs of oil&water/oil/water double emulsion with increased number and ratio of encapsulated inner droplets.These are observed in the outer flow channel.The big one is aqueous droplet and the other is oil droplet.The number ratios of oil droplets to aqueous droplets are(a)1:1;(b)2:1;(c)3:1;and(d)4:1.The fluids are water+1%PVA+2%SDS&TPGDA/silicon oil(50 mPa·s)+10%Dow corning/water+1%PVA+2%SDS.The scale bar is 200 μm[25].
5.The Applications of the Multiphase Emulsions

Fig.8.The microscopic images of the Janus droplets.Here ETPTA is dyed red with Sudan Red.(a)The formation process of Janus droplets and the monodispersed droplets dispersed in the Petri dish.(b)The different Janus morphologies with different flow rate ratios of water to ETPTA.From the left to the right,the flow rate ratios are 1:10,1:1,and 10:1,respectively.(c)The different Janus morphologies with different surfactant mass fractions.From the left to the right,the surfactant mass fraction is:0 wt.%,0.4 wt.%and 0.8 wt.%,respectively[26].

Fig.9.The microscopic images of the four-phase system.(a)The evolution from core–shell structure to different morphologies.(b)The four typical morphologies of four-phase system.They are(1)separately-engulfing structure,(2)triple emulsion structure,(3)inner-Janus structure and(4)Janus-engulfing structure.The scale bar here represents 100 μm[25].
Because the emulsionscould be highly adjusted in the size and structure through a microfluidic device,it could be utilized together with the various functional raw materials to form functional particles with specific morphologies.In this part,the progress in forming particles applied in the fields such as optical particle templates,biomedicine,hollow and porous particle templates,targeted drug delivery and high efficiency extraction process would be introduced.
5.1.Optical materials
Orderly self-assembled materials could have special optical properties because the building blocks are organized spontaneously into bulk thermodynamic phases.However,undesired defects of selfasse mbled structures such as random distributed holes or humps inside or outside of self-assembled structures would strongly influence its optical properties which is difficult to avoid.Furthermore,it is hard to control the quality of the ordered arrangement and packing structure.In the past decade,researchers showed successful fabrication of relatively well-ordered structures of colloids with the confined geometry effect of microchannels or capillary tubes[27].Confined colloidal particles in droplets could be self-organized into a spherical colloidal crystal(or photonic crystal ball)[28],exhibiting an optical stop band for any normal incident light homogeneously over the spherical surface because of the isotropic shrinking of droplets with slow evaporation of the liquid phase of the droplets.Since then,an emulsion-based method for creating spherical-shaped monodispersed colloidal crystals and more complicated structures has been developed.In this method,the self-assembly process of colloidal particles is wellconfined geometrically within monodispersed emulsion droplets.Thus,the formation of high quality colloidal crystal beads requires a specific and controllable size,a small size distribution,and good reproducibility.
Here by using a microfluidic device and a solvent-extraction method,Xu et al.[29]demonstrated that monodispersed colloidal particles inside emulsion droplets can be organized into core–shell colloidal crystal microbeads.In this work,monodispersed water-in-oil emulsions are formed,in which the oil phase acts as an extractant and the water in the aqueous droplets diffuses into the oil phase.This leaves the insoluble materials inside the shrinking droplet to consolidate to form a solid bead.The diameter of aqueous droplets produced could be easily controlled by changing the velocity of the two streams.Optical and SEM micrographs of the microbe ads are shown in Fig.10.The microbeads were identified by a characteristic iridescent opaline colour.We could find from Fig.10(a)and(d)that the particles are highly monodispersed and well arranged.Fig.10(b)and(d)shows clear evidence of the formation of spherical colloidal crystals or photonic balls.
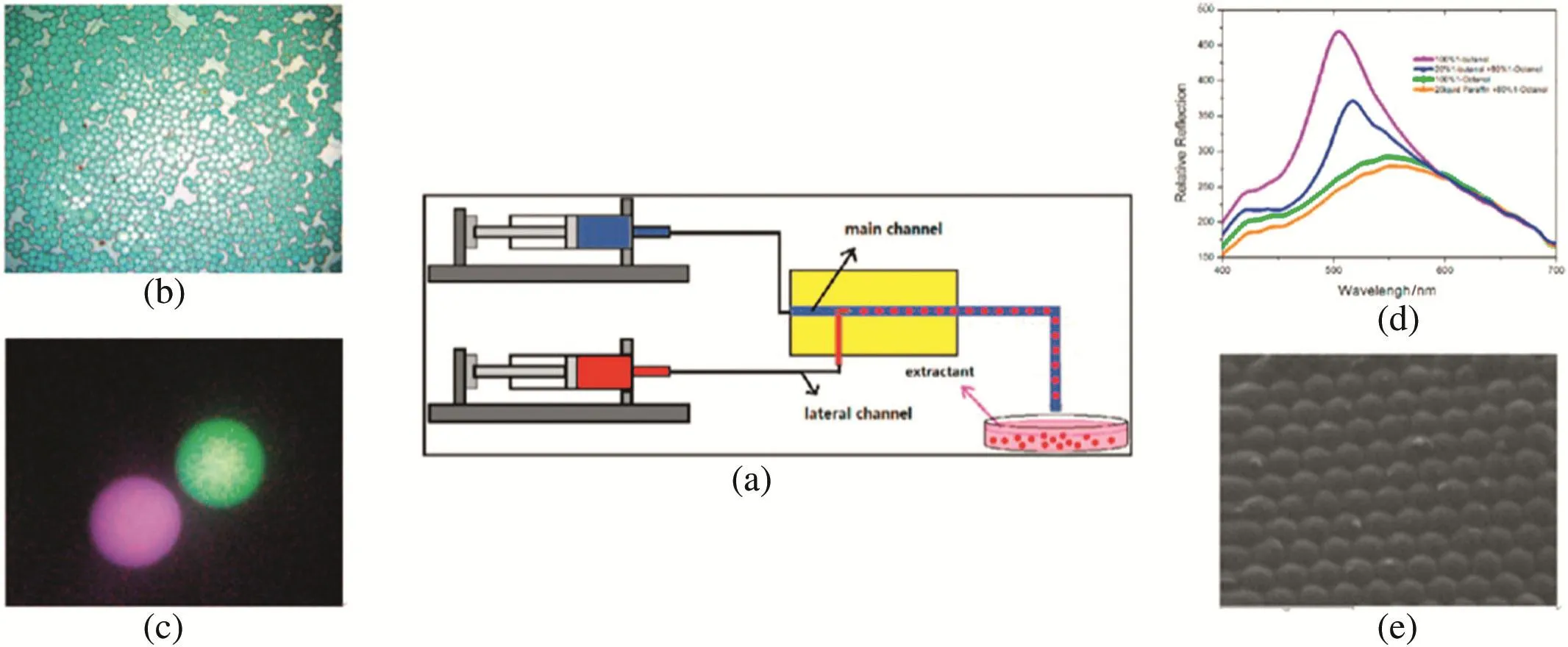
Fig.10.(a)The schematic diagram of the device for fabricating photonic microbeads in an extraction-derived method.(b)Optical image of monodispersed photonic beads constructed by colloidal PS particles of 200 nm in diameter and extracted by 20 wt.%n-butyl alcohol in 80 wt.% n-octyl alcohol.(c)An optical image of microbeads which are sized 45 μm in diameter.(d)Reflectance spectrum of microbeads extracted by 20 wt.%n-butyl alcohol in n-octyl alcohol,by pure n-octyl alcohol and by 30 wt.%hexadecane in n-octyl alcohol.(e)SEM images of microbeads constructed by colloidal PS particles of 200 nm in diameter and extracted by 20 wt.%n-butyl alcohol in 80 wt.%n-octyl alcohol[29].
Moreover,they introduced gas bubbles to attach on the aqueous colloidal suspension droplets when forming the droplets in a double coaxial microfluidic device[30].The microbubble and the attached liquid formed Janus droplets,creating a triphase interface on different sides.Thus the mass transfer rates across the interface are not the same,leading to a “coffee ring”effect.This creates circumflux inside the aqueous droplets which might lead to a concentrating of colloidal particles towards the triphase interface.The optical properties of close-packed polystyrene colloidal crystal particles could be improved and controlled via adjusting their shape.By adjusting the structure of Janus droplets,they could control the shape of colloidal microparticles.In Fig.11,four typical shapes could be observed,as plate-like,bowl-like,potato-like,and spherical,respectively.All of them have a smooth shape and bright reflection of certain wavelengths.When investigating the reflection spectra of particles of different shapes,it could be found that the relative strength of the reflection peak increases remarkably with the decrease of sphericity of microparticles.It shows that the nonspherical particles have stronger optical properties than the sphericalones.There is a noteworthy phenomenon that the area where the curve radius is the smallest,namely the initial triphase interface,always has the best optical property,the relative area of which is positive to the integral optical properties.This progress could be useful in optimizing the performance of biosensors and in developing other fields related to photonic crystals.
5.2.Fluorescence-encoded microparticles
High-throughput multiplexed detection has high value in many biomedical applications such as clinical diagnostic,drug discovery,and genetic analysis[31].Optical barcoding technology which can be rapidly processed using conventional flow cytometry,has become an emerging platform[32].It is based on fluorescence-encoded microparticles and semiconductor quantum dots(QDs)are ideal fluorophores compared with traditional fluorescent dyes.QDs have excellent optical properties for encoding including minimal emission width,size-tunable fluorescence,remarkable photo stability against bleaching and broad excitation range[33].Furthermore,microparticles with a large number of unique spectral barcodes can be produced by embedding QDs with different sizes at precisely controlled concentrations.This can be used to identify the recognition molecules conjugated on the surface of microparticles[34].Remarkably,the mono-dispersity and stability of the coded microparticles would strongly influence the recognizable and diversified coding.By using capillary microfluidics,Chen et al.[22]demonstrated a facile method to prepare multicolour QD-encoded core–shell microparticles with precise coding and enhanced stability.These microparticles are composed of QD-trapped polymer cores and hydrogel shells.By using O/W/O double emulsions with multi-cores as templates,QDs were dispersed and embedded in different polymer cores after UV polymerization,respectively.Then they measure the fluorescence spectrum of the whole microparticles.Fig.12 shows the formation process of emulsion templates with two QDs and the spectra of the whole microparticles.The process of making particles with three QDs is similar.From Fig.12(h),we could find that the fluorescence spectrum of the whole microparticles fitted well with those of the cores,which meant that no interaction existed between the two kinds of QDs.Especially,from Fig.12(i),the peak intensities of the encoded microparticles are in good accordance with the original encoding ratios,showing a linear relationship between the fluorescence intensity and the concentration of embedded QDs.
Furthermore,tri-colour QD-encoded microparticles with three different cores were generated,and the fluorescence spectrum of microparticles was also in good accordance with that of each core,as shown in Fig.13.Thus,by changing the core number and the QD concentration in each core,precise codes could be generated.Significantly,the fluorescence spectrum of each kind of QDs could be recorded by detecting each core respectively,with no need for the help of spectral deconvolution or signal processing methods.As a result,a spectral overlapping problem could be avoided and the embedded QD species could be greatly increased.Furthermore,the hydrogel shells could prevent the leakage of QDs and thus the stability of the fluorescent microparticles is improved significantly.

Fig.12.(a)Scheme of the microfluidic device for bi-core double emulsion preparation.(b)High-speed micrograph of the formation of the emulsions.(c)Optical micrograph of the core–shell microparticles with bi-cores.(d–g)Laser scanning confocal microscope(LSCM)images in 558 nmand 607 nm;bright- field microscope image;combination of the above images of the core–shell microparticles with bi-cores.(h)Representative spectra of multicolour QD-encoded microparticles containing two types of QDs mixed in ratios of 1.75:1,1.17:1,and 0.58:1.(i)The fluorescence intensities as a function of the concentration of QDs in the QD-encoded microparticles[22].
5.3.Functional microspheres based on chitosan
Chitosan,with an abundance of–NH2on its chain,has great adsorption capability of protein and metal ions.Therefore,chitosan has the potential to be used in adsorption,drug controlled release,and catalysis in the form of microspheres.
Xu et al.[35]have made progress in forming chitosan microparticles and apply them into drug release and heavy metal ion adsorption.
Different drug release patterns are obtained through loading drug in microspheres with various diameters or structures.Chitosan microspheres with controllable structures,from porous,core–shell to solid,are prepared in one-step and the diameter is controlled between 25 μm and 65 μm which is shown in Fig.14[36].Chitosan microspheres with different structures are obtained simply through controlling the residence time of droplets in a solidification bath.From Fig.14(a),it could be observed that denser structure is obtained with longer solidification time.Then BSA is in situ encapsulated in the microspheres with different structures to obtain different BSA release profiles.From Fig.14(b),we could find that a porous structure could lead to faster release velocity.With different release profiles,various requirements of different drugs can be met,which lead to good foundation for specific applications.
Even though chitosan microspheres have high adsorption potential for ions,their poor mechanical property has bad effect on its practical applications.To solve this problem,many researchers try to composite chitosan with inorganic materials.Due to synergistic effects resulting from the physical or chemical interactions between inorganic and organic components,these hybrid materials can show improved catalytic,optical,thermal and mechanical properties[37].Lan et al.[38]developed a one-step microfluidic approach to prepare chitosan–silica hybrid microspheres in co-axial microchannel.Chitosan–silica sol prepared by dissolving tetraethoxysilane(TEOS)in a chitosan/acetic acid aqueous solution and stirring overnight was emulsified in an organic phase containing n-octanoland 30 wt.%TOA.Through extraction of acetic acid and water to the organic phase,the droplets were solidified to microspheres whose structure could be controlled through the amount of oleic acid.Compared with chitosan microspheres,chitosan–silica hybrid microspheres shown in Fig.15(a)show higher mechanical intensity.But the hybrid microspheres in this work are relatively dense to impede the adsorption.Then Zhao et al.[39]improved the hybrid chitosan–silica microspheres by using n-octanol with glutaraldehyde and TOA as a solidification bath in co-axial microchannel to increase the specific surface area.The hybrid microspheres show spongy pore and could adsorb 50 mg·g-1Cu(II)in 6 h,as shown in Fig.15(b).Xu et al.[40]improved the adsorption capability to 72 mg·g-1in 25 h by preparing porous chitosan–poly(acrylic acid)hybrid microspheres using poly(ethylene glycol)(PEG)as pore template shown in Fig.15(c).Then chitosan/silica core–shell hybrid microspheres were prepared with chitosan aqueous solution and silica sol as inner fluid and middle fluid[41].The droplets were first pre-solidified for 20 min in a solidification bath and then immersed in n-octane for 24 h to make silica solgelated.Due to the protection of–NH2from excessively crosslinking by silica shell,the core–shell hybrid microspheres could adsorb Cu(II)above 90 mg·g-1in 10 h.
Furthermore,chitosan could also be used as catalyst support in biocatalytic and organic catalytic reactions.In Zhao's work,they adsorbed Cu(II)and then reduced it to Cu(I)to catalyse the click reaction between benzyl azide and phenylacetylene[41].From Table 1,it could be concluded that the reaction could be completed in water within 3 h at room temperature with recyclable and reproducible catalyst.Silica/chitosan core–shell hybrid microspheres loaded with CuI could also be used to catalyse the terminal alkyne homocoupling reaction[42].Chitosan hybrid microspherescan also be used to immobilize enzyme to form compound catalyst in biological reaction.Xu et al.[43]prepared chitosan–poly(acrylic acid)composite microspheres with porous surface structure and apply it in immobilization of glucose isomerase on the microspheres.They showed better pHand temperature stability compared with free glucose isomerase(GI).In addition,over 90%enzyme activity was kept after using 10 times,as shown in Fig.16.
5.4.Stimulation-responsive microcapsules for targeted release
Stimulation-responsive microcapsules have considerable applications in agricultural industries[34],medical technology[44],and drug delivery[45].They are always taking advantage of temperature[46],pH[47],ultrasound irradiation and magnetic fields[48].Among all the abovementioned advantages,thermo-responsive materials such as PNIPAm have been widely used due to its simplicity and quick responsibility.
In addition,targeted release is essential in drug release to maximize the drug efficiency and minimize the possible side effects to healthy cells or issues.Recently,researchers found that monodispersed drug barriers could also increase the drug dosage efficiency.Thus,microfluidics whose advantages include high control in monodispersed emulsions has become a promising method in this field.Asymmetrical morphology emulsions made by a microfluidic device have been used to enhance orientation.For instance,Chen et al.[49]and Jeong et al.[50]utilized branched polymers and phase separation to prepare magnetic Janus particles.These Janus microgels with magnetic nanoparticles offered an orientation property by applying a magnetic field.However,accuracy and efficiency are still lacking because the microgels could only release the active substances in a certain area rather than reach a specific site.

Fig.14.The mechanism of chitosan microsphere generation process and in vitro BSA release profiles for microspheres with different structures[36].
Then,Ge et al.[51]developed a novel and facile microfluidic approach to achieve an accurate and efficient targeted release method of the active substance in the core by utilizing the asymmetric advantage and magnetic ability of magnetic decentred core–shell microcapsules.The microcapsules could burstrelease the drug directly towards the target,targeting the tissue or cells efficiently.The synthetic process for these microcapsules is shown in Fig.17.They developed a novel and simple microfluidic approach for achieving targeted burst release with good controlofthe release location and direction by preparing magnetic decentred core–shell microcapsules.The temperature-responsive nature of the PNIPAm microcapsule,with an asymmetric distribution of the shell thickness,contributes to the asymmetric shrinkage of the shell,thus realizing the burst release of the active substance in the thinner shell membrane direction.
The microphotographs and the corresponding schematic diagrams of the targeted release process are shown in Fig.18.The burstrelease direction could be simply controlled towards the right(Fig.18(a))or towards the top(Fig.18(b))by positioning the magnet either at the left or bottom of the microcapsule,respectively.As the ambient temperature increased,the microcapsule shrank immediately and burstreleased the active substance directly towards the target.Five stages could be observed in the burst release of the microcapsule.Firstly,the decentred core–shell microcapsule was the original stage.Secondly,in an extruding stage,the microcapsule started to shrink when the temperature was increased.The thicker side of the shell shrank faster than the thinner side,pushing the inner core even more eccentric.Then,approaching the deformation stage,the difference in the shrinkage forces enlarged the differences in the shell thickness,deforming the inner core.When deformation increased,the thinnest side of the shell started to rupture,the shrinkage force arrived at the maximum,and so it was the rupture stage.Finally,the microcapsule reached the burst release stage,in which the inner core burst out directly with a high speed and a straight ejection trace in the opposite direction to the magnet.The transformation through the stages takes less than 10 s.
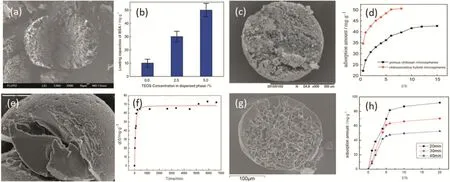
Fig.15.Different chitosan-based hybrid microspheres and their adsorption properties.(a)(b)Chitosan–silica hybrid microspheres;(c)(d)chitosan–silica hybrid microspheres;(e)(f)porous chitosan–poly(acrylic acid)hybrid microspheres;(g)(h)chitosan/silica core–shell hybrid microspheres[39].

Table 1 Recycling of the catalyst
This method,with accurate targeting and facile control,offers a novel approach to control the stimuli-responsive targeted release of an active substance.Furthermore,these microcapsules combining two or more environmental responses and asymmetric structures can greatly contribute to the development of barrier synthesis with highlytailored drug release.
5.5.Enhancement of high phase ratio liquid–liquid extraction

Fig.16.Operating stability of immobilized GI[43].

Fig.17.The synthetic process for magnetic decentred core–shell microcapsules[51].
A one-step microfluidic capillary device for the enhancement of the mass transfer process with high phase ratio by a hollow droplet was developed[52].During the droplet formation stage,the fluorescent brightness of the droplets becomes stronger for a droplet with and without a bubble in it.During the droplet moving stage,for both flow conditions,the fluorescence intensity increases along the outlet channel.At the latter half of the outlet channel,the addition of gas greatly enhances the mass transfer process.The mean overall volumetric mass transfer coefficients kLa increase with increasing gas flow rate;this is mostly because of the sharply increased specific area.Moreover,the kLa value of gas–liquid–liquid hollow droplet flow increases around 10–60 times compared to that of a liquid–liquid droplet flow system.The length of extraction equipment needed to reach 95%extraction efficiency is reduced to around 10–1000 times when gas microbubbles are introduced.Based on the experimental data,a theoretical model has been built up for the potential prediction of the enhancement of the extraction by adding gas microbubbles.The effective diffusion coefficient is introduced to combine the convective mass transfer factor into this model.The modelling results fit well with the experimental data.All the abovementioned results present a practical method for the enhancement of the extraction process with high phase ratio systems,which has potential applications in analytical chemistry,micro-extraction,and biological extraction.Fig.19 shows the comparison of the extraction process without and with gas in the emulsion.From top to bottom,they are emulsions without gas and with gas of different frequencies while fixing the emulsion flow rates.When the gas frequency increases,the emulsion distance between each other decreases and boosts the extraction.Fig.19(a)shows the microscopic images,Fig.19(b)is the sketch and Fig.19(c)shows the fluorescent images.The fluorescence intensity which represents the mass of Rhodamine B could show the extraction efficiency of the process.From Fig.19(c),it could be observed clearly that the existence of the gas would increase the extraction and with the frequency of gas increasing,the extraction process could be intensified.
6.Conclusions and Outlook
Emulsions which are natural templates for particles,have been researched for decades.The improvement of the microfluidic device makes emulsion formation more accurate and controllable.The research on the liquid flow patterns,the basic force equilibrium equations and the scaling up of the microfluidic devices is widely carried out.
Here in this review,we describe the recent progress in the device making,the flow pattern control and the multiphase emulsion formation and their applications.Multiphase emulsions with controlled sizes,complex structures,and various phase combinations by introducing gas and more immiscible phases have been produced.The emulsion morphologies of the three phase system,including core–shell and Janus structures have been deeply depicted through the minimum energy theory and experimental methods.In addition,the four-phase system has been explored for the first time by observing the evolution process of the emulsions from the initialstate to the equilibrium state.Four typical morphologies of the four-phase system have been discovered and the primary theory in predicting the four-phase system has been built.
We also depict the great application potentials for the multiphase emulsions in fields such as optical particle templates,biomedicine,hollow and porous particle templates,targeted drug delivery and high efficiency extraction process.By taking the advantages of high monodispersity and manoeuvrability in the microfluidic devices,the wide variety of emulsion morphologies and the functional materials and components,these researches have made great progress in their specific areas.
In the future work,more efforts would be focused on the application in the wider fields,deeper understanding of the dynamic interfacial phenomena and the structure–function relationships for functional material design.Moreover,the large-scale production of multiphase emulsions and functional materials,which is the major restriction of their development,is highly imperative for more efficient applications in real industry.
[1]C.Laugel,et al.,Oil–water–oil multiple emulsions for prolonged delivery of hydrocortisone after topical application:comparison with simple emulsions,Int.J.Pharm.160(1)(1998)109–117.
[2]B.Raghuraman,et al.,Emulsion liquid membranes for waste-water treatmentequilibrium-models for some typical metal–extractant systems,Environ.Sci.Technol.28(6)(1994)1090–1098.
[3]K.J.Lissant,et al.,Structure of high internal phase ratio emulsions,J.Colloid Interface Sci.47(2)(1974)416–423.
[4]M.Gallarate,et al.,On the stability of ascorbic acid in emulsified systems for topical and cosmetic use,Int.J.Pharm.188(2)(1999)233–241.
[5]C.Kaewsaneha,et al.,Janus Colloidal Particles:Preparation,Properties,and Biomedical Applications,ACS Appl.Mater.Interfaces 5(6)(2013)1857–1869.
[6]J.Bibette,et al.,Emulsion Science—Basic Principles.An Overview—Introduction,Emulsion Science:Basic Principles– An Overview,1812002 1–4.
[7]S.Matsumoto,et al.,Attempt at preparing water-in-oil-in-water multiple-phase emulsions,J.Colloid Interface Sci.57(2)(1976)353–361.
[8]L.Y.Chu,et al.,Controllable monodisperse multiple emulsions,Angew.Chem.Int.Ed.46(47)(2007)8970–8974.
[9]H.C.Shum,et al.,Multicompartment polymersomes from double emulsions,Angew.Chem.Int.Ed.50(7)(2011)1648–1651.
[10]L.L.A.Adams,et al.,Single step emulsification for the generation of multi-component double emulsions(vol 8,pg 10719,2012),Soft Matter 8(48)(2012)12132.
[11]S.K.Lee,et al.,Synthesis,assembly and reaction of a nanocatalyst in microfluidic systems:a general platform,Lab Chip 12(20)(2012)4080–4084.
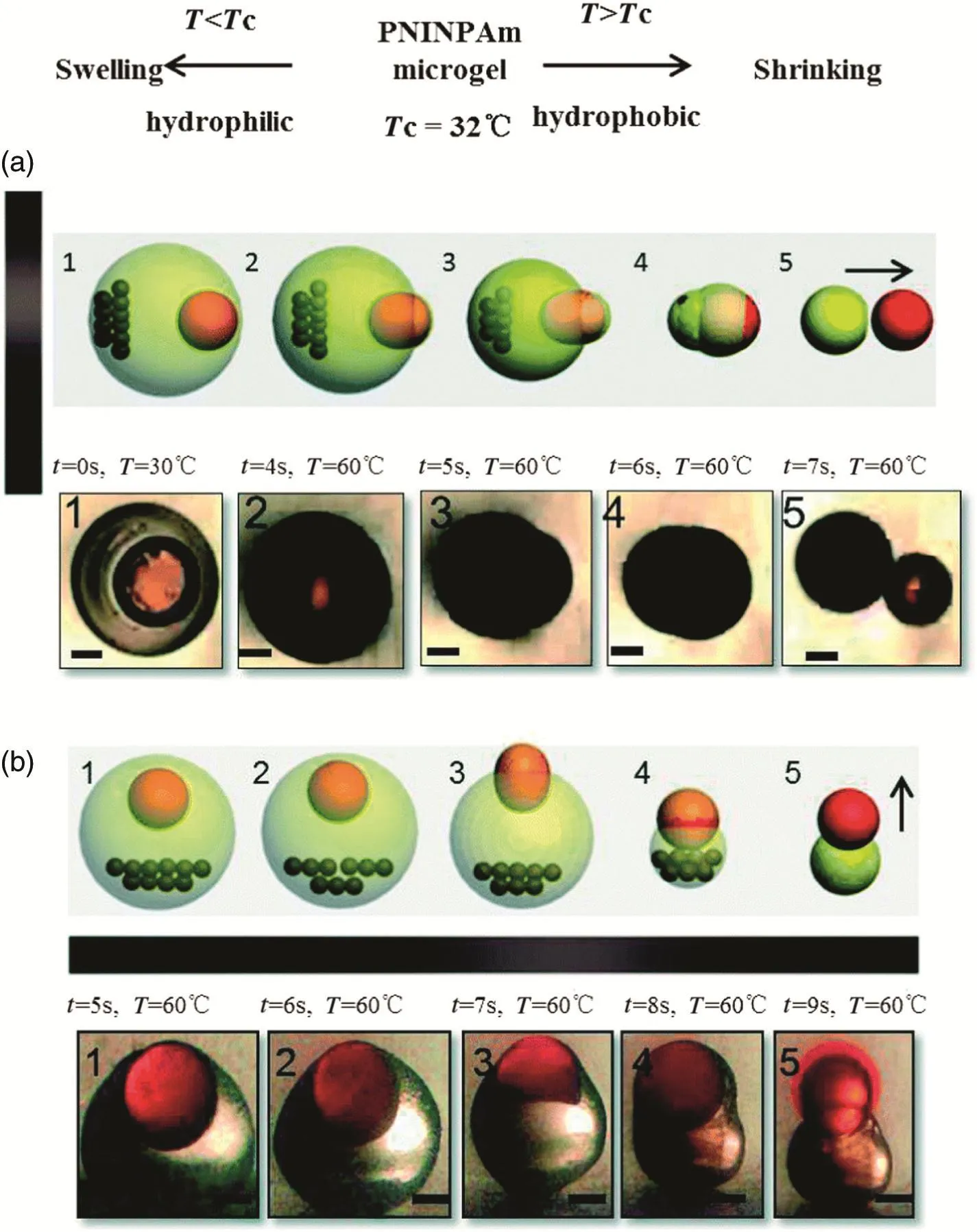
Fig.18.The process of burst release with controlled direction.(a)and(b)are the top view and the side view of the targeted burstrelease process,respectively.The magnetin(a)is on the left while in(b)it is at the bottom of the microcapsule,and the arrows show that the burst release direction was towards the right and up,respectively,contrary to the direction of the magnetic force.These micrographs depicted the five stage transformation of targeted burst release in less than 10 s.The five stages are:1-originalstage;2-extruding stage;3-deformation stage;4-rupture stage and 5-burst release stage.The black bars represent 200 μm[51].

Fig.19.The comparison of the extraction process without and with gas in the emulsion core.(a)The images show the different generation frequencies when the inner gas flow rates are increased from 0 to a high value.(b)It is a sketch of the dispersion states inside the outlet channel before and after the gas microbubble is introduced.(c)The images show the increase of fluorescence intensity when the gas phase is added[52].
[12]M.Shaohua,et al.,Fabrication of microgel particles with complex shape via selective polymerization of aqueous two-phase systems,Small 8(15)(2012)2356–2360.
[13]W.Wang,et al.,Thermo-driven microcrawlers fabricated via a microfluidic approach,J.Phys.D.Appl.Phys.(2013)46(11).
[14]M.Seo,et al.,Microfluidic consecutive flow-focusing droplet generators,Soft Matter 3(8)(2007)986–992.
[15]Z.H.Nie,et al.,Polymer particles with various shapes and morphologies produced in continuous microfluidic reactors,J.Am.Chem.Soc.127(22)(2005)8058–8063.
[16]N.Pannacci,et al.,Equilibrium and nonequilibrium states in microfluidic double emulsions,Phys.Rev.Lett.101(16)(2008).
[17]S.Torza,et al.,3-phase interactions in shear and electrical fields,J.Colloid Interface Sci.33(1)(1970)67–71.
[18]J.H.Xu,et al.,Correlations of droplet formation in T-junction microfluidic devices:from squeezing to dripping,Microfluid.Nano fluid.5(6)(2008)711–717.
[19]J.H.Xu,et al.,Controllable preparation of monodisperse O/W and W/O emulsions in the same microfluidic device,Langmuir 22(2006)7943–7946.
[20]T.Ward,M.Faivre,H.A.Stone,Drop production and tip-streaming phenomenon in a microfluidic flow-focusing device via an interfacial chemical reaction,Langmuir 26(2010)9233–9239.
[21]J.H.Xu,et al.,The dynamic effects of surfactants on droplet formation in coaxial microfluidic devices,Langmuir 28(2012)9250–9258.
[22]Y.Chen,et al.,Microfluidic generation of multicolor quantum-dot-encoded core–shell microparticles with precise coding and enhanced stability,Langmuir 30(2014)8538–8542.
[23]J.H.Xu,et al.,Controllable gas/liquid/liquid double emulsions in a dual-coaxial microfluidic device,Lab Chip 12(2012)2029–2036.
[24]R.Chen,et al.,Controllable microfluidic production of gas-in-oil-in-water emulsions for hollow microspheres with thin polymer shells,Lab Chip 12(2012)3858–3860.
[25]J.H.Xu,et al.,Microfluidic preparation and structure evolution of double emulsions with two-phase cores,RSC Adv.4(4)(2014)1900–1906.
[26]K.Xu,et al.,A region-selective modified capillary microfluidic device for fabricating water–oil Janus droplets and hydrophilic-hydrophobic anisotropic microparticles,RSC Adv.5(58)(2015)46981–46988.
[27]J.H.Moon,et al.,Fabrication of ordered macroporous cylinders by colloidal templating in microcapillaries,Langmuir 20(2004)2033–2035.
[28]A.Gunther,et al.,Multiphase microfluidics:from flow characteristics to chemical and materials synthesis,Lab Chip 6(2006)1487–1503.
[29]K.Xu,et al.,Extraction-derived self-organization of colloidal photonic crystal particles within confining aqueous droplets,Cryst.Growth Des.13(2013)926–935.
[30]K.Xu,et al.,A novel method of fabricating,adjusting,and optimizing polystyrene colloidal crystal nonspherical microparticles from gas–water Janus droplets in a double coaxial microfluidic device,Cryst.Growth Des.14(2014)401–405.
[31]R.Wilson,et al.,Encoded microcarriers for high-throughput multiplexed detection,Angew.Chem.Int.Ed.45(2006)6104–6117.
[32]D.C.Pregibon,et al.,Multifunctional encoded particles for high-throughput biomolecule analysis,Science 315(2007)1393–1396.
[33]X.H.Ji,et al.,On-demand preparation of quantum dot-encoded microparticles using a droplet microfluidic system,Lab Chip 11(2011)2561–2568.
[34]M.Y.Han,et al.,Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules,Nat.Biotechnol.19(2001)631–635.
[35]J.H.Xu,et al.,Preparation of monodispersed chitosan microspheres and in situ encapsulation of BSA in a co-axial microfluidic device,Biomed.Microdevices 11(2008)243–249.
[36]J.H.Xu,et al.,A novel microfluidic approach for monodispersed chitosan microspheres with controllable structures,Adv.Healthcare Mater.1(2012)106–111.
[37]M.R.Gandhi,et al.,Preparation and characterization of silica gel/chitosan composite for the removal of Cu(II)and Pb(II),Int.J.Biol.Macromol.50(2012)650–657.
[38]L.Yu,et al.,Synthesis of monodisperse zeolite A/chitosan hybrid microspheres and binderless zeolite A microspheres,Ind.Eng.Chem.Res.51(2012)2299–2308.
[39]H.Zhao,et al.,Microfluidic production of porous chitosan/silica hybrid microspheres and its Cu(II)adsorption performance,Chem.Eng.J.229(2013)82–89.
[40]J.H.Xu,et al.,Microfluidic preparation of chitosan microspheres with enhanced adsorption performance of copper(II),Sensors Actuators B Chem.183(2013)201–210.
[41]H.Zhao,et al.,A novel microfluidic approach for preparing chitosan–silica core–shell hybrid microspheres with controlled structures and their catalytic performance,Lab Chip 14(2014)1901–1906.
[42]H.Zhao,et al.,Silica/chitosan core–shell hybrid-microsphere-supported CuI catalyst for terminal alkyne homocoupling reaction,Appl.Catal.A Gen.502(2015)188–194.
[43]X.M.Xu,et al.,Micro fl uidic preparation of chitosan–poly(acrylic acid)composite microspheres with a porous surface structure,RSC Adv.4(2014)37142–37147.
[44]A.Fang,et al.,Smart swelling biopolymer microparticles by a microfluidic approach:synthesis,in situ encapsulation and controlled release,Colloids Surf.B Biointerfaces 82(2011)81–86.
[45]M.Marquis,et al.,Microfluidic generation and selective degradation of biopolymerbased Janus microbeads,Biomacromolecules 13(2012)1197–1203.
[46]B.Maheswari,et al.,Role of N-vinyl-2-pyrrolidinone on the thermoresponsive behavior of PNIPAm hydrogel and its release kinetics using dye and vitamin-B12 as model drug,J.Biomater.Sci.Polym.Ed.25(2014)269–286.
[47]J.Wei,et al.,Multi-stimuli-responsive microcapsules for adjustable controlled-release,Adv.Funct.Mater.24(2014)3312–3323.
[48]B.L.Zhang,et al.,Preparation of thermoresponsive Fe3O4/P(acrylic acid–methyl methacrylate–N-isopropylacrylamide)magnetic composite microspheres with controlled shell thickness and its releasing property for phenolphthalein,J.Colloid Interface Sci.398(2013)51–58.
[49]Y.Chen,et al.,Multicompartmental Janus microbeads from branched polymers by single-emulsion droplet microfluidics,Langmuir 29(2013)12657–12662.
[50]J.Jeong,et al.,One-step preparation of magnetic Janus particles using controlled phase separation of polymer blends and nanoparticles,RSC Adv.3(2013)11801.
[51]X.H.Ge,et al.,Controlled stimulation-burst targeted release by smart decentered core–shell microcapsules in gravity and magnetic field,Lab Chip 14(2015)4451–4454.
[52]W.T.Wang,et al.,The enhancementof liquid–liquid extraction with high phase ratio by micro fluidic-based hollow droplet,RSC Adv.5(100)(2015)82056–82064.
 Chinese Journal of Chemical Engineering2016年6期
Chinese Journal of Chemical Engineering2016年6期
- Chinese Journal of Chemical Engineering的其它文章
- Mixture temperature prediction of waxy oil–water two-phase system flowing near wax appearance temperature☆
- Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange☆
- Online process monitoring for complex systems with dynamic weighted principal component analysis☆
- A stepwise optimal design of water network☆
- Integration of coal pyrolysis process with iron ore reduction:Reduction behaviors of iron ore with benzene-containing coal pyrolysis gas as a reducing agent☆
- Multiple linear equation of pore structure and coal–oxygen diffusion on low temperature oxidation process of lignite☆
