circRNA_000203对小鼠心肌成纤维细胞纤维化表型的影响*
朱文思, 唐春梅, 朱杰宁, 林秋雄, 符永恒, 邓春玉, 杨 慧, 饶 芳, 吴书林, 单志新△
(1南方医科大学, 广东 广州510515; 2广东省心血管病研究所, 广东省人民医院, 广东省医学科学院 广东 广州 510080)
circRNA_000203对小鼠心肌成纤维细胞纤维化表型的影响*
朱文思1,2, 唐春梅1,2, 朱杰宁2, 林秋雄2, 符永恒2, 邓春玉2, 杨 慧2, 饶 芳2, 吴书林2, 单志新2△
(1南方医科大学, 广东 广州510515;2广东省心血管病研究所, 广东省人民医院, 广东省医学科学院 广东 广州 510080)
目的: 检测糖尿病小鼠心肌中环形RNAs (circRNAs)的表达谱,探讨circRNA_000203对心肌成纤维细胞纤维化表型的影响。方法: 采用Masson染色对糖尿病db/db小鼠和db/m对照小鼠心肌组织进行胶原纤维的染色观察;用circRNAs表达谱芯片检测糖尿病心肌中circRNAs的表达谱;实时荧光定量PCR技术(RT-qPCR)验证小鼠心肌中circRNA_000203的表达水平;构建重组circRNA_000203腺病毒载体,并感染小鼠心肌成纤维细胞;利用RT-qPCR和Western blot检测过表达circRNA_000203后,小鼠心肌成纤维细胞中纤维化相关基因Col1a2、Col3a1、α-SMA的mRNA和蛋白表达变化。结果: Masson染色结果显示,与对照db/m小鼠相比,糖尿病db/db小鼠心肌组织出现明显纤维化。circRNAs芯片检测结果表明circRNAs在糖尿病心肌中异常表达,其中circRNA_000203表达显著上调。RT-qPCR结果证明重组circRNA_000203腺病毒载体构建成功,RT-qPCR和Western blot结果均显示过表达circRNA_000203后,心肌成纤维细胞中Col1a2、Col3a1、α-SMA的表达均显著增强。结论: circRNA_000203在糖尿病心肌中显著上调,其可特异地促进心肌成纤维细胞中纤维化相关基因的表达和成纤维细胞向肌成纤维细胞表型的转化。
环形RNAs; 心肌纤维化; 细胞外基质
糖尿病性心肌病(diabetic cardiomyopathy,DCM)是由糖尿病引起的心脏微血管的病变、心肌纤维化和心肌代谢紊乱等所导致的心肌结构异常[1],最终引起左心室肥厚、舒张期和(或)收缩期功能障碍的一种疾病状态。以往的研究认为,糖尿病性心肌病的主要病理改变包括心肌肥大和心肌纤维化[2-3]。糖尿病性心肌病引起胶原合成和降解的动态失衡,促使大量细胞外基质(extracellular matrix,ECM)沉积于心室壁,导致心脏僵硬度增加,心室顺应性降低,引起心功能不全,严重时甚至心衰。但是,糖尿病性心肌纤维化的发病机制复杂,人们对于其发生发展的确切分子和细胞信号转导机制还不完全清楚,尚未找到有效的干预方法。
环形RNAs(circular RNAs, circRNAs)主要来源于外显子或内含子,分别通过外显子反向剪接、索套内含子2种剪接形成[4]。与线性RNAs相比,circRNAs不含3′或 5′末端,呈闭合环状结构,不受RNA外切酶影响,表达更稳定,不易降解,大量存在于真核细胞的细胞质中。circRNAs可作为miRNAs海绵体,竞争性结合miRNAs位点,调节miRNAs活性,使其功能减弱或丧失,最终影响miRNAs下游靶基因的表达[5-10]。近年来, 越来越多的证据表明,circRNAs在动脉粥样硬化、神经系统紊乱、朊病毒病以及癌症等多种疾病的发生与发展过程中发挥了一定的作用[11-13],有可能成为新型的疾病分子标志物。深入了解circRNAs的作用机制及其功能,有助于我们了解疾病本身,也为疾病的治疗和诊断提供科学依据和资料。
本研究中,我们选取16周龄的糖尿病db/db和对照db/m小鼠,用circRNAs芯片检测各组心肌组织circRNAs表达谱,并选取了糖尿病心肌中表达显著上调的circRNA_000203,通过构建重组circRNA_000203腺病毒,进一步研究了其与糖尿病性心肌纤维化的关系。
材 料 和 方 法
1 主要试剂
限制性内切酶XhoI、EcoR I、PacI和PmeI(NEB); BJ5183E.Coli、载体pAd-Track-cmv vector和pAdEasy-I(Coloncancer); Lipofectamine 2000、TRIzol、逆转录试剂盒、4×SDS loading buffer(Invitrogen);2×SYBR Green Mix及RNase free water(TaKaRa);BCA蛋白定量试剂盒(Thermo);SDS-聚丙烯酰胺凝胶配置试剂盒(碧云天);抗体胶厚(collagen,Col)1a2、Col3a1和GAPDH(Protein Technology);α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)(Abcam);蛋白Marker (Fermentas); PVDF膜(Whatman);ECL发光液(Bioword);Masson三色染色试剂盒(北京索莱宝科技有限公司);DMEM/F12细胞培养基(HyClone);特级澳洲胎牛血清(Gibco);胰蛋白酶粉末(广州威佳科技有限公司);其它生化试剂均为进口分装或国产分析纯。所用引物由Invitrogen公司根据设计合成,具体序列见表1。
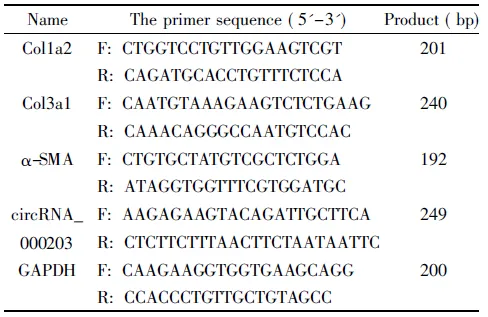
表1 PCR引物序列
F: forward; R: reverse.
2 主要方法
2.1 Masson 三色染色 腹腔注射50 mg/kg 戊巴比妥钠麻醉小鼠,取左心室心肌组织,4%甲醛固定,石蜡包埋并切成4 μm厚度的连续切片,Masson胶原纤维染色,观察心脏组织的胶原纤维增生情况,分析评价糖尿病db/db和对照db/m小鼠心脏的纤维化程度。
2.2 circRNAs芯片检测 留取糖尿病db/db小鼠和db/m对照小鼠心肌组织各8份,提取总RNA,对应标本合并成2份总RNA。circRNAs表达谱检测、分析由上海康成公司完成。简要步骤如下:先用RNase R消化总RNA,去除线性RNA,富集circRNAs。将富集后的circRNAs采用随机引物转录,扩增为荧光标记的cRNA探针(Arraystar Super RNA Labeling Kit)。cRNAs探针与Arraystar Human circRNA Array(8×15K)表达谱芯片上的寡核苷酸片段杂交,Agilent Scanner G2505C扫描,Agilent Feature Extraction Software (Version 11.0.1.1)分析杂交结果。
2.3 心肌成纤维细胞的分离、培养和处理 心肌成纤维细胞从刚出生1~3 d的C57BL/6小鼠心脏中原代分离、培养,具体方法参考文献[14]进行,并有所改进。成纤维细胞由于贴壁速度不同而与心肌细胞分离,将其接种于10 cm培养皿中,用含有10%胎牛血清及1×105U/L青霉素和100 mg/L链霉素的DMEM/F12培养基,置于37 ℃、5% CO2培养箱中培养。当细胞生长融合度达到约90%时,用0.25%含EDTA的胰蛋白酶消化传代,传至P3代时按所需浓度接种培养板中,分别感染重组circRNA_000203腺病毒和对照GFP标记的腺病毒(MOI=10)。
2.4 重组circRNA_000203腺病毒的制备 circRNA_000203包含了Myo9a基因的外显子7~15序列。我们合成了对应的1 412 nt双链DNA模版,包含Myo9a基因的外显子7~15序列,内含子6和15序列,并将限制性内切酶XhoI 和EcoR I的识别序列加在双链DNA模版末端,见图1。按照我们报道的方法[15],将circRNA_000203的DNA模版分别定向插入到pAd-Track-cmv载体的多克隆位点。接下来,进一步用pAd-Track-cmv-circRNA_000203与pAdEasy-I在BJ5183E.Coli中形成重组载体。最后,重组circRNA_000203腺病毒载体被限制性内切酶PmeI线性化,转染至293T细胞中,包装重组腺病毒。

Figure 1.Template DNA sequence of circRNA_000203.
图1 circRNA_000203的DNA序列模版
2.5 RT-qPCR检测circRNA_000203以及纤维化相关基因的表达 TRIzol法提取总RNA后,取1.5 μg总RNA逆转录为cDNA。以GAPDH为内参照,vii A7 Quantitative PCR System(Applied Biosystems)进行PCR。2-ΔΔCt法计算目的基因的相对表达量。
2.6 Western blot 检测蛋白水平 收集处理后的成纤维细胞,加入RIPA蛋白裂解液,冰上裂解,于4 ℃、12 000 r/min离心10 min,取上清进行蛋白定量后分装,加入4×上样缓冲液,100 ℃加热10 min使蛋白变性,然后进行聚丙烯酰胺凝胶电泳。用PVDF膜转膜,5%脱脂奶粉封闭2 h,分别用相应的抗体anti-Col1a2(1∶1 000)、anti-Col3a1(1∶1 000)、anti-α-SMA(1∶2 000)4 ℃孵育过夜。TBST洗膜后, II 抗(1∶5 000)4 ℃孵育2 h。ECL发光试剂盒显影,以GAPDH(1∶ 2 000)作内参照,扫描灰度值并分析蛋白表达相对含量。
3 统计学处理
用SPSS 21.0统计软件进行分析。数据均采用均数±标准误(mean±SEM)表示,组间差异采用t检验,以P<0.05为差异有统计学意义。
结 果
1 糖尿病心肌组织中circRNA_000203表达上调
Masson染色的结果显示,与db/m对照小鼠相比,糖尿病心肌组织中血管周围和心肌间质纤维化程度加剧。circRNAs芯片检测结果显示,糖尿病心肌中circRNAs表达异常,有45个circRNAs上调2倍以上,而有31个circRNAs下调2倍以上。RT-qPCR证实,与db/m对照小鼠相比,db/db小鼠心肌组织中circRNA_000203显著上调。琼脂糖凝胶电泳鉴定显示PCR产物大小与预期相符。DNA测序的结果也一致性证实PCR产物是circRNA_000203的片段,见图2。
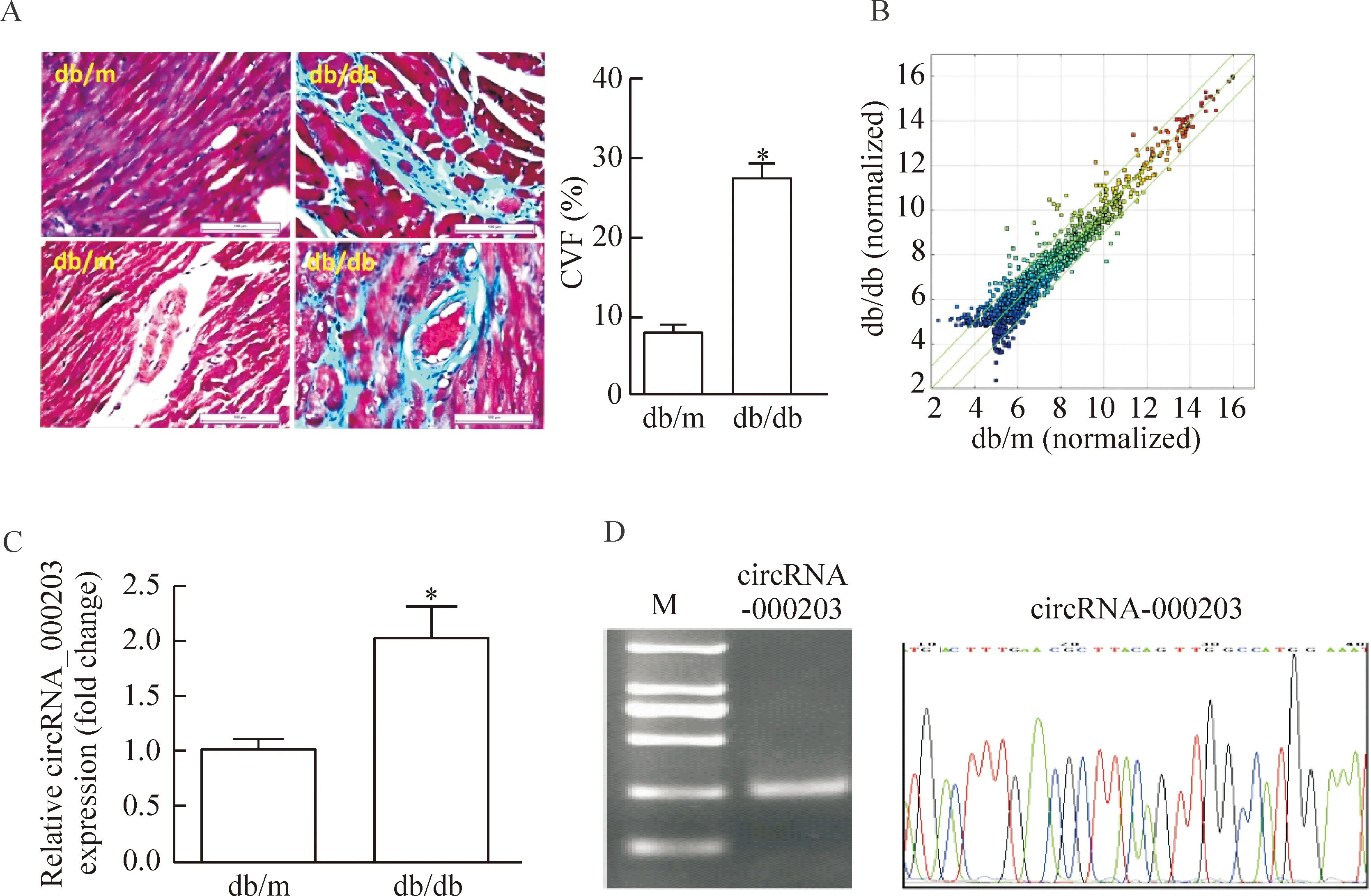
Figure 2.circRNA_000203 expression in the diabetic mouse myocardium. A: Masson trichrome staining. The scale bar=100 μm. B: the scatter plot figure showed the representative dysregulated circRNAs in the diabetic mouse myocardium. The values of X and Y axes in the scatter-plot are the normalized signal values of the samples (log2 scaled). The green lines are fold change lines. The circRNAs above the top green line and below the bottom green line indicated more than 2 fold change of circRNAs between the 2 compared samples. C: the expression of circRNA_000203 by RT-qPCR. D: PCR product of circRNA_000203 was identified by 1.5% agarose gel electrophoresis and DNA sequencing, respectively. M: DL2000 DNA marker (2 000, 1 000, 750, 500, 250,100 bp). Mean±SEM.n=8.*P<0.05vsdb/m.
图2 CircRNA_000203在糖尿病小鼠心肌中的表达
2 重组circRNA_000203腺病毒的构建
我们成功构建了穿梭质粒pAd-Track-cmv-circRNA_000203,并进一步用于构建重组腺病毒质粒。线性化后转染入293T细胞,包装重组circRNA_000203腺病毒。病毒感染心肌成纤维细胞24 h后,荧光倒置显微镜下观察,可见感染效率与对照空载体病毒rAd-GFP相当。RT-qPCR结果证实感染rAd-circRNA_000203后,成纤维细胞中circRNA_000203表达显著上调,见图3。
3 腺病毒介导过表达circRNA_000203后心肌成纤维细胞中纤维化相关基因的表达
为进一步研究circRNA_000203对心肌成纤维细胞中纤维化表型的影响,我们检测了过表达circRNA_000203后,纤维化相关基因Col1a2、Col3a1和α-SMA的变化。RT-qPCR及Western blot 结果显示,与对照组相比,rAd-circRNA_000203感染组心肌成纤维细胞中,Col1a2、Col3a1和α-SMA的mRNA及蛋白表达均显著增强,见图4。
讨 论
近年来,基于生物信息学和实验分析方法,多种circRNAs在哺乳动物转录组中被发现[5,10,16-1,7]。越来越多的证据表明,circRNAs参与了转录、mRNA剪接、RNA降解和翻译等生物学过程[18],且与癌症、神经系统紊乱、心血管病等多种人类疾病的发生发展密切相关[19]。Burd等[11]研究发现编码基因位于染色体INK4的circRNA表达水平与人动脉粥样硬化的发生风险显著相关。Wang等[20]报道,circRNA-HRCR可作为海绵体,竞争性结合miR-223位点,抑制其对靶基因ARC的调控作用,发挥抗心肌肥厚和心脏衰竭作用。此外,Du等[21]报道,circRNA-Foxo3在衰老小鼠和人的心肌组织中显著上调表达,circRNA-Foxo3通过与抗衰老蛋白ID-1、E2F1、抗压力蛋白FAK、HIF1α相互作用,阻止上述转录因子入核,促进小鼠胚胎成纤维细胞衰老。

Figure 3.Preparation of recombinant circRNA_000203 adenovirus and its infection in cardiac fibroblasts. A: restriction endonuclease digestion of rAd-circRNA_000203 by electrophoresis through an 8 g/L agarose gel. Lane M: DL15000 DNA ladder marker (from up to down, 15, 10, 7.5, 5, 2.5, 1.0, 0.25 kb in size); lane 1: pAdTrack-circRNA_000203 digested byPacI; lane 2: rAd-circRNA_000203 digested byPacI; B: packaging of rAd-Cap8 shRNA in HEK293 cells (×200); C: determination of circRNA_000203 in cardiac fibroblasts with infection of rAd-circRNA_000203. Mean±SEM.n= 3.△△P<0.01vsrAd-GFP.
图3 重组circRNA_000203腺病毒的构建及感染心肌成纤维细胞

Figure 4.The expression of Col1a2, Col3a1 and α-SMA in the cardiac fibroblasts with enforced-expression of circRNA_000203. Mean±SEM.n=3.*P<0.05vsrAd-GFP.
图4 过表达circRNA_000203后心肌成纤维细胞中Col1a2、Col3a1 以及 α-SMA的表达
本研究中,我们发现circRNAs在纤维化的糖尿病心肌中表达异常,提示circRNAs有可能参与了糖尿病性心肌纤维化过程。基于糖尿病db/db小鼠心肌组织差异表达的circRNAs芯片检测和实时定量PCR结果,本文选择circRNA_000203进行研究,试图揭示其在心肌纤维化中的作用。
目前普遍认为circRNAs来自剪接体介导的前体mRNA(pre-mRNA)剪接,而剪接体也被认为参与了外显子环化过程[11,22-23]。某些circRNAs是由两侧的内含子互补配对驱动环化的。例如,人类的这些互补配对序列常包含反向的Alu重复序列[24-25]。本研究合成了circRNA_000203的双链DNA模版,包含Myo9a基因的外显子7~15序列,以及两端的内含子6和15序列,并进一步克隆到腺病毒表达载体上。RT-qPCR结果显示,重组腺病毒可有效介导心肌成纤维细胞中circRNA_000203过表达。这些结果也说明位于Myo9a基因内含子4和15上的旁侧序列对circRNA_000203的形成十分重要。
心肌纤维化是细胞外基质大量沉积,成纤维细胞增殖并向肌成纤维细胞转化的过程[26]。肌成纤维细胞是主要的效应细胞,以表达α-SMA为标志[27]。本文发现过表达circRNA_000203可上调α-SMA表达,提示circRNA_000203能促进心肌成纤维细胞向肌成纤维细胞的转化。同时,我们也证实心肌成纤维细胞中过表达circRNA_000203后,纤维化相关基因Col1a2和Col3a1表达均一致性上调。因此,上述结果提示circRNA_000203通过调控纤维化相关基因表达,以及促进成纤维细胞向肌成纤维细胞表型转化,发挥促心肌纤维化作用,但其具体的作用机制还有待进一步研究。
[1] Murarka S, Movahed MR. Diabetic cardiomyopathy[J]. J Card Fail, 2010, 16(12):971-979.
[2] Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study[J]. Circulation, 2000, 101(19):2271-2276.
[3] Bella JN, Devereux RB, Roman MJ, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study)[J]. Am J Cardiol, 2001, 87(11):1260-1265.
[4] Li J, Yang J, Zhou P, et al. Circular RNAs in cancer: novel insights into origins, properties, functions and implications[J]. Am J Cancer Res, 2015, 5(2):472-480.
[5] Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency[J]. Nature, 2013, 495(7441):333-338.
[6] Hentze MW, Preiss T. Circular RNAs: splicing's enigma variations[J]. EMBO J, 2013, 32(7):923-925.
[7] Zhang Y, Zhang XO, Chen T, et al. Circular intronic long noncoding RNAs[J]. Mol Cell, 2013, 51(6):792-806.
[8] Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus[J]. Nat Struct Mol Biol, 2015, 22(3):256-264.
[9] Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing[J]. Mol Cell, 2014, 56(1):55-66.
[10]Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges[J]. Nature, 2013, 495(7441):384-388.
[11]Burd CE, Jeck WR, Liu Y, et al. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk[J]. PLoS Genet, 2010, 6(12):e1001233.
[12]Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer[J]. Cancer Res, 2013, 73(18):5609-5612.
[13]Li F, Zhang L, Li W, et al. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/beta-catenin pathway[J]. Oncotarget, 2015, 6(8):6001-6013.
[14]Communal C, Singh K, Pimentel DR, et al. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway[J]. Circulation, 1998, 98(13):1329-1334.
[15]Liang Y, Lin Q, Zhu J, et al. The caspase-8 shRNA-modified mesenchymal stem cells improve the function of infarcted heart[J]. Mol Cell Biochem, 2014, 397(1-2):7-16.
[16]Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats[J]. RNA, 2013, 19(2):141-157.
[17]Salzman J, Gawad C, Wang PL, et al. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types[J]. PLoS One, 2012, 7(2):e30733.
[18]Wang PL, Bao Y, Yee MC, et al. Circular RNA is expressed across the eukaryotic tree of life[J]. PLoS One,2014,9(6):e90859.
[19]Chen Y, Li C, Tan C, et al. Circular RNAs: a new frontier in the study of human diseases[J]. J Med Genet,2016,53(6):359-365.
[20]Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223[J]. Eur Heart J,2016.[Epub ahead of print]
[21]Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses[J]. Eur Heart J,2016.[Epub ahead of print]
[22]Chao CW, Chan DC, Kuo A, et al. The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis[J]. Mol Med, 1998, 4(9):614-628.
[23]Hansen TB, Wiklund ED, Bramsen JB, et al. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA[J]. EMBO J, 2011, 30(21):4414-4422.
[24]Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production[J]. Genes Dev, 2014, 28(20):2233-2247.
[25]Zhang XO, Wang HB, Zhang Y, et al. Complementary sequence-mediated exon circularization[J]. Cell, 2014, 159(1):134-147.
[26]赵 飞,王思博,褚 鹏,等. 钙网织蛋白促进心脏瓣膜病患者心房重构[J]. 中国病理生理杂志,2016,32(01):33-40.
[27]Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases [J]. J Pathol,2003,200(4):500-503.
(责任编辑: 林白霜, 余小慧)
Effect of circRNA_000203 on fibrotic phenotypes in mouse cardiac fibroblasts
ZHU Wen-si1, 2, TANG Chun-mei1, 2, ZHU Jie-ning2, LIN Qiu-xiong2, FU Yong-heng2, DENG Chun-yu2, YANG Hui2, RAO Fang2,WU Shu-lin2, SHAN Zhi-xin2
(1SouthernMedicalUniversity,Guangzhou510515,China;2GuangdongCardiovascularInstitute,GuangdongGeneralHospital,GuangdongAcademyofMedicalSciences,Guangzhou510080,China.E-mail:zhixinshan@aliyun.com.cn)
AIM: To determine circular RNA (circRNA) profiles in the diabetic mouse myocardium, and to investigate the effect of circRNA_000203 on fibrotic phenotypes in cardiac fibroblasts.METHODS: Masson trichrome staining was performed on the myocardium of the diabetic db/db mice and the non diabetic db/m control mice. circRNA expression profile in the diabetic myocardium was detected by circRNAs microarray. The expression of circRNA_000203 was determined by real time fluorescence quantitative PCR (RT-qPCR). Recombinant circRNA_000203 adenovirus was prepared for enforced the expression of circRNA_000203 in mouse cardiac fibroblasts. The expression of Col1a2, Col3a1and α-SMA was determined in circRNA_000203-modified cardiac fibroblasts, respectively. RESULTS: Masson trichrome staining showed that fibrosis was increased in the diabetic mouse myocardium. The results of circRNA array detection revealed that circRNAs were dysregulated in the diabetic myocardium. circRNA_000203 was up-regulated in the diabetic myocardium. Significant over-expression of circRNA_000203 was achieved in the cardiac fibroblasts after infection with the recombinant circRNA_000203 adenovirus. The mRNA and protein expression of Col1a2, Col3a1 and α-SMA was significantly increased in the cardiac fibroblasts with over-expression of circRNA_000203.CONCLUSION: circRNA_000203 is up-regulated in the diabetic mouse myocardium. It has pro-fibrotic effect on the cardiac fibroblasts.
Circular RNAs; Cardiac fibrosis; Extracellular matrix
1000- 4718(2016)08- 1351- 06
2016- 02- 03
2016- 04- 26
国家自然科学基金资助项目 (No.81470439; No.81270222);广东省自然科学基金资助项目 (No.2014A030313635)
R587.1; R363
A
10.3969/j.issn.1000- 4718.2016.08.002
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 020-83827812-51158; E-mail: zhixinshan@aliyun.com.cn

