What is the Optimal Duration of Dual Antiplatelet Therapy After Stenting?
Udaya S. Tantry, PhD, Eliano P. Navarese, MD, PhD and Paul A. Gurbel, MD
1Inova Center for Thrombosis Research and Drug Development, Inova Heart and Vascular Institute, Falls Church, VA, USA
Introduction
The optimal duration of dual antiplatelet therapy(DAPT) of aspirin and a P2Y12receptor blocker after stenting is not yet clearly defined. Acute coronary syndromes (ACS) are associated with injured arterial vessel walls in the setting of a highly prothrombotic state. The stimulus for thrombosis is further heightened by the frequent implantation of a foreign body (a stent) at the site of coronary artery damage. Complete stent endothelialization, the most desired outcome, has been observed within a month with bare metal stent (BMS) implantation,whereas drug-eluting stent (DES) implantation has been associated with highly suppressed early healing and poor endothelial cell coverage that may persist for years. In the presence of heightened platelet activation and platelet response to agonists following ACS and percutaneous coronary intervention (PCI), the risk for recurrent thrombotic event occurrences is high during the first three months and thrombotic events continue to increase for at least 3 years [1–3]. Therefore, in the presence of endothelial dysfunction, denuded vessel and potentially exposed stent struts, the risk of stent thrombosis and events occurring outside the target vessel from plaque rupture is increased when there is inadequate platelet inhibition. In this line, it has been demonstrated that premature discontinuation of DAPT and high on-treatment platelet reactivity to adenosine diphosphate are associated with stent thrombosis and mortality. Moreover, stent thrombosis is associated with a high rate of mortality(~20–45%) [4, 5]. These observations provide a strong rationale for uninterrupted DAPT in patients treated with DES.
Extensive use of complete revascularization,newer-generation DES associated with complete and earlier endothelial healing and more effective P2Y12inhibition have challenged the relevance of data on the optimal duration of DAPT derived from patients studied more than even 3 years ago. The newest stents have been associated with very low early and late thrombosis rates, and recent studies have questioned the need for DAPT for longer than 3–6 months [6–12]. Moreover, the accruing evidence addressing the cost and morbidity associated with bleeding during long-term DAPT has stimulated great interest in shortening the duration of DAPT. However, recent randomized clinical trials (RCT’s) of longer duration of DAPT suggested a continued divergence of event curves with time extending more than 3 years and also occurrence of ischemic events in non-culprit lesion vessels beyond stent thrombosis. In this line, the bottom line statement remains, ‘The optimal duration of DAPT after DES implantation is not known’ [13].
In this article, we review the current guidelines,and evidence on duration of DAPT based on randomized controlled trials and meta-analyses. We then discuss potential future directions.
Current Guidelines
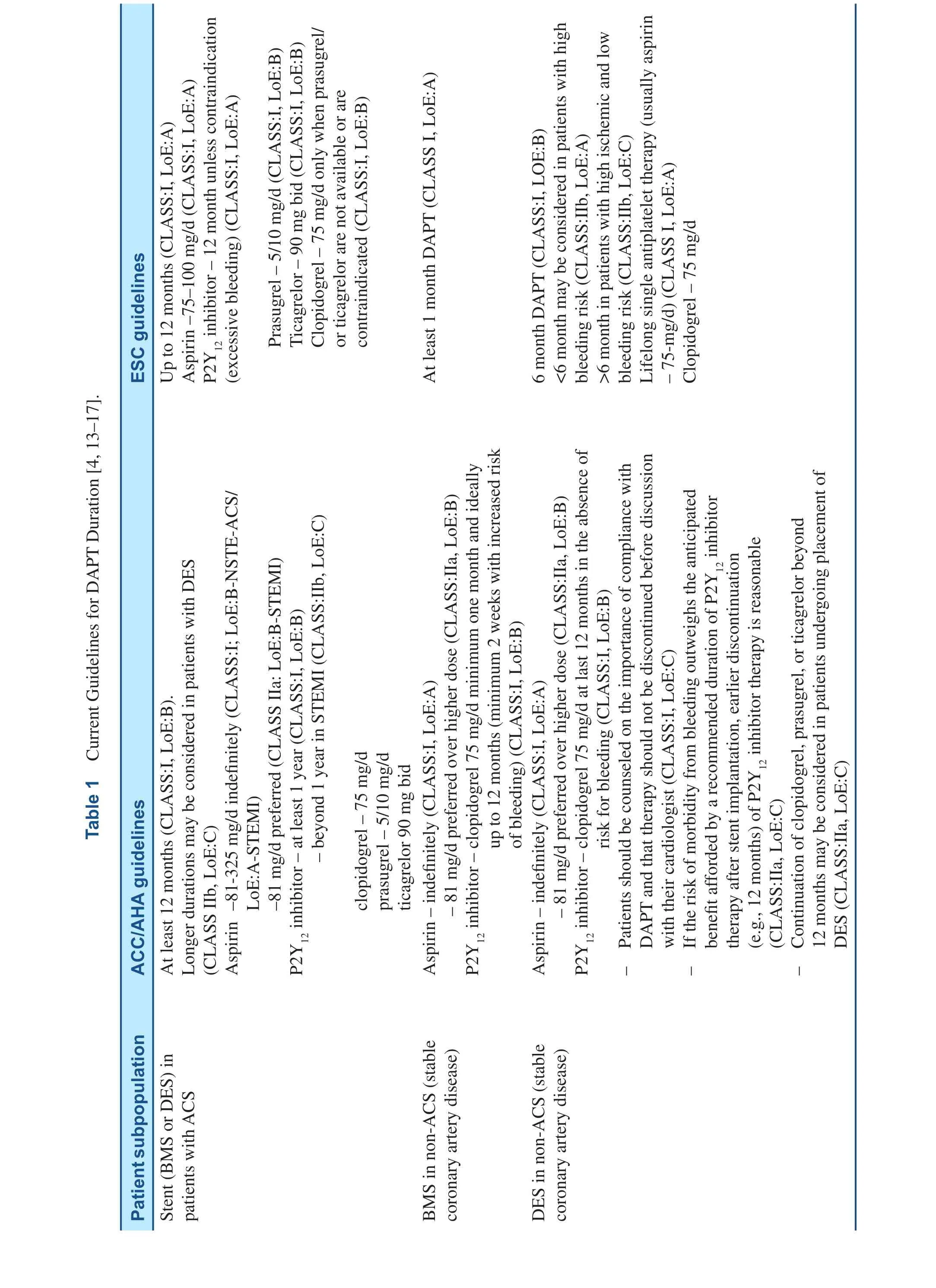
The current DAPT guidelines in patients treated with stenting are presented in the Table 1 [4, 13–16].
Evidence from Randomized Trials(Table 2)
Three month vs. 1 year DAPT
Two RCTs, the REal Safety and Efficacy of a 3-month DAPT following E-ZES implantation(RESET) trial and the Optimized Duration of Clopidogrel Therapy Following Treatment With the Zotarolimus-Eluting Stent in Real-World Clinical Practice (OPTIMIZE) trial, have compared 3-month vs. 1-year DAPT [6, 7]. In the RESET trial (n=2117, ~55% with ACS), the primary combined endpoint occurrence at 1 year of follow-up was non-inferior between 3-month DAPT after a ZES vs. 12-month DAPT with another DES [6].Similarly in the OPTIMIZE trial (n=3119, ~32%ACS), non-inferiority for the primary combined endpoint of net adverse clinical and cerebral events was demonstrated with 3 vs. 12 months of DAPT[7]. In both trials, (a) patients were randomized at the time of PCI and not at the time of DAPT discontinuation, thus including events occurring while both treatment groups were on DAPT, potentially diluting treatment effects, (b) majority of patients are of low risk presenting with stable angina or troponin-negative ACS patients, and (c) event rates were low and the studies were thus underpowered. The authors of the RESET study stated that, “the generalized application of these results to the entire population demands careful attention”.However, despite underpowering, the authors of the OPTIMIZE trial concluded that short-term DAPT may be sufficient in a low-risk population after DES placement.
Six month vs. one year DAPT
Six-month DAPT vs. 1-year DAPT was evaluated in three RCTs: (1) the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting(EXCELLENT) trial, (2) the SEcond-Generation Drug-Eluting Stent Implantation Followed by Six-Versus Twelve-Month Dual Antiplatelet Therapy(SECURITY) trial, and (3) the Intracoronary Stenting and Antithrombotic Regimen: Safety And Efficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting study (ISAR-SAFE) trial [8–10].In the EXCELLENT trial (n=1443, ~50% with ACS), the primary combined endpoint of target vessel failure [defined as the composite of cardiac death, myocardial infarction (MI), or ischemiadriven target vessel revascularization at 12 months]was non-inferior between 6-month DAPT vs.12-month DAPT (P<0.001 for noninferiority with a predefined noninferiority margin of 4.0%) [8].In the SECURITY trial (n=1404, ACS=38%), the primary composite end point of cardiac death, MI,stroke, definite or probable stent thrombosis, or Bleeding Academic Research Consortium (BARC)type 3 or 5 bleeding at 12 months, was non-inferior between 6-month DAPT vs. 12-month DAPT(P<0.05, upper 95% CI limit was lower than the pre-set margin of 2%) [9]. Similarly, in the ISARSAFE trial, 6 months vs. 12 months clopidogrel therapy was associated with similar rates of the primary composite endpoint of death, MI, definite or probable stent thrombosis, stroke, or thrombolysis in myocardial infarction (TIMI) major bleeding (1.5 vs. 1.6%), which met the criterion for noninferiority (P non-inferiority<0.001) [10]. Once again, all of these trials were underpowered due to lower than expected event rates. SECURITY and ISAR-SAFE were prematurely interrupted due to slow enrolment or logistic and economic constraints. In addition, patients were randomized at the time of PCI rather than at the time of DAPT discontinuation in the short treatment arm (6 months after PCI) in the EXCELLENT and SECURITY trials [8–10].
?
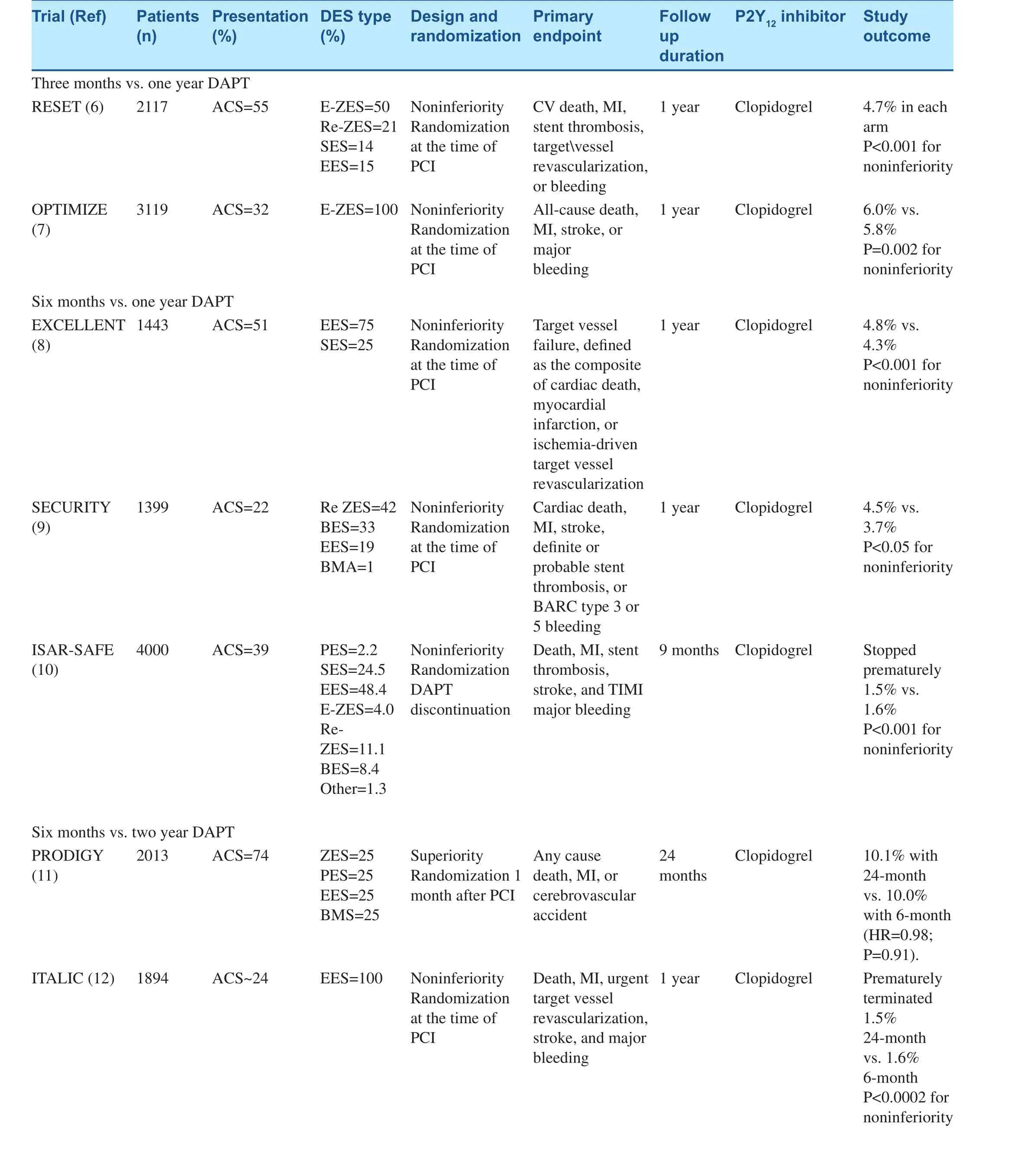
Table 2 Evidence for Duration of DAPT from Randomized Trials.
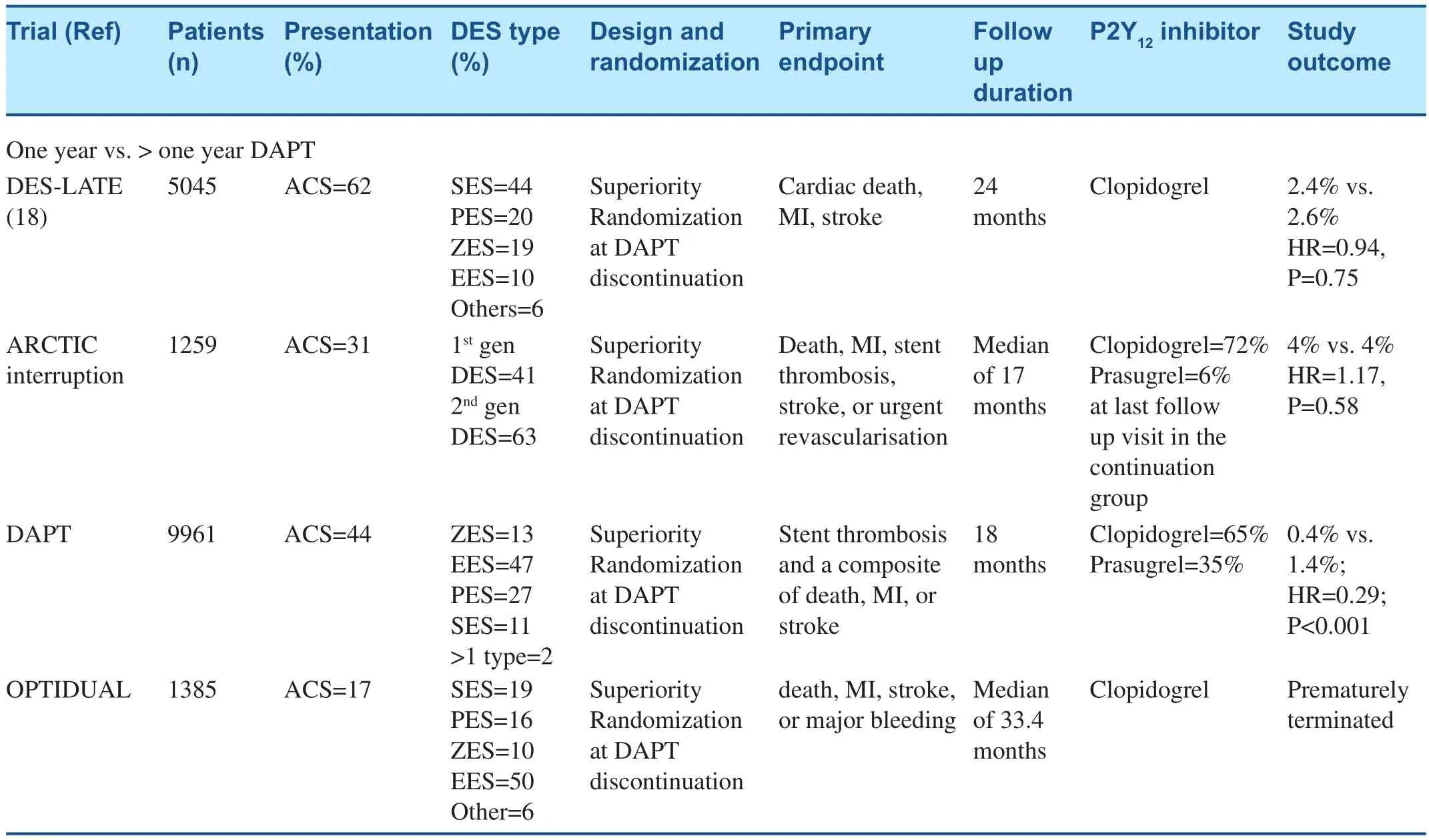
Table 2 (continued)
Six-month vs. 2-year DAPT
The Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study(PRODIGY) trial and the Is There A LIfe for DES after discontinuation of Clopidogrel (ITALIC) trial evaluated the six month vs. 2 year DAPT. Similar to previous trials, no significant difference in the primary endpoint was observed at 1-year followup in the ITALIC trial and 2-year follow-up in the PRODIGY trial [11, 12]. In the ITALIC trial, lowrisk patients were enrolled and it was prematurely interrupted due to slow recruitment [11]. Both studies were underpowered. In addition, patients were randomized either at the time of PCI in the ITALIC trial or 1-month after PCI in the PRODIGY trial,thereby potentially diluting the treatment effect[11, 12].
One year vs. >1 Year DAPT
Four RCTs, the DAPT trial, the Optimal Duration of Clopidogrel Therapy with DES to Reduce Late Coronary Arterial Thrombotic Event (DES-LATE)trial, the Assessment by a double Randomization of a Conventional antiplatelet strategy versus a monitoring-guided strategy for drug-eluting stent implantation and of Treatment Interruption versus Continuation 1 year after stenting (ARCTIC-Interruption) trial,and the OPTImal DUAL Antiplatelet Therapy(OPTIDUAL) trial [1, 17–19]. In these trials, eventfree patients on DAPT 1 year after stent placement were randomized to either aspirin monotherapy or to continue clopidogrel or prasugrel therapy. Another analysis of two RCTs, Correlation of Clopidogrel Therapy Discontinuation in Real-World Patients Treated with Drug- Eluting Stent Implantation and Late Coronary Arterial Thrombotic Events (REALLATE) and Evaluation of the Long-Term Safety after Zotarolimus-Eluting Stent, Sirolimus-Eluting Stent,or Paclitaxel-Eluting Stent Implantation for Coronary Lesions-Late Coronary Arterial Thrombotic Events(ZESTLATE), also evaluated the benefit of continuing DAPT beyond 1 year [20]. Event-free patients on DAPT for 1 year after stent placement were randomized to either aspirin monotherapy or to continue clopidogrel or prasugrel therapy in all of these trials.Except for the DAPT trial, in all of the other trials the primary endpoint was similar in both arms.
The DAPT trial was powered to detect an absolute 0.725% difference in death, MI, or stroke, and an absolute 0.275% difference in definite or probable stent thrombosis with a non-inferiority margin of 0.8% in Global Use of Strategies to Open Occluded Arteries (GUSTO) moderate or severe bleeding compared at an expected rate of 2.2%. In the DAPT study, 11,648 patients who were treated with aspirin and a thienopyridine for 12 months after successful coronary stenting were then randomly assigned to placebo vs. continuation on DAPT for another 18 months. Thirty-one per cent of patients enrolled were stented for acute MI. Prolonged DAPT was associated with a significant reduction in major adverse cardiovascular and cerebrovascular events in those with and without MI, but the treatment effect was more pronounced in the ‘clot formers’,i.e. those with MI (Pinteraction=0.03). Bleeding was also more pronounced in the group treated with prolonged DAPT, but, perhaps not surprisingly, the absolute occurrences and relative risk of bleeding were numerically lower in the clot formers, suggesting that they have an inherent prothrombotic state[21]. Overall, GUSTO moderate or severe bleeding was higher in the prolonged arm vs. the control arm(2.5 vs. 1.6: HR=1.61, P=0.001). Importantly, allcause mortality was higher in the prolonged therapy group (2.0 vs. 1.5%, HR=1.36, P=0.05) and cardiac mortality was similar (0.9% vs. 1.0%). Increased mortality in the extended DAPT arm was attributed to bleeding, trauma and cancer. Prasugrel therapy(n=1745, 35%) was associated with 48% reduction in the occurrence of the primary endpoint compared with a 20% reduction with clopidogrel (n=3725,65%) in drug-eluting stent (DES)-treated patients[1]. The latter observation lends further support for the ‘platelet hypothesis’ – superior platelet inhibition results in a more favorable reduction in ischemic outcomes [1]. Moreover, there was an increased risk of MI (both stent-related and non-stent-related)and stent thrombosis during the first 3 months after discontinuation of P2Y12inhibitor therapy. The latter observation suggests that heightening of platelet reactivity to ADP plays an important role in these cessation-related ischemic event occurrences even at 33 months post-stenting for the index event [1].
To better identify those patients who benefit from extended DAPT, a risk score was developed based on the results of the DAPT trial that was presented at American Heart Association (AHA) 2015 Scientific Sessions in Orlando. The DAPT Score is composed of following clinical factors- age, diabetes status, smoking status, PCI or MI history,presence of chronic heart failure or left ventricular ejection fraction <30% and index procedural characteristics: MI at presentation, vein-graft PCI, and stent diameter. This risk score may help to differentiate between patients most likely to benefit without increased bleeding risk, from those most likely to have bleeding risks that exceed the expected benefit of continued thienopyridine therapy.
Meta-Analyses of Trials of DAPT Duration
All of the RCTs described above, with the exception of the DAPT trial, were underpowered for detecting differences between treatment arms in stent thrombosis and were largely heterogeneous in study design, with some being prematurely terminated.The latter evidence has stimulated numerous metaanalyses to address the perplexing issue of optimal DAPT duration in the stented patient. Most of these meta-analyses have nearly arrived at the same conclusion- compared with a standard 12 month duration, short term DAPT (<12 months) after DES is associated with reduced bleeding with no apparent increase in ischemic complications. However, in selected patients with low bleeding risk and very high ischemic risk, extended DAPT (>12 months)should be considered. The increase in all cause but not cardiovascular death with extended DAPT requires further investigation [22–25].
Giustino et al. extensively analyzed the ischemic and bleeding endpoints according to DES type and report a significant interaction (P<0.008) between DAPT duration and the type of DES where a short DAPT was associated with an attenuated risk for stent thrombosis with the use of second-generation DES (OR=1.54) compared with first-generation DES (OR=3.94) [23]. Earlier large scale registries suggested that shorter (3 month) DAPT duration was as safe with 2ndgeneration DES as long term DAPT. In the DAPT trial the primary endpoint of MACE between 12 and 30 months was reduced with prolonged DAPT in patients receiving first-generation DES (~50% of patients) but not CoCr-EES (Pinteraction=0.05) whereas, the relative rate of stent thrombosis was reduced to a similar degree with 30-month vs.12-month DAPT for all DES types. The increase in bleeding with prolonged DAPT compared with shorter DAPT was, as expected, consistent with all DES [1]. In the Patient Related OuTcomes with Endeavor vs. Cypher stenting Trial (PROTECT) an interaction between the type of DES and DAPT duration was observed –very late (>1 year) stent thrombosis rates were increased with SES compared with ZES only if DAPT had been discontinued [26].
Another important meta-analysis of 33,435 patients analyzed the impact of the prior MI on long term DAPT [27]. In this analysis of high-risk patients stabilized following an MI, extended DAPT beyond 1 year was associated with a 22% relative and 1.1% absolute risk reduction in major adverse cardiovascular events over a mean 31 months of follow-up as compared to overall aspirin alone therapy. This result suggested that stabilized patients with a history of prior MI (known “clot formers”),at expected higher ischemic risk compared with patients with stable coronary artery disease treated with elective PCI, are indeed benefited by extended duration DAPT. Furthermore the benefit of extended duration DAPT was observed in patients with prior MI irrespective of therapy with PCI or not, indicating that the benefit of extended duration DAPT was related to risk reduction in the culprit as well as non-culprit vessels. The latter observation is supported by recent studies suggesting that in addition to the ruptured plaque that results in MI occurrence,patients with prior MI have more vulnerable plaques in the coronary vasculature. These patients may also experience heightened inflammation, dysfunctional endothelium and delayed stent endothelialization.These patients would therefore be expected to be more susceptible to the occurrence of thrombotic events in the setting of insufficient platelet inhibition following recurrent plaque rupture.
The DAPT and the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54(PEGASUS) trials demonstrated a reduction in stentand non-stent-related MI with extended duration of DAPT with thienopyridines (i.e. clopidogrel or prasugrel) or ticagrelor, respectively [1, 3]. However,a recent meta-analyis of three trials of thienopyridines (i.e. clopidogrel/prasugrel) and one of ticagrelor was conducted (n=37,427) to address potential differences in all-cause mortality between P2Y12inhibitors. All-cause mortality was increased by 30%with prolonged thienopyridines therapy [odds ratio(OR)=1.30] but not with prolonged ticagrelor therapy(OR=0.94) with a significant interaction between duration and type of treatment (Pinteraction=0.02). The differential impact of thienopyridines vs. ticagrelor on all-cause mortality was driven by both cardiovascular and non-cardiovascular death [28].
Other Evidence for Extended Duration DAPT
Despite being the gold standard that leads to Class I recommendations in the guidelines, there remain pitfalls of RCTs that include stringent enrolment criteria precluding generalizability (real-world experience) and high cost. The benefits of RCTs are balanced groups, limited selection bias, and well monitored follow-up including confirmation of patient compliance. Sample size and the cost of performing large-scale RCTs can be addressed by well-planned registries defining pre-specified demographic, medication, and outcome variables.Although extensive measures are taken to adjust for multiple known confounders, a major limitation of registry studies is the inability to adjust for unknown confounders [29, 30]. An important example would be the implicit decision-making of the clinician regarding the duration of DAPT in a given patient. Other limitations include incomplete follow-up with the risk of missing important ‘silent’endpoints and unknown patient compliance regarding study medications.
Numerous factors influence the duration of DAPT. In consideration of the latter, it would be useful to take into account the recent findings of the patterns of non-adherence to antiplatelet regimens in stented patients (PARIS) registry [31].In PARIS, the influence of DAPT cessation on clinical outcomes was evaluated with respect to physician-recommended discontinuation, brief interruption (for surgery), or disruption (noncompliance or because of bleeding). Physicianguided discontinuation was the major reason(41%) for DAPT cessation and was associated with significantly lower major adverse cardiovascular events (MACEs) compared with other strategies. Also, brief interruption lasting up to 14 days was not associated with increased risk for thrombotic event occurrence, whereas disruptions due to bleeding or non-compliance were associated with significantly increased risk of MACEs that was largely attenuated after 30 days. The evidence from PARIS strongly suggested that the mode of DAPT cessation greatly influences risk[31]. Among the most widely reported registry is the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART). Varenhorst et al.report findings from SWEDEHEART and other Swedish registries in 56,440 patients. Extensive measures were taken to adjust for baseline variables potentially associated with occurrence of the primary endpoint (all-cause death, stroke,or re-infarction) including revascularization.Outcomes were compared with respect to the duration of DAPT based on the number of prescribed clopidogrel tablets (3 months, 84–100 clopidogrel tablets; >3 months, >100 tablets; 6 months, 168–200 tablets; >6 months, >200 tablets). The study demonstrated a significantly lower incidence of the combined primary effi-cacy endpoint in patients treated with DAPT for>3 months compared with 3 months [adjusted hazard ratio (HR) 0.84, P=0.0042]. The adjusted HR (HR=0.75; P=0.0155) was also in favor of>6 months DAPT vs. 6 months for the same endpoint [32].
The potential benefit of DAPT up to median of 33 months after a MI was studied in the PEGASUSTIMI 54 trial. In this trial of 21,162 patients who had a MI 1–3 years earlier to Ticagrelor at a dose of 90 mg twice daily, Ticagrelor at a dose of 60 mg twice daily, or placebo. Both the Ticagrelor doses reduced the primary efficacy end point of 33 month composite of cardiovascular death, MI, or stroke as compared to aspirin alone therapy (HR for 90 mg Ticagrelor=0.85;P=0.008; and HR for 60 mg Ticagrelor=0.84;P=0.004). TIMI major bleeding rate was higher with Ticagrelor (2.6% with 90 mg and 2.3% with 60 mg)than with placebo (1.06%) (P<0.001 for each dose vs.placebo) and the rates of intracranial hemorrhage or fatal bleeding in the three groups were 0.63%, 0.71%,and 0.60%, respectively [3].
A large subset of patients (n=18,761) in PEGASUS had the timing of their last P2Y12inhibitor dose recorded and formed the group analyzed by Bonaca et al. The investigators extensively explored the question of whether the time interval between P2Y12cessation and the reintroduction of P2Y12inhibition using Ticagrelor influenced the anti-ischaemic benefit of the drug. Not surprisingly, this interval was closely linked to when the patients had their index clotting event: those with the shortest interval (≤30 days) had the shortest median time from index clot formation to randomization (16 months), whereas those with the longest interval (>1 year) had the longest median time from index clot formation to randomization (29 months). The former group is most similar to the clot former group in the DAPT trial [33].
The investigators made important observations. First, in the placebo group, a 47% increase in MACE was observed in those who discontinued P2Y12inhibitor in ≤30 days as compared with those who discontinued P2Y12inhibitor after >1 year.However, the former group also had more risk factors. Secondly, the benefit of Ticagrelor over placebo appeared largely confined to patients who discontinued the P2Y12inhibitor after ≤30 days,with a 27% relative risk reduction and 2.2% absolute risk reduction (P<0.001) in MACE compared with placebo. This benefit appeared mainly due to a significant reduction in MI with the 90 mg bid dose and was similar regardless of time from index MI (27% and 29% relative reductions in the MI<24 months and MI≥24 months previously groups, respectively). Finally, TIMI major bleeding increased with Ticagrelor therapy in all groups regardless of time from last dose of P2Y12inhibitor vs. placebo [33].
Conclusions
Clinicians are frequently uneasy about stopping the P2Y12inhibitor and, thus, subjecting the patient to a clotting stress test, while at the same time fear the definite risk of bleeding associated with continued potent P2Y12blockade. The available data support the anti-ischemic benefit of prolonged DAPT in patients with prior MI. These patients have a demonstrated altered pathophysiology that allows robust thrombus formation in the setting of high arterial shear that can withstand the disruptive effects of blood flow. In these patients the net benefit appears to favor uninterrupted DAPT by reducing events in the stented and non-stented vessel. These patients may also be less prone to bleeding since they appear hypercoagulable. A useful tool that improves the identification of candidates for prolonged DAPT is the DAPT risk calculator. Future work that analyzes intrinsic thrombogenicity and atherosclerotic coronary burden may further identify the optimal candidate for prolonged DAPT. Emerging evidence in the elective stent patient treated with late generation stents suggests the benefit of shorter term DAPT.However, there have been neither adequately powered trials for stent thrombosis in this population nor validated risk prediction tools.
Disclosures
Dr. Gurbel reports personal fees from AstraZeneca,Boehringer Ingelheim, Daiichi Sankyo/Lilly,Merck, Janssen Pharmaceuticals, New Haven Pharmaceuticals, Bayer, and Haemonetics;grants from Haemonetics, Merck, Duke Clinical Research Institute, Harvard Clinical Research Institute, National Institutes of Health, New Haven Pharmaceuticals, Coramed Technologies,MedImmune, and Sinnowa; a patent for platelet function testing; and stock options in Merck.
REFERENCES
1. Mauri L, Kereiakes DJ, Yeh RW,Driscoll-Shempp P, Cutlip DE,Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–66.
2. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ,Metzger DC, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drugeluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 2013;382:614–23.
3. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800.
4. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B,et al. ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–122.
5. Grines CL, Bonow RO, Casey DE Jr,Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association,American College Of Cardiology,Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol 2007;49:734–9.
6. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimuseluting stent implantation). J Am Coll Cardiol 2012;60:1340–8.
7. Feres F, Costa RA, Abizaid A,Leon MB, Marin-Neto JA, Botelho RV, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. J Am Med Assoc 2013;310:2510–22.
8. Gwon HC, Hahn JY, Park KW,Song YB, Chae IH, Lim DS, et al.Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT)randomized, multicenter study.Circulation 2012;125:505–13.
9. Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, et al. Secondgeneration drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014;64:2086–97.
10. Schulz-Schupke S, Byrne RA, Ten Berg JM, Neumann FJ, Han Y,Adriaenssens T, et al. ISAR-SAFE:a randomized, double-blind,placebo-controlled trial of 6 vs.12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252–63.
11. Valgimigli M, Campo G, Monti M,Vranckx P, Percoco G, Tumscitz C, et al. Short versus long-term duration of dual-antiplatelet therapy after coronary stenting:a randomized multicenter trial.Circulation 2012;125:2015–26.
12. Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol 2014;65:777–86.
13. Amsterdam EA, Wenger NK,Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–228.
14. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2016;133:1135–47.
15. Windecker S, Kolh P, Alfonso F,Collet J-P, Cremer J, Falk V, et al.2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2014;35:2541–619.
16. Montalescot G, Sechtem U,Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003.
17. Lee CW, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial.Circulation 2013;129:304–12.
18. Collet JP, Silvain J, Barthelemy O,Rangé G, Cayla G, Van Belle E,et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTICInterruption): a randomised trial.Lancet 2014;384:1577–85.
19. Helft G, Steg PG, Le Feuvre C,Georges JL, Carrie D, Dreyfus X,et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365-74.
20. Park SJ, Park DW, Kim YH,Kang S-J, Lee S-W, Lee CW,et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med 2010;362:1374–82.
21. Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ,Gershlick AH, et al. DAPT Study Investigators. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol 2015;65:2211–21.
22. Pandit A, Giri S, Hakim FA,Fortuin FD. Shorter (
23. Giustino G, Baber U, Sartori S,Mehran R, Mastoris I, Kini AS,et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65:1298–1310.
24. Palmerini T, Benedetto U, Bacchi-Reggiani L, Della Riva D, Biondi-Zoccai G, Feres F, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371–82.
25. Navarese EP, Andreotti F, Schulze V, Kolodziejczak M, Buffon A,Brouwer M, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. Br Med J 2015;350:h1618.
26. Camenzind E, Boersma E, WijnsW,Mauri L, Rademaker-Havinga T,Ordoubadi FF, et al. Modifying effect of dual antiplatelet therapy on incidence of stent thrombosis according to implanted drugeluting stent type. Eur Heart J 2014;35:1932–48.
27. Udell JA, Bonaca MP, Collet JP,Lincoff AM, Kereiakes DJ, Costa F, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction:a collaborative meta-analysis of randomized trials. Eur Heart J 2016;37:390–9.
28. Costa F, Adamo M, Ariotti S,Navarese EP, Biondi-Zoccai G,Valgimigli M. Impact of greater than 12-month dual antiplatelet therapy duration on mortality:Drug-specific or a class-effect?A meta-analysis. Int J Cardiol 2015;201:179–81.
29. Granger CB, Gersh BJ. Clinical trials and registries in cardiovascular disease: competitive or complementary? Eur Heart J 2010;31:520–1.
30. Gitt AK, Bueno H, Danchin N,Fox K, Hochadel M, Kearney P,et al. The role of cardiac registries in evidence based medicine. Eur Heart J 2010;31:525–529.
31. Mehran R, Baber U, Steg PG,Aritic, Weisz G, Witzenbichler B,et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study.Lancet 2013;382:1714–22.
32. Varenhorst C, Jensevik K, Jernberg T, Sundström A, Hasvold P, Held C, et al. Duration of dual antiplatelet treatment with clopidogrel and aspirin in patients with acute coronary syndrome. Eur Heart J 2014;35:969–78.
33. Bonaca MP, Bhatt DL, Steg PG,Storey RF, Cohen M, Im K, et al.Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12inhibitor withdrawal in patients with prior myocardial infarction: insights from PEGASUS-TIMI 54. Eur Heart J 2015;37:1133–42.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Coronary Artery Chronic Total Occlusion
- The Future of Transcatheter Therapy for Mitral Valve Disease
- The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
- Carotid Artery Stenting: 2016 and Beyond
- Identification and Management of Iatrogenic Aortocoronary Dissection
- Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
