Identification and Management of Iatrogenic Aortocoronary Dissection
Shao-Ping Nie, MD, PhD, FESC, FSCAI and Xiao Wang, MD
Introduction
Iatrogenic aortocoronary dissection (IACD) is a rare but potentially life-threatening complication during coronary catheterizations [1]. Although the reported incidence was low (<0.1%) [2–7], it represents one of the most common causes of procedure failure with increased risk of myocardial infarction and death. Depending on whether the dissection flap extends antegradely and/or retrogradely, the clinical manifestation varies from an asymptomatic angiographic finding to a complete hemodynamic collapse [8–10]. Thus, it is important to recognize and manage IACD promptly to prevent dissection propagation and improve outcomes. The purpose of this review is to describe the causes and characteristics of IACD, and to propose potential strategies for identification and management of this lethal complication.
Incidence
Dunning et al. [2] analyzed data from 43,143 cardiac catheterizations and found nine aortocoronary dissections for an incidence of 0.02%. In another report, the overall incidence of IACD was 0.04%,with 0.12% in interventional procedures and 0.01%in diagnostic procedures [6]. About 40% of aortic dissection propagates rapidly to the ascending aorta, and the in-hospital mortality was 11–35% [2,11]. In particular, left main (LM) ostial dissection is a severe type of IACD. In a single-center experience of 42,345 cardiac catheterizations, 30 cases(0.071%) died within 24 hours, 20 (67%) of which were caused by LM dissections during diagnostic coronary angiography [12]. Recent studies showed the incidence of iatrogenic LM dissection was about 0.07% [3, 4].
Causes of IACD
IACD is mainly caused by disruption of intima at the ostia of LM or right coronary artery (RCA)during coronary catheterizations. Dissection at the ostium could extend antegradely and lead to subtotal or total occlusion of the coronary lumen without distal antegrade blood flow. Similarly, it could extend retrogradely into the sinus of Valsalva and cusp, compromising the other ostium of the coronary artery, and propagate to the ascending aorta,aortic arch, or even the descending aorta [3, 10, 13].The following factors might contribute to the presence and progression of IACD.
Device and Technique Factors
IACD can be induced during manipulation of guiding catheter (GC) into coronary artery or retrieval of balloon catheter or stent into GC. When the catheter is unfit, the tip is often pressed against the vessel wall, which causes damping of the catheter. The direct injury and blind contrast injection may lead to dissection. Data from Dunning et al. [2] revealed that Amplatz GC were being used in 44% of cases at the time of dissection. Goldstein et al. [14] retrospectively analyzed 28 cases of IACD, and found 11 cases (39%) were caused by balloon dilatation, 8 cases (29%) by injury by GC, and 4 cases (14%) by manipulation of guidewires.
Vascular Pathology
Coronary dissection may be partly associated with extensive atherosclerosis. Catheter-induced plaque ulceration may also serve as an entry point for the pulsatile flow of blood [15]. Degenerative medial diseases, such as cystic medial necrosis,might also play a role in the development of IACD[16, 17]. Susceptibility to the development of intimal tears and propagation of the hematoma may be related to an underlying structural weakness of the media. Predisposing factors include Marfan syndrome, congenitally unicuspid and bicuspid aortic valves [18].
Vascular Anatomy
Previous studied have indicated that the risk of dissection is higher in RCA than left coronary artery(LCA). The proportion of RCA dissection is more than two thirds in IACD [2, 6, 14]. In contrast to those of the RCA, the periostial wall and sinotubular junction of the LCA were formed by more smooth muscle cells and by a dense matrix of collagen type I fibers [19]. The fewer side branches in the RCA compared to the left anterior descending(LAD) coronary artery could also partially explain why dissection occurs more often in the RCA [15].In contrast, observations from autopsy study suggested an angulated LCA may be a risk factor for dissection at angiography [20].
Identification and Diagnosis
Early identification and prompt management is crucial to the prognosis of patients with IACD.Experienced operators can identify the dissection at first time, in which case no changes in hemodynamics or ischemic symptoms have occurred.Immediate bail-out stenting could prevent propagation of dissection and severe complications.
Dissection appears as filling defects with the coronary lumen or persistence of contrast (“extraluminal cap”) or intimal tear outside the coronary lumen. Dissection could disseminate antegradely and lead to subtotal or total lumen occlusion, and is associated with complaints of severe chest distress,chest pain, nausea or vomiting, as well as hemodynamic compromise including slowing down of heart rate and blood pressure. Electrocardiogram often presents with ST segment elevation in culprit vessel-related leads or severe arrhythmia. It is to be noted that some IACD patients may have no symptoms or changes in hemodynamics, and are prone to be ignored. Cheng et al. [4] reported that about half of all 13 cases of iatrogenic LM dissection were initially asymptomatic. Kovac and de Bono [21] showed 72% of severe LM dissection occurred during or immediately after the procedure while the patient was still in the catheterization laboratory, and 28% after return to a ward or recovery area.
In some cases (dissection just in the sinus of Valsalva), intravascular ultrasound could be used for the diagnosis of suspicious IACD. Moreover,multi-slice computed tomography (CT), magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography could also be used to evaluate the progression of IACD.
Classifications
National Heart, Lung, an d Blood Institute(NHLBI) Classification
NHLBiclassifies dissections into six types (A–F),which could predict risk of acute coronary occlusion[3, 22, 23]. The NHLBiclassification and examples are presented in Figure 1. Huber et al. [23] reported 691 dissections during percutaneous transluminal coronary angiography, which were categorized according to NHLBiclassification. Five hundred and forty three patients with type B dissections had a similar clinical success rate (93.7%) compared with patients without dissection, with no increase in morbidity and mortality. One hundred and forty eight procedures with type C–F dissections had significant increased rates of in-hospital complications, including acute closure (31%), need for emergency coronary bypass surgery (37%), myocardial infarction (13%) and repeat angioplasty (24%),with an overall success rate of 38%. However, the NHLBiclassification is limited by unavailability to entirely show coronary ostia and aortic dissections in patients with IACD.
Dunning Classification
Dunning et al. [2] proposed a classification for coronary dissection with retrograde extension into the aortic root. Class I is defined as a focal dissection restricted to the coronary cusp. Class II involves the cusp and extends up the ascending aorta but is less than 40 mm. Class III extends from the coronary cusp up the ascending aorta greater than 40 mm(Figure 2). Gomez-Moreno et al. [6] identified 18 cases of IACD and there were 11 patients with class I, 4 patients with class II, and 3 patients with class III. Compared to class I and II dissections,class III dissections were more often associated with exclusive retrograde extension (61% vs. 27%).Thus, this classification could help to predict prognosis and guide therapeutic strategy, but it did not take into account the antegrade extension of dissection into the coronary tree.
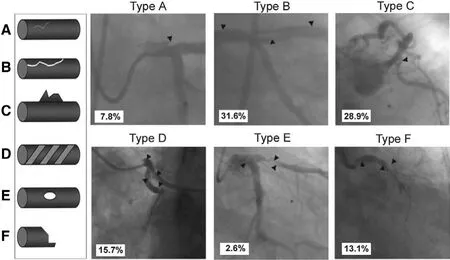
Figure 1 Left Main Dissection According to the NHLBiclassification.Left: schematic diagram (A–F). Right: examples and relative incidence (percentage) of each type of dissection. Type A dissections indicate minor radiolucent areas within the coronary lumen during contrast injection with little or no persistence after dye clearance. Type B dissections appear as parallel tracts or a double lumen separated by a radiolucent area during contrast injection, with minimal or no persistence of contrast after the dye has cleared from the lumen. Type C dissections present as contrast outside the lumen with persistence of contrast after dye clearance. Type D dissections appear as spiral luminal filling defects,frequently with excessive contrast retention of the dissected false lumen. Type E dissections present as new, persistent filling defects within the coronary lumen. Type F dissections represent those that lead to total occlusion of the coronary lumen without distal antegrade flow. Arrowheads indicate dissection. (Modified from Eshtehardi et al. Am Heart J 2010;159:1147–53.)
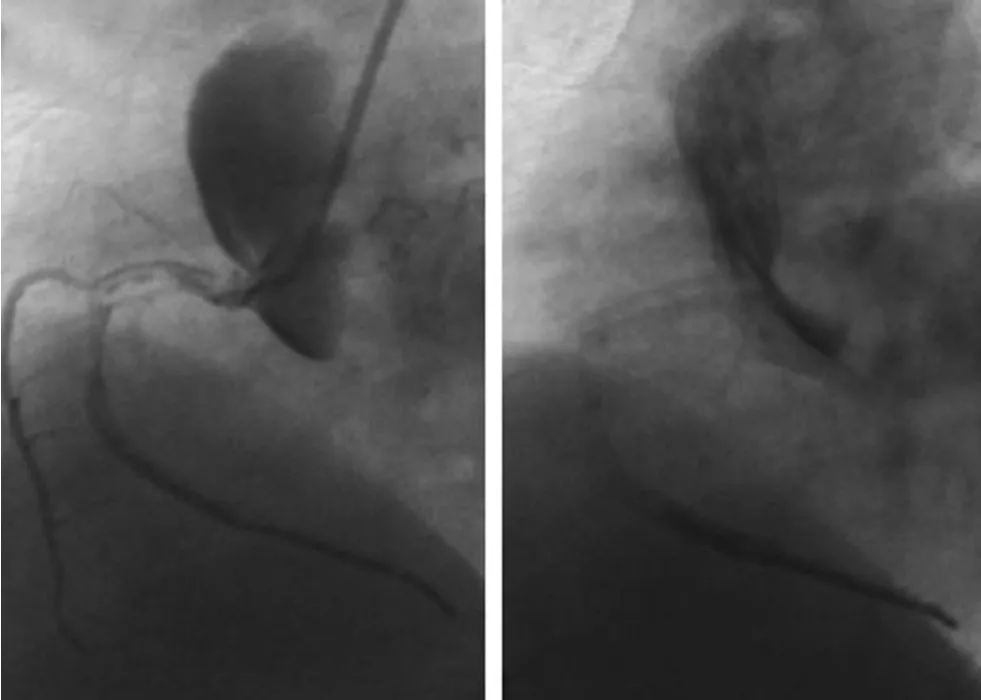
Figure 2 Example of Dunning Class III Dissection.Blind contrast injection while damping of the catheter leads to severe dissection of aorta and RCA (left). The dissection extends antegradely into the proximal to distal RCA and also involves right sinus of Valsalva and ascending aorta(>40 mm). After catheter extraction, extensive contrast retention could be observed (right).
Eshtehardi’s Simplified Classification
According to the extension of LM ostial dissection, Eshtehardi et al. [3] put forward a simplified classification (Figure 3). A localized dissection in the LM ostium is defined as type I. Extension of the dissection from the LM into the LAD or left circumflex artery (LCX) is defined as type II, and extension of the dissection flap into the aortic root is classified as type III. According to the classifi-cation, type I dissection was found in 21 patients(55%), type II in 16 patients (42%), and type III in 1 patient (3%). No hemodynamic collapse was observed in patients with type I dissection. In contrast, 7 (41%) of 17 patients with type II or III dissections developed hemodynamic instability, and 5 patients (29%) required cardiopulmonary resuscitation. A patient with type III dissection died before emergency intervention was performed.Overall, this classification involves both antegrade and retrograde extension of dissection. However,it is only applicable to LM ostial dissection, and cannot precisely reflect extension of the dissection flap.
There are some limitations regarding the aforementioned three classifications. They cannot be applied to all patients with IACD (including aortic-LM/RCA ostial dissection) and reflect the scope and degree of antegrade and retrograde extension.In this case, we can use the NHLBiclassification to evaluate the severity of dissection and the risk of acute occlusion, and according to the Dunning or Eshtehardiclassifications, we can identify the extension of dissection to guide therapeutic strategy and predict prognosis.
Management
Initial Therapeutic Strategies
Immediate bail-out stenting and emergency coronary artery bypass grafting (CABG) are two initial strategies available for the management of IACD [3, 7].Carstensen and Ward reviewed 67 cases of dissection including 28 cases (42%) with rapid progression.Four of the 28 cases (14%) were treated conservatively and two of these four died suddenly. Immediate stenting was performed in 13 cases (46%) with no deaths. Eleven cases received emergency surgical repair (39%) and three of these cases died eventually [13]. Actually, in most cases, the dissection flap and hematoma can deteriorate within minutes or hours, and propagate abruptly in an uncontrollable way. Therefore, unless in some selected cases (for example, NHLBI type A or B), the so-called “wait and see” conservative strategy is scarcely considered as the best therapeutic option [24].
Dissections primarily occur in the catheterization laboratory. Therefore, immediate bail-out stenting is superior to emergency surgical intervention in terms of time and technique. Several reports have showed that bail-out stenting to seal the entry site of dissection can improve clinical outcomes [2, 5,6, 13, 25–30]. Lee et al. [5] reported ten cases of LM dissection which were successfully treated with stenting with no deaths. Carstensen and Ward [13]presented four cases which were immediately sealed with stenting of the coronary ostium. Long-term outcome was universally event free.
Compared to bail-out stenting, emergency surgery needs skilled operators with high technique level, thus increasing the mortality risk. It was reported that the in-hospital mortality for emergency CABG ranges from 7.8% to 14% [31]. When taking full anticoagulation and antiplatelet setting into consideration, as well as the unstable hemodynamic condition, the risk of surgery may be even higher. Moreover, hemodynamic deterioration may progress with fatal results during transportation or when the patients were waiting for surgery.
Once coronary dissection was identified, the key point is to maintain blood flow in the true lumen.Successful treatment of dissection relies on prompt sealing of the entry site to terminate progression of dissection or even abrupt occlusion due to intramural coronary hematoma. Dunning et al. [2]summarized the results from nine patients and proposed that patients might be managed by bail-out stenting if the dissection extends <40 mm from the coronary ostium and that surgical intervention might be required if the dissection extends >40 mm from the ostium. Generally, immediate bail-out stenting does not appear to lose the chance of surgical success. A review of the literature showed that bail-out stenting was successfully performed on 32 of 36 patients (88.9%). With back-up CABG for failure of catheter-based treatment, overall survival rate was 94.4% (34/36) [4]. Thus, immediate bail-out stenting should be performed as rapidly as possible in cases of severe dissection (NHLBI type C–F), even when significant propagation has already occurred. Surgery should only be considered when stenting failed to seal the dissection and the patients had hemodynamic compromise.
Stenting for Bilateral Aortocoronary Ostial Dissection
When the dissection was not managed promptly, it can extend retrogradely into the sinus of Valsalva,or even propagate to the contralateral ostium of the coronary artery, leading to bilateral aortocoronary ostial dissection or even occlusion. Thus, in patients with probable bilateral involvement of the sinus of Valsalva, it is suggested to apply stenting in the contralateral ostium of the coronary artery immediately after the initial stenting of ipsilateral ostium.Sekiguchi et al. [32] presented one case of RCA dissection with extension to the left sinus of Valsalva,leading to simultaneous occlusion of RCA and LCA and cardiogenic shock. Immediate bail-out stenting of RCA and LCA respectively restored the coronary flow, and CT scan showed no dissection progression after seven days.
Bifurcation Stenting Strategy of LM Dissection
In patients with Eshtehardi type II dissection involving both the LAD and LCX, the decision should be made according to dissection anatomy and hemodynamic condition. First, it should be ensured that both the LAD and LCX are open with stable hemodynamics. When the two vessels are both involved with subtotal occlusion, the operators should perform stenting in the vessel with more hemodynamic significance. Second, stents should be implanted from the distal to proximal segment and up to the ostium of the coronary artery, ensuring full coverage of the ostium (protruding 2–3 mm outside the coronary artery). Third, ostial stenting should be dilated as a “bellmouth” to the greatest extent to ensure complete stent apposition and to prevent retrograde extension of dissection. Fourth,the operator should choose the following stenting technique, including Step-crush, modified T, TAP or Culottes technique. The classic Crush or kissing stenting technique should not be used unless the hemodynamics are stable, because these techniques need GC with large size and are more complicated and time-consuming. Fifth, in cases of LM bifurcation dissection during percutaneous coronary intervention via radial access, if the GC and guidewire was inserted and bifurcation stenting was unavoidable, it is not advisable to change arterial access or GC size. It is possible that the guidewire will be difficult to reenter into the true lumen, thus prolonging the operation time. In addition, reinsertion of GC may exacerbate ostial dissection. In reality,bifurcation stenting (except classic Crush or kissing stenting) can be performed with 6F GC using the present types of stents and balloons. Sixth, when the operators have limited experience of bifurcation stenting, they can preferentially perform stenting in the major artery to achieve full coverage of the ostium of coronary artery and to stabilize the hemodynamics. Bifurcation stenting can be left for senior operators. We recently presented a case of iatrogenic LM dissection with occlusion of both braches. Immediate bail-out TAP-stenting was successfully performed and the patient was discharged without any complications. One-year follow-up indicated stent patency and favorable clinical result [33].
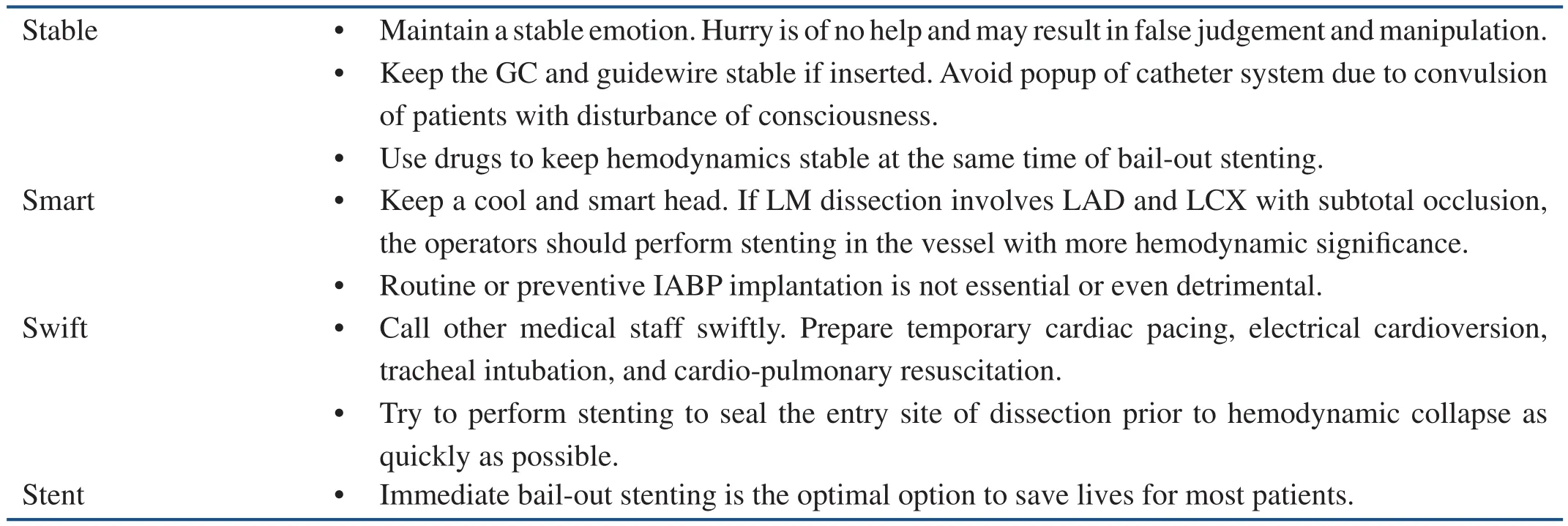
Table 1 The “4S” Law for the Management of Iatrogenic Aortocoronary Dissection.
The “4S” Law for Emergency Management of IACD
Management of IACD requires considerable personal experience, strong ability of emergency reaction, and good psychological qualities. The operators should stay clear-minded, calm, and responsive to rapidly-changing situations. From the evidences of the literature and also our local experiences [33], in the condition of severe dissection, if the operators can keep a cool head and manage their emotions (Smart), maintain the catheter system stability (GC and guidewire) (Stable),manipulate stably and rapidly (Swift), and perform Stent implantation immediately (“4S” law), most patients will be out of danger and avoid emergency CABG, thereby decreasing mortality risk(Table 1).
Conclusions
IACD is a rare but potentially disastrous complication during coronary catheterizations. It has typical angiographic findings and represents one of the most common causes of procedure failure with increased risk of myocardial infarction and death.Early identification and prompt management is crucial to the patients with IACD. Immediate bail-out stenting should be the first choice and performed as promptly as possible in most cases of severe dissection. Surgery should only be considered when stenting failed to seal the dissection and the patients had hemodynamic collapse.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This study was supported in part by grants from the Beijing Natural Science Foundation (7141003)and Beijing Municipal Science & Technology Commission (Z141107002514014).
REFERENCES
1. Bonow RO, Mann DL, Zipes DP,Libby P. Braunwald’s Heart disease: a textbook of cardiovascular medicine. 9th ed. Philadelphia, PA:Elsevier Saunders; 2012.
2. Dunning DW, Kahn JK, Hawkins ET, O’Neill WW. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv 2000;51:387–93.
3. Eshtehardi P, Adorjan P, Togni M,Tevaearai H, Vogel R, Seiler C,et al. Iatrogenic left main coronary artery dissection: incidence,classification, management, and long-term follow-up. Am Heart J 2010;159:1147–53.
4. Cheng CI, Wu CJ, Hsieh YK, Chen YH, Chen CJ, Chen SM, et al.Percutaneous coronary intervention for iatrogenic left main coronary artery dissection. Int J Cardiol 2008;126:177–82.
5. Lee SW, Hong MK, Kim YH,Park JH, Rhee KS, Lee CW, et al. Bail-out stenting for left main coronary artery dissection during catheter-based procedure: acute and long-term results. Clin Cardiol 2004;27:393–5.
6. Gomez-Moreno S, Sabate M,Jimenez-Quevedo P, Vazquez P,Alfonso F, Angiolillo DJ, et al.Iatrogenic dissection of the ascending aorta following heart catheterisation: incidence, management and outcome. EuroIntervention 2006;2:197–202.
7. Nunez-Gil IJ, Bautista D, Cerrato E, Salinas P, Varbella F, Omede P, et al. Incidence, management,and immediate- and long-term outcomes after iatrogenic aortic dissection during diagnostic or interventional coronary procedures.Circulation 2015;131:2114–9.
8. Awadalla H, Sabet S, El Sebaie A,Rosales O, Smalling R. Catheterinduced left main dissection incidence, predisposition and therapeutic strategies experience from two sides of the hemisphere.J Invasive Cardiol 2005;17:233–6.
9. Rao GK, Ayyanthan A, Davis G. Catheter induced aortocoronary dissection. Acute Card Care 2008;10:58–9.
10. Liao MT, Liu SC, Lee JK, Chiang FT, Wu CK. Aortocoronary dissection with extension to the suprarenal abdominal aorta: a rare complication after percutaneous coronary intervention. JACC Cardiovasc Interv 2012;5:1292–3.11. Yip HK, Wu CJ, Yeh KH, Hang CL, Fang CY, Hsieh KY, et al.Unusual complication of retrograde dissection to the coronary sinus of valsalva during percutaneous revascularization: a single-center experience and literature review.Chest 2001;119:493–501.
12. Devlin G, Lazzam L, Schwartz L. Mortality related to diagnostic cardiac catheterization. The importance of left main coronary disease and catheter induced trauma. Int J Card Imaging 1997;13:379–84;discussion 85–6.
13. Carstensen S, Ward MR. Iatrogenic aortocoronary dissection: the case for immediate aortoostial stenting.Heart Lung Circ 2008;17:325–9.
14. Goldstein JA, Casserly IP,Katsiyiannis WT, Lasala JM,Taniuchi M. Aortocoronary dissection complicating a percutaneous coronary intervention. J Invasive Cardiol 2003;15:89–92.
15. Gur M, Yilmaz R, Demirbag R,Kunt AS. Large atherosclerotic plaque related severe right coronary artery dissection during coronary angiography. Int J Cardiovasc Imaging 2006;22:321–5.
16. Carlson RG, LilleheicW, Edwards JE. Cystic medial necrosis of the ascending aorta in relation to age and hypertension. Am J Cardiol 1970;25:411–5.
17. Schlatmann TJ, Becker AE.Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol 1977;39:13–20.
18. Spittell PC, Spittell JA, Jr., Joyce JW, Tajik AJ, Edwards WD, Schaff HV, et al. Clinical features and differential diagnosis of aortic dissection: experience with 236 cases(1980 through 1990). Mayo Clin Proc 1993;68:642–51.
19. Lopez-Minguez JR, Climent V,Yen-Ho S, Gonzalez-Fernandez R, Nogales-Asensio JM, Sanchez-Quintana D. Structural features of the sinus of valsalva and the proximal portion of the coronary arteries: their relevance to retrograde aortocoronary dissection. Rev Esp Cardiol 2006;59:696–702.
20. Curtis MJ, Traboulsi M, Knudtson ML, Lester WM. Left main coronary artery dissection during cardiac catheterization. Can J Cardiol 1992;8:725–8.
21. Kovac JD, de Bono DP. Cardiac catheter complications related to left main stem disease. Heart 1996;76:76–8.
22. Dorros G, Cowley MJ, Simpson J, Bentivoglio LG, Block PC,Bourassa M, et al. Percutaneous transluminal coronary angioplasty: report of complications from the National Heart, Lung, and Blood Institute PTCA Registry.Circulation 1983;67:723–30.
23. Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol 1991;68:467–71.
24. Celik M, Yuksel UC, Yalcinkaya E,Gokoglan Y, Iyisoy A. Conservative treatment of iatrogenic left main coronary artery dissection: report of two cases. Cardiovasc Diagn Ther 2013;3:244–6.
25. Patel TM, Shah SC, Ranjan A.Unusual retrograde aortic arch dissection during percutaneous coronary intervention: a case report.Angiology 2006;57:501–5.
26. Wykrzykowska JJ, Carrozza J,Laham RJ. Aortocoronary dissection with acute left main artery occlusion: successful treatment with emergent stenting. J Invasive Cardiol 2006;18:E217–20.
27. Saito T, Noguchi K, Oikawa T. Iatrogenic dissection of the anomalous-origin right coronary artery and left sinus of Valsalva. J Invasive Cardiol 2011;23:E51–3.
28. Vatrano M, Dattilo G, Mandraffino G,Gangemi S, Ciconte VA, Quartuccio S, et al. A quick bailout ongoing of cardiogenic shock and iatrogenic dissection of the left main coronary artery. Int J Cardiol 2015;184:473–4.
29. Davlouros PA, Kontoprias K,Alexopoulos D. Iatrogenic left main coronary artery dissection mimicking complete proximal occlusion of the left main branches.Hellenic J Cardiol 2015;56:100–2.
30. Boukhris M, Tomasello SD,Marza F, Azzarelli S, Galassi AR. Iatrogenic aortic dissection complicating percutaneous coronary intervention for chronic total occlusion. Can J Cardiol 2015;31:320–7.
31. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–122.
32. Sekiguchi M, Sagawa N, Miyajima A, Hasegawa S, Yamazaki M,Kurabayashi M. Simultaneous right and left coronary occlusion caused by an extensive dissection to the coronary sinus of Valsalva during percutaneous intervention in right coronary artery. Int Heart J 2009;50:663–7.
33. Lao EP, Nie SP, Ma CS. Immediate bail-out TAP-stenting for the treatment of iatrogenic aortocoronary dissection involving left main bifurcation. J Geriatr Cardiol 2013;10:202–4.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
- Cardiovascular Abnormalities Among Patients with Spontaneous Subarachnoid Hemorrhage.A Single Center Experience
- Coronary Artery Chronic Total Occlusion
- Carotid Artery Stenting: 2016 and Beyond
- The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
- The Future of Transcatheter Therapy for Mitral Valve Disease
