The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
Dhaval Pau, MD, Nileshkumar J. Patel, MD, Nish Patel, MD and Mauricio G. Cohen, MD,FACC, FSCAI
1Department of Medicine, Staten Island University Hospital, Staten Island, NY, USA
2Cardiovascular Division and Elaine and Sydney Sussman Cardiac Catheterization Laboratory, University of Miami Miller School of Medicine, Miami, FL, USA
Introduction
Cardiac catheterization and percutaneous coronary intervention (PCI) play an important role in the diagnosis and treatment of coronary artery disease. The transfemoral approach to cardiac catheterization has been the dominant technique utilized by interventional cardiologists in the past decades. However, the transradial approach has emerged as an effective alternative since the first successful coronary angiography and PCI using this method were performed in 1989 and 1993 respectively [1–3]. The transradial approach has become increasingly popular in light of multiple studies which suggest advantages of this vascular access site over the transfemoral approach; including, reduced access site bleeding, lower rates of vascular complications, early sheath removal, improved patient comfort, fast recovery, and decreased costs [4].Despite these advantages, the transradial approach has been associated with longer procedure times, a prolonged learning curve, higher crossover rates, and inability to use large bore sheaths, which has led to variability in its adoption worldwide [5–7]. Both the ACC/AHA/SCAI and European guidelines include a class IIA recommendation for transradial approach to decrease access site complications [8, 9].
In this review, the history, observational trends,efficacy, and technical aspects of transradial cardiac catheterization and PCI will be discussed.
History
Initial attempts at central arterial catheterization and coronary angiography via the radial artery were first reported by Radner in 1948 [10]. Although there was interest in the transradial approach, equipment related limitations led to a shift of most catheterbased procedures at the time, to larger vessels. The radial artery remained a site for monitoring arterial pressure [1]. Early PCI in the 1970s were performed using larger 9F guiding catheters [11]. Campeau was the first to report successful coronary angiography using a transradial approach in 1989, with successful PCI performed by Kiemeneij in 1993 utilizing smaller 6F guiding catheters [2, 3]. There were early enthusiastic adopters and as utilization grew,improvements in patient comfort and reduction in bleeding complications were noted.
The Problem with Access
Bleeding complications after both diagnostic and interventional cardiac catheterization are most commonly related to the access site and are associated with significantly higher morbidity, mortality and cost [12–15]. One large study has reported that major bleeding occurred in 2.8% of all patients hospitalized for acute myocardial infarction [16]. Intracranial bleeding and gastrointestinal bleeding are well acknowledged potentially fatal events, however, bleeding complications related to the access site have been historically viewed as benign complications. Studies conducted by Doyle et al. and Yatskar et al. have shown that major femoral bleeding complications after cardiac catheterization including major hematoma, external bleeding, and retroperitoneal bleeding are associated with an increased short and long term mortality [12, 17]. Consequently,femoral access site bleeding complications should not be disregarded. It has been reported that using a transradial instead of a transfemoral approach is the most effective method of reducing major bleeding [18].
The frequency of bleeding complications is significantly higher in the setting of ST-elevation myocardial infarction. One study conducted using the CathPCI registry noted that bleeding complications in the ST-elevation myocardial infarction (STEMI)subgroup were more than twice as likely when compared to the non-ST elevation myocardial infarction(NSTEMI) subgroup and close to 4 times as likely in comparison to patients undergoing PCI electively[19]. The same study provided a few explanations for this. Firstly, the STEMI subgroup had lower utilization of the transradial approach in comparison to the NSTEMI and elective PCI subgroups. Secondly, the STEMI subgroup was more aggressively anticoagulated. Lastly, there was higher utilization of intraaortic balloon pumps, which have been associated with a larger bleeding risk [20].
In addition to the morbidity and mortality associated with post PCI bleeding, a significant economic impact can also be noted. One study by Kugelmass et al. showed vascular complications had an added cost of approximately $6400 and added close to 3 days of hospitalization [14]. Studies comparing transfemoral and transradial approaches have shown significantly lower hospital costs with the transradial approach. An early study in published in 1999 showed that among patients undergoing diagnostic cardiac catheterization, the transradial approach was associated with savings of $290 per case [21]. It is likely that these savings were due to decreased procedural complications and shorter hemostasis times. The savings would be expected to be higher in the case of PCI due to the more aggressive utilization of antiplatelet and anticoagulant agents. Disadvantages of the transradial approach include longer procedure times and higher crossover rates. The question that needed to be answered was whether the potentially higher costs of longer procedure times and higher crossover rates were counterbalanced by fewer complications. According to a systematic review conducted by Mitchell et al., which accounted for these variables, the transradial approach was favored in all conditions tested and resulted in a $275 less cost per patient[5]. Another reason for the economic advantages of the transradial approach is the increased likelihood of same day discharge. One study involving over 100,000 Medicare beneficiaries showed that same day discharges occur in only 1.25% of elective PCicases. A significantly higher proportion of those patients underwent PCI using the transradial approach [22]. Studies have shown that same day discharge after uncomplicated elective transradial PCI leads to a relative cost reduction of 50% [23].
Transradial catheterization is also associated with increased patient comfort. From the time when early studies were published, there has been strong patient preference for transradial catheterization and an improved post procedure quality of life has been noted in comparison to the transfemoral approach. One small study has shown transradial PCI led to reduced pain, and improved physical health and walking ability [21]. Results from the OCEAN RACE trial also showed that the transradial approach is associated with improved psychological outcomes and fewer mobility-related problems [24]. The RIVAL trial also demonstrated that when patients were asked regarding their preference for subsequent procedures, transradial was the more frequent choice [25]. The transradial and transfemoral approaches are compared in Table 1.
Trends in Utilization
Despite the many advantages of the transradial approach, its adoption varies significantly across the United States and internationally. The use of the transradial approach was adopted quickly in Europe and Asia. Studies have reported that a significant num-ber of PCI procedures performed in Japan (60%),France (55%), Canada (50%), Spain (43%), the United Kingdom (35%), India (32%), Italy (25%),Germany (25%), China (25%) and Poland (22%)are performed via the transradial approach [26, 27].Caputo et al. report that an estimated 20% of PCI procedures worldwide are performed transradially.This number increases to 29% if the United States is excluded from the estimate [27]. The low utilization of this approach in the United States is confirmed by a few studies. One study conducted using the National Cardiovascular Data Registry from 2004 to 2007 showed that only 1.32% of all PCI procedures were performed using the transradial approach [6].Results from a subsequent study using 2007–2012 data from the same registry showed that utilization of this approach has increased from 1.2% in the first quarter of 2007 to 16.1% in the third quarter of 2012 and accounts for 6.3% of all PCI procedures performed during the study period [28]. The same study also noted that the transradial approach was more frequently utilized in teaching hospitals and in the northeast region of the United States. Another study conducted by Baklanov et al. from the same registry noted that in the setting of STEMI, the utilization of transradial PCI increased from 0.9% to 6.4% between 2007 and 2011. The study also noted that the transradial approach was associated with a longer median door-to-balloon time but at the same time had lower risk of bleeding and in-hospital mortality rates [29]. A study conducted by Hannan et al.noted that the utilization of the transradial approach for PCI in the setting of STEMI, increased from 4.9% to 11.9% between 2009 and 2010 in the state of New York [30]. Figure 1 illustrates the trends in utilization of the transradial approach for PCI from the National Cardiovascular Data Registry between years 2007 and 2012 [28].
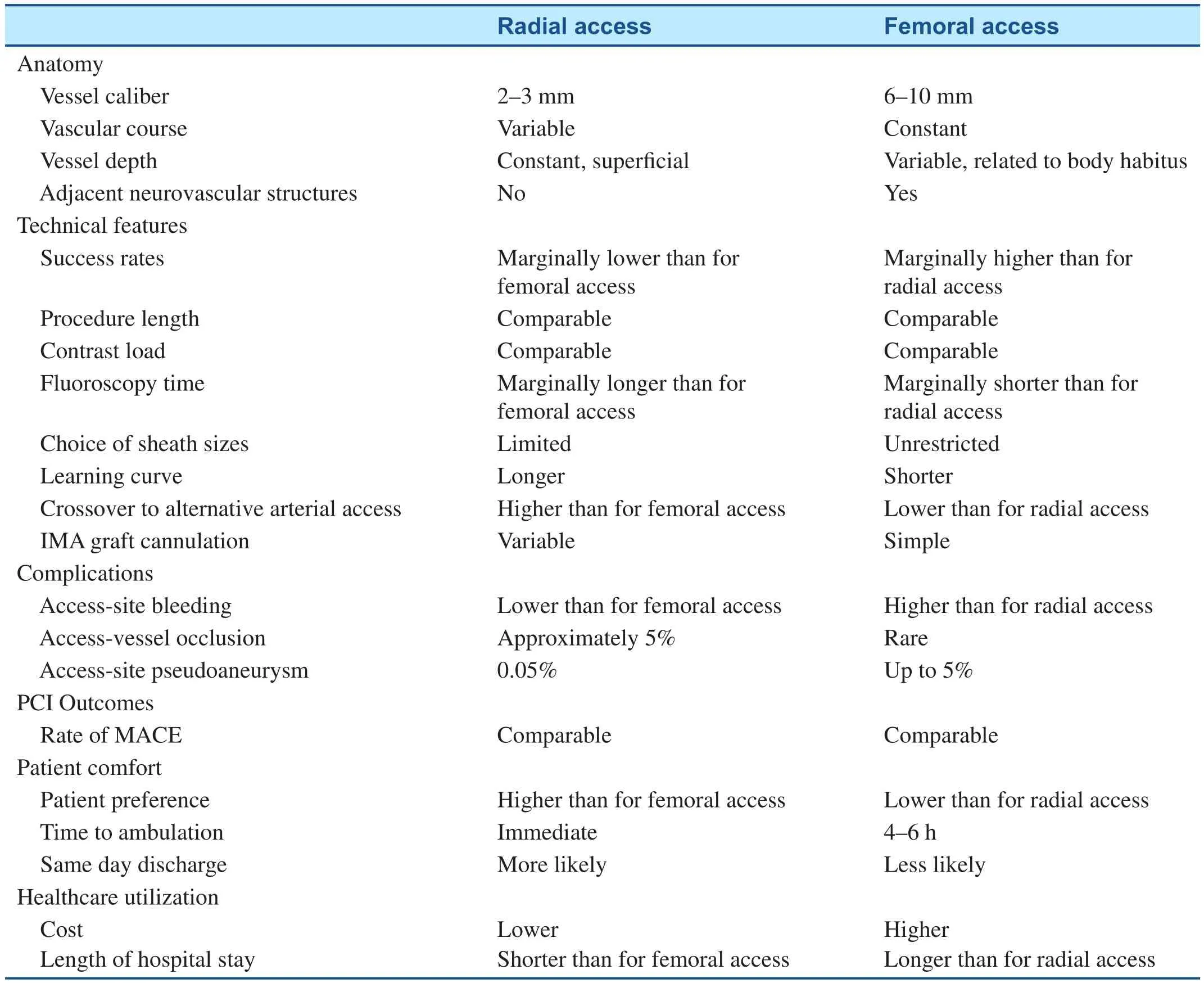
Table 1 Comparison of Transradial and Transfemoral Access.
Figure 1 Trends in Utilization of the Transradial Approach for PCI from the National Cardiovascular Data Registry between 2007 and 2012.
PCI, percutaneous coronary intervention; UA, unstable angina; NSTEMI, non-ST elevation myocardial infarction; STEMI,ST-elevation myocardial infarction.
Obtained from Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007–2012). Circulation 2013;127:2295–306.
Randomized Clinical Trials
Multiple randomized clinical trials have been conducted to compare transradial and transfemoral approaches. The RIVAL trial randomly assigned patients with acute coronary syndromes who underwent PCI to either transradial or transfemoral approaches. Findings showed no differences in patients with NSTEMI, however, in STEMI patients,the transradial approach reduced mortality, myocardial infarction, stroke, and non-coronary bypass graft-related major bleeding. One confounding factor for RIVAL was that operator experience with transradial PCI was greater in the STEMI group when compared to the NSTEMI group; this could be a possible explanation for the lack of positive findings in the NSTEMI group [31]. The recently conducted MATRIX trial randomized a total of approximately 8400 patients with acute coronary syndromes, both STEMI and NSTEMI, who underwent PCI across multiple centers to either femoral or radial approaches. Results showed that the transradial approach was associated with a net reduction in adverse clinical events including major bleeding and all-cause mortality [32]. The SAFE-PCI for Women trial, which randomized female patients to transradial or transfemoral approaches showed conflicting results [33]. A total of 1787 were recruited at 60 sites. Findings of the study showed that the radial approach did not significantly reduce bleeding or vascular complications in women undergoing PCI. However, when results for both diagnostic and interventional procedures were combined, there were better outcomes for the transradial approach.The conflicting findings from this trial may also be explained by its early termination due to a lower than expected rate of bleeding and vascular complications. Investigators reported that the originally planned sample size would not be able to show a difference between approaches. RIFLE-STEACS was a multicenter study involving 1001 patients with STEMI randomized to transradial or transfemoral approaches for PCI, which noted that the transradial approach was associated with fewer adverse clinical events, shorter hospital stay, as well as lower overall morbidity and cardiac mortality [34]. Table 2 summarizes the findings of randomized clinical trials involving the transradial approach.
Technical Aspects
Despite the many benefits of the transradial approach, it should be emphasized that it is not always feasible, as many technical aspects need to be considered. In most cases, there is an anastomosis between ulnar and radial arteries, with the predominant blood supply being provided by the ulnar artery [37]. However, the vascular anatomy of the hand can have significant variability, making handischemia a possible complication. Studies have suggested that radial artery access can lead to vessel occlusion in 0.8–30.0% of cases [38]. Thus, it may be beneficial to confirm the integrity of palmar arches, prior to utilizing the transradial approach.Guidelines from both the European Society of Cardiology and the Society for Cardiac Angiography and Interventions recommend testing for the integrity of blood supply to the hand prior to utilizing the transradial approach for cardiac catheterization. In 1929, the Allen test was introduced to evaluate the blood supply to the hand in patients with Buerger disease. This test is performed by compressing the radial and ulnar arteries simultaneously while the patient clenches his/her fist, which causes the patient’s hand to blanch. Next, the patient is asked to unclench his/her fist while the ulnar artery is released. Return of normal color to the patient’s hand is thought to indicate the presence of adequate collateral circulation. The Allen test has since been modified in several ways to test circulation prior to radial artery access [39]. Whether or not this test predicts the likelihood of ischemic complications after transradial access is a controversial issue, with some studies suggesting that many centers no longer utilize it [36]. An international survey, showed that hand circulation is assessed in most cases however no prior testing is performed in 23.4% of cases [7].Results from the RADAR trial suggest that normal Allen test results are not required to identify patients in whom the transradial approach can be safely utilized [36]. Thus far, a large number of patients have undergone transradial access without Allen testing and only a minimal number of cases of hand ischemia have been reported [40–42]. Therefore, it is important to emphasize that abnormal Allen test results should not exclude patients from undergoing procedures utilizing the transradial approach.
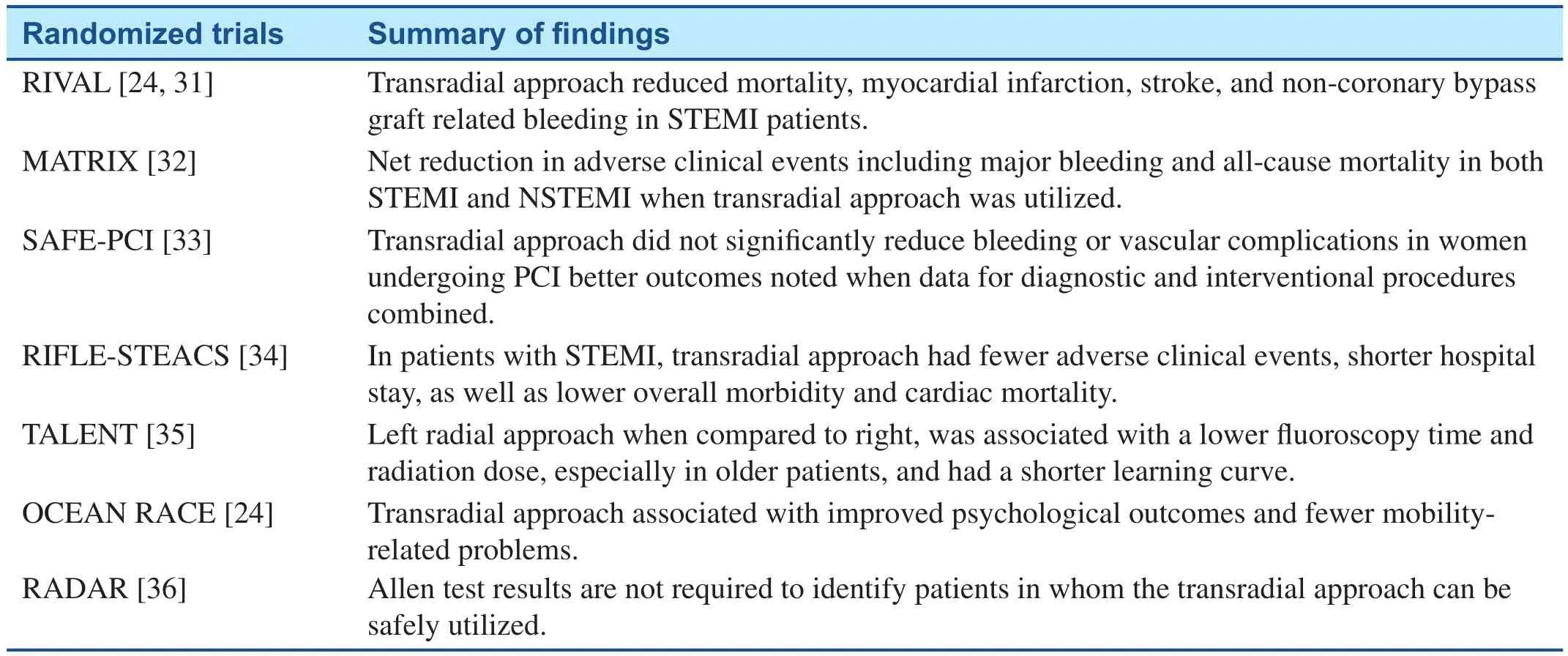
Table 2 Randomized Trials Related to the Transradial Approach.
Both left and right radial arteries can be utilized as access for cardiac catheterization and PCI. The decision of which side to use can depend largely on physician preference. Comfortable positioning for both patient and physician is essential to performing safe and successful procedures. The TALENT study reported that the left radial approach was associated with a lower fluoroscopy time and radiation dose,especially in older patients [35]. This may be attributable to more tortuosity of the right subclavian artery and radial loops, making navigation more difficult [43]. The findings being amplified in older patients are likely due to increased atherlosclerosis, tortuosity and calcification. Another reason for increased difficulty in catheter navigation on the right is that the right subclavian artery does not directly feed into the aortic root. Right-sided catheters have to pass through both the right subclavian as well as the brachiocephalic trunk prior to accessing the aortic root. In contrast, the left subclavian artery arises directly from the aortic root allowing for easier navigation. One recent meta-analysis of randomized trials noted that right radial access was associated with a significantly larger risk of crossover to femoral access when compared to left. However, no significant overall differences were present in terms of procedural time, contrast use, fluoroscopy time, or major complications [44]. Although increased procedure time, fluoroscopic time and radiation exposure have been demonstrated with the transradial approach, studies have shown that this significantly decreases with operator experience[25, 45].
The manipulation of catheters to navigate vasculature and engage coronary arteries can be more challenging from a transradial approach. Regular guidewires with a J shaped tip (3 mm radius)can often be larger than the diameter of the radial artery leading to spasm. A better choice for the navigation of small and sometimes tortuous vessels is a guidewire with a smaller J tip (1.5 mm radius). Utilizing hydrophilic angle-tipped wires can be associated with accidental perforation of small arterial branches, especially in the anticoagulated patients. As a result, close fluoroscopic guidance is required when these wires are utilized.It is essential for instruments not to be advanced against resistance due to the smaller diameter of the arteries in the upper extremity. Thinner 0.014-inch guidewires and smaller catheter sizes can be utilized to navigate radial ulnar loops and tortuosity with subsequent exchange with the standard 0.035-inch guidewire when it has been advanced past the brachial artery.
Other limitations of the transradial approach are related to smaller diameter of the radial in comparison to the femoral artery. One study involving patients that underwent radial artery ultrasonography showed that it has a mean size of approximate 2.5 mm [46]. This makes 6F sheaths, which are 2 mm in diameter the largest that can be properly utilized. In most cases, PCican be adequately performed with guide catheters of this size. However, in some instances involving complex coronary artery disease such as bifurcating lesions or situations requiring dual stent techniques, larger catheters can become necessary. Reports have indicated that a sheathless technique can be safely utilized to allow for larger guiding catheters up to 7–8F in size [47].There have been efforts to miniaturize the catheter size for transradial catheterization and intervention in order to decrease radial artery occlusion, facilitate navigation, and improve patient comfort with less spasm. A study conducted by Masutani et al.has demonstrated the successful and safe use of a“slender system” in which 0.010-inch guidewires are utilized along with 3-F catheters for the purposes of treating complex lesions [48, 49]. Future studies and improvements in the transradial technique need to be conducted to refine this method further and reduce limitations.
Learning Curve and Operator Volume
The significant learning curve associated with the transradial approach has been well described in the literature. A meta-analysis conducted by Jolly et al. reported a high procedure failure rate among inexperienced physicians utilizing the transradial approach, however, in experienced operators success rates were comparable to transfemoral approach [50]. Several studies have shown a strong association between operator volume and outcomes with the transradial approach. One substudy of the RIVAL trial showed a strong correlation between institutional volumes and outcomes with the transradial approach but no such relationship was demonstrated with the transfemoral approach [51]. A study conducted by Ball et al. showed that a minimum case volume of 50 is required to achieve acceptable outcomes and odds of failure of this approach decrease significantly with increases in operator volume [52]. Another study utilizing the CathPCI registry showed that operators experienced with the transradial approach are more likely to utilize it in higher risk patients [53]. Approaches from the right radial artery can be significantly more challenging when compared to the left due to the right subclavian artery not feeding directly into the aortic root as well as other factors that have been mentioned previously. For newer operators, the left radial approach may be best during their learning phase.Investigators of the TALENT trial also reported that among trainees, a left radial approach was associated with a much shorter learning curve with reductions in access and fluoroscopy times as operator volume increased [35]. To summarize, these findings highlight the significant learning curve associated with the transradial approach and emphasize the impact of experience on the outcomes associated with this approach.
Radiation Exposure
As mentioned previously, there have been concerns regarding increased radiation exposure for both patients and operators, which may have contributed to the suboptimal utilization of the transradial approach. In a meta-analysis and systematic review conducted by Plourde et al., the transradial approach was associated with a small yet significant increase in radiation exposure by 1–2 min for both diagnostic and interventional procedures [54]. Recent studies have shown that this gap is much smaller, with the transradial approach adding about 30 s in fluoroscopy time [54]. It is possible that this reduction is due to the advent of dedicated devices for the transradial approach, improvement in techniques, and an increase in operator experience. It has been consistently demonstrated that the increased radiation dose and fluoroscopy times significantly decreases with operator experience, and no differences in radiation exposure are observed when expert operators perform transradial procedures [25, 45, 54].
Conclusion
In conclusion, although the transfemoral approach to PCI has been traditionally dominant, there is an increasing utilization of the transradial approach. In light of significant benefits shown by observational studies as well as randomized clinical trials including fewer bleeding complications, reduced morbidity and mortality, improved quality of life, as well as better economic outcomes; the transradial approach has surpassed the transfemoral approach to become the dominant method for performing PCI in some countries. Its utilization in other countries like the United States remains suboptimal due to a prolonged “learning curve”, longer procedure times, and a higher crossover rate, mostly among older operators that did not receive transradial training and are unwilling to change their practices.Further efforts need to be made to increase its utilization as well as refine this method and reduce limitations for the purposes of improving patient outcomes and comfort while simultaneously reducing costs.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
1. Rao SV, Cohen MG, Kandzari DE,Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts,and future directions. J Am Coll Cardiol 2010;55(20):2187–95.
2. Campeau L. Percutaneous radial artery approach for coronary angiography.Cathet Cardiovasc Diagn 1989;16(1):3–7.
3. Kiemeneij F, Laarman GJ. Percutaneous transradial artery approach for coronary stent implantation. Cathet Cardiovasc Diagn 1993;30(2):173–8.
4. Louvard Y, Kumar S, Lefevre T.[Percentage of transradial approach for interventional cardiology in the world and learning the technique].Ann Cardiol Angeiol (Paris)2009;58(6):327–32.
5. Mitchell MD, Hong JA, Lee BY,Umscheid CA, Bartsch SM, Don CW. Systematic review and costbenefit analysis of radial artery access for coronary angiography and intervention.Circ Cardiovasc Qual Outcomes 2012;5(4):454–62.
6. Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS,et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry.JACC Cardiovasc Interv 2008;1(4):379–86.
7. Bertrand OF, Rao SV, Pancholy S,Jolly SS, Rodés-Cabau J, Larose E,et al. Transradial approach for coronary angiography and interventions:results of the first international transradial practice survey. JACC Cardiovasc Interv 2010;3(10):1022–31.
8. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2012;79(3):453–95.
9. Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, et al.ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 2012;33(20):2569–619.
10. Radner S. Thoracal aortography by catheterization from the radial artery; preliminary report of a new technique. Acta radiol 1948;29(2):178–80.
11. Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis:percutaneous transluminal coronary angioplasty. N Engl J Med 1979;301(2):61–8.
12. Doyle BJ, Rihal CS, Gastineau DA,Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009;53(22):2019–27.
13. Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM,Bassand JP. Bleeding and blood transfusion issues in patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28(10):1193–204.
14. Kugelmass AD, Cohen DJ, Brown PP, Simon AW, Becker ER, Culler SD. Hospital resources consumed in treating complications associated with percutaneous coronary interventions. Am J Cardiol 2006;97(3):322–7.
15. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003;92(8):930–5.
16. Spencer FA, Moscucci M, Granger CB, Gore JM, Goldberg RJ, Steg PG, et al. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction? Circulation 2007;116(24):2793–801.
17. Yatskar L, Selzer F, Feit F, Cohen HA, Jacobs AK, Williams DO,et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung,and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv 2007;69(7):961–6.
18. Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol 2004;44(2):349–56.
19. Subherwal S, Peterson ED, Dai D,Thomas L, Messenger JC, Xian Y,et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention:a report from the National Cardiovascular Data CathPCI Registry. J Am Coll Cardiol 2012;59(21):1861–9.
20. Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J,Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE).Eur Heart J 2003;24(20):1815–23.
21. Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, et al., Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J 1999;138(3 Pt 1):430–6.
22. Rao SV, Kaltenbach LA, Weintraub WS, Roe MT, Brindis RG,Rumsfeld JS, et al. Prevalence and outcomes of same-day discharge after elective percutaneous coronary intervention among older patients. J Am Med Assoc 2011;306(13):1461–7.
23. Rinfret S, Kennedy WA, Lachaine J, Lemay A, Rodés-Cabau J, Cohen DJ, et al. Economic impact of sameday home discharge after uncomplicated transradial percutaneous coronary intervention and bolus-only abciximab regimen. JACC Cardiovasc Interv 2010;3(10):1011–9.
24. Koltowski L, Koltowska-Haggstrom M, Filipiak KJ, Kochman J, Golicki D, Pietrasik A, et al.Quality of life in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention--radial versus femoral access (from the OCEAN RACE Trial). Am J Cardiol 2014;114(4):516–21.
25. Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes(RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377(9775):1409–20.
26. Hamon M, Pristipino C, Di Mario C, Nolan J, Ludwig J, Tubaro M,et al. Consensus document on the radial approach in percutaneous cardiovascular interventions:position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention 2013;8(11):1242–51.
27. Caputo RP, Tremmel JA, Rao S,Gilchrist IC, Pyne C, Pancholy S,et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv 2011;78(6):823–39.
28. Feldman DN, Swaminathan RV,Kaltenbach LA, Baklanov DV,Kim LK, Wong SC, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007–2012). Circulation 2013;127(23):2295–306.
29. Baklanov DV, Kaltenbach LA,Marso SP, Subherwal SS, Feldman DN, Garratt KN, et al. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol 2013;61(4):420–6.
30. Hannan EL, Farrell LS, Walford G,Berger PB, Stamato NJ, Venditti FJ, et al. Utilization of radial artery access for percutaneous coronary intervention for ST-segment elevation myocardial infarction in New York. JACC Cardiovasc Interv 2014;7(3):276–83.
31. Mehta SR, Jolly SS, Cairns J,Niemela K, Rao SV, Cheema AN, et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST-segment elevation. J Am Coll Cardiol 2012;60(24):2490–9.
32. Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015;385(9986):2465–76.
33. Rao SV, Hess CN, Barham B,Aberle LH, Anstrom KJ, Patel TB,et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention:the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv 2014;7(8):857–67.
34. Romagnoli E, Biondi-Zoccai G,Sciahbasi A, Politi L, Rigattieri S,Pendenza G, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLESTEACS (Radial Versus Femoral Randomized Investigation in STElevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012;60(24):2481–9.
35. Sciahbasi A, Romagnoli E, Burzotta F, Tranic, Sarandrea A, Summaria F, et al. Transradial approach(left vs right) and procedural times during percutaneous coronary procedures: TALENT study. Am Heart J 2011;161(1):172–9.
36. Valgimigli M, Campo G, Penzo C, Tebaldi M, Biscaglia S, Ferrari R, et al. Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. J Am Coll Cardiol 2014;63(18):1833–41.
37. Wallach SG. Cannulation injury of the radial artery: diagnosis and treatment algorithm. Am J Crit Care 2004;13(4):315–9.
38. Rao SV, Bernat I, Bertrand OF.Clinical update: remaining challenges and opportunities for improvement in percutaneous transradial coronary procedures. Eur Heart J 2012;33(20):2521–6.
39. Fuhrman TM, Pippin WD, Talmage LA, Reilley TE. Evaluation of collateral circulation of the hand.J Clin Monit 1992;8(1):28–32.
40. Rademakers LM, Laarman GJ.Critical hand ischaemia after transradial cardiac catheterisation:an uncommon complication of a common procedure. Neth Heart J 2012;20(9):372–5.
41. de Bucourt M, Teichgraber U.Digital ischemia and consecutive amputation after emergency transradial cardiac catheter examination. Cardiovasc Intervent Radiol 2012;35(5):1242–4.
42. Taglieri N, Galie N, Marzocchi A.Acute hand ischemia after radial intervention in patient with CREST-associated pulmonary hypertension:successful treatment with manual thromboaspiration. J Invasive Cardiol 2013;25(2):89–91.
43. Norgaz T, Gorgulu S, Dagdelen S.Arterial anatomic variations and its influence on transradial coronary procedural outcome. J Interv Cardiol 2012;25(4):418–24.
44. Biondi-Zoccai G, Sciahbasi A, Bodí V, Fernández-Portales J, Kanei Y,Romagnoli E, et al. Right versus left radial artery access for coronary procedures: an international collaborative systematic review and meta-analysis including 5 randomized trials and 3210 patients.Int J Cardiol 2013;166(3):621–6.
45. Pristipino C, Tranic, Nazzaro MS,Berni A, Patti G, Patrizi R, et al.Major improvement of percutaneous cardiovascular procedure outcomes with radial artery catheterisation: results from the PREVAIL study. Heart 2009;95(6):476–82.
46. Loh YJ, Nakao M, Tan WD, Lim CH, Tan YS, Chua YL. Factors influencing radial artery size.Asian Cardiovasc Thorac Ann 2007;15(4):324–6.
47. Li Q, He Y, Jiang R, Huang D.Using sheathless standard guiding catheters for transradial percutaneous coronary intervention to treat bifurcation lesions. Exp Clin Cardiol 2013;18(2):73–6.
48. Masutani M, Yoshimachi F, Matsukage T, Ikari Y, Saito S. Use of slender catheters for transradial angiography and interventions.Indian Heart J 2008;60(1 Suppl A):A22–6.
49. Kiemeneij F, Yoshimachi F, Matsukage T, Amoroso G, Fraser D,Claessen BE, et al. Focus on maximal miniaturisation of transradial coronary access materials and techniques by the Slender Club Japan and Europe: an overview and classification. EuroIntervention 2015;10(10):1178–86.
50. Jolly SS, Amlani S, Hamon M,Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials.Am Heart J 2009;157(1):132–40.
51. Jolly SS, Cairns J, Niemela K, Steg PG, Natarajan MK, Cheema AN,et al. Effect of radial versus femoral access on radiation dose and the importance of procedural volume:a substudy of the multicenter randomized RIVAL trial. JACC Cardiovasc Interv 2013;6(3):258–66.
52. Ball WT, Sharieff W, Jolly SS,Hong T, Kutryk MJ, Graham JJ,et al. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv 2011;4(4):336–41.
53. Hess CN, Peterson ED, Neely ML, Dai D, Hillegass WB, Krucoff MW, et al. The learning curve for transradial percutaneous coronary intervention among operators in the United States: a study from the National Cardiovascular Data Registry. Circulation 2014;129(22):2277–86.
54. Plourde G, Pancholy SB, Nolan J,Jolly S, Rao SV, Amhed I, et al.Radiation exposure in relation to the arterial access site used for diagnostic coronary angiography and percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 2015;386(10009):2192–203.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
- Identification and Management of Iatrogenic Aortocoronary Dissection
- Cardiovascular Abnormalities Among Patients with Spontaneous Subarachnoid Hemorrhage.A Single Center Experience
- Coronary Artery Chronic Total Occlusion
- Carotid Artery Stenting: 2016 and Beyond
- The Future of Transcatheter Therapy for Mitral Valve Disease
