Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
Yan Chaowu, PhD, MD, Xu Zhongying, PhD, MD, Zhang Gejun, PhD, MD, Zheng Hong,PhD, MD, Jin Jinglin, PhD, MD, Li Shiguo, PhD, MD, Lv Jianhua, MD, Hu Haibo, PhD,MD, Song Huijun, PhD, MD, and Zhao Shihua, PhD, MD, FESC
1Interventional Radiology, National Center for Cardiovascular Diseases, Cardiovascular Institute and Fuwai Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China
2Radiology Imaging Center, National Center for Cardiovascular Diseases, Cardiovascular Institute and Fuwai Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China
Introduction
In pediatric cardiac catheterization, ketamine has been applied widely for sedation in spontaneously breathing children and there was no report about the respiratory complication [1, 2]. However, a rare and unreported respiratory complication, transient and reversible atelectasis of lungs (TRAL), was identified in our institute. To our knowledge, TRAL constitutes a distinct subgroup, which was different from currently known complications in pediatric cardiac catheterization. In this study, we present data from 33 sick children with TRAL.
Materials and Methods
Patients
From January 2004 to July 2015, 4474 sick children (1643 cyanotic CHD and 2831 acyanotic CHD) were sedated with ketamine, and pediatric cardiac catheterization was carried out under spontaneous breathing. Thirty three patients (17 males,16 females) had onset of TRAL in cardiac catheterization. All patients underwent detailed evaluation of electrocardiogram (ECG), chest X-ray, and echocardiography. Patients requiring ventilatory treatment or intravenous inotropic support were excluded. The study was approved by our hospital research ethics committee, and informed consent was obtained from the parents of each child.Consent for the anaesthetic was obtained independently by the anaesthetist who was involved with the procedure.
Procedure
The patients were admitted to the catheterization laboratory and an intravenous cannula was placed and an infusion of one-third saline solution was started. Ketamine was applied for general anaesthesia (1 mg/kg at induction followed by an infusion of 3–5 mg/kg/h) in spontaneously breathing children. Concurrently, atropine was administered intravenously (0.01 mg/kg) or intramuscularly(0.02 mg/kg). In all patients, local anaesthesia with 1% lidocaine was administered before percutanous puncture of femoral artery and/or vein. The power injector (Medrad100, Pittsburgh, PA, USA)was used for angiocardiography. Nonionic contrast medium was applied in this study (Iopromide,Ultravist, Schering, Germany; Iohexol, Omnipaque,Nycomed, Norway; Iopamidol, Bracco Diagnostics,Plainsboro, NJ, USA).
The first sign of TRAL was defined as the occurrence of opacity lungs. Once TRAL attacked in the examination, supportive 100% oxygen was administrated by bag and mask via a clear airway (Endotracheal intubation was not the routine procedure in the general anaesthesia). If adequate oxygenation failed to be maintained, endotracheal intubation (volume-limited ventilator) followed immediately after muscular relaxation.The images of chest fluoroscopy were recorded promptly to evaluate the radiographic features of TRAL before, during and after the onset. Standard guidelines for monitoring during delivery of general anesthesia and cardiac catheterization were carried out, including 3-lead surface ECG, pulse oximetry and so on. SpO2was determined by means of pulse oximetry. The clinical manifestation, radiological findings and treatment were also recorded.
Among the 33 sick children, 13 patients had exact records of SpO2and heart rates before and during the onset of TRAL. Their approximate durations of TRAL, which ranged from the emergence to the disappearance of opacity lungs, were recorded too. When the left cardiac catheterization was performed, systolic aortic pressure was recorded by catheter.
Statistical Analysis
The data in the study were expressed as mean value±SD. The results were compared between before and during onset of TRAL using pairedsamples T test, and P<0.05 was considered significant. Statistical software used in this study was SPSS 12.0.
Results
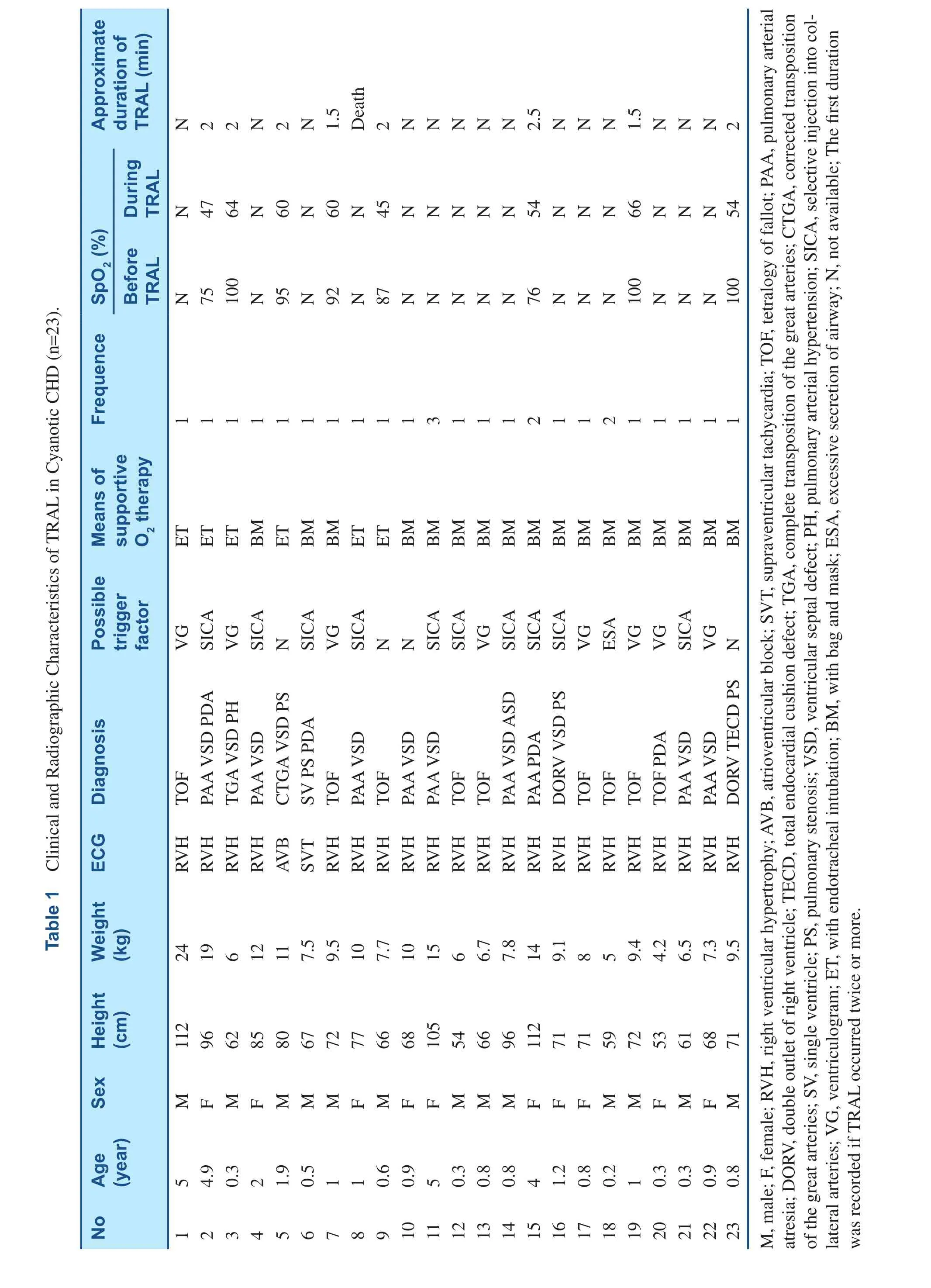
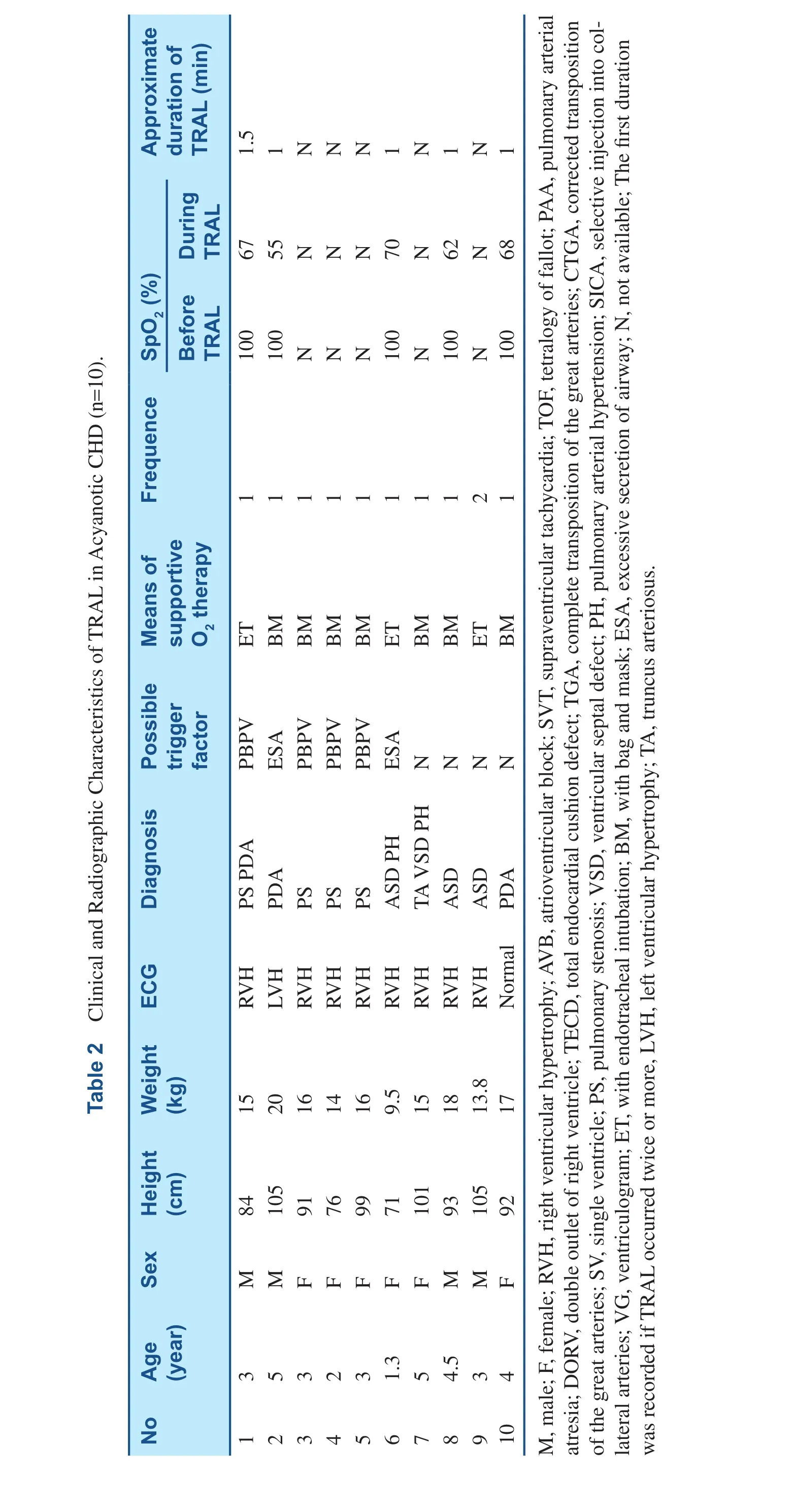
Thirty three sick children (17 males, 16 females)presented with TRAL in cardiac catheterization.Cyanotic CHD were 23 (Table 1), and acyanotic CHD were 10 (Table 2). The age were 2.1±1.7 years, weight were 11.5±4.9 kg and height were 80.6±17.3 cm. Angiocardiography was performed in 30 patients, and right cardiac catheterization in 3 children. The ages of patients with cyanotic CHD was younger than those with acyanotic CHD,1.5±1.6 years vs. 3.4±1.2 years (P<0.05). Except for one child, abnormalities of ECG can be found in the other patients, including right ventricular hypertrophy (29 patients), left ventricular hypertrophy (one patient), supraventricular tachycardia (one patient)and atrioventricular block (one patient). The fluoroscopy time was 20±10.2 minutes.
?
?
TRAL was precipitated by selective injection of collateral vessels in ten patients (Figure 1A–C),PBPV in four patients (Figure 2A–C), ventriculogram in eight patients (Figure 3A–D), and excessive secretion of airway in three patients. In the other eight cases, however, no obvious stimulus was apparent. Interventional therapy was performed in nine patients, including PBPV (four patients), transcatheter closure of PDA (two patients) and transcatheter closure of ASD (three patients). TRAL occurred twice or more in four patients.
After TRAL occurred, SpO2decreased quickly from 94.2±9.2% to 59.4±2.2% (P<0.05) and heart rates fell from 143.5±14.3 bpm to 58.3±9.7 bpm (P<0.05). The duration of TRAL was 1.6±0.5 minutes (the duration of diffuse pulmonary opacity under X-ray fluoroscopy). Systolic aortic pressure recorded by catheter was 115.3±17.5 mmHg.TRAL was relieved by supportive oxygen with face mask in 24 patients, and by mechanical ventilation with endotracheal intubation in nine patients. Except for one child, all patients were relieved completely by the administration of 100% oxygen.
Common Clinical Course
TRAL had common clinical and radiographic features in all patients: the diffuse opacity of bilateral lungs developed rapidly, associated with decrease in lung volume. And then the decrease in SpO2and heart rates followed quickly. The sequence of opacity lungs was from center to peripheral area, and the opacity of the upper lung zone was often earlier and more apparent than that of lower lung. There was no evidence of cardiac enlargement. In particular, the opacity of lungs developed within one minute. During the onset of TRAL, there was no reflex increase in heart rates and breath sounds were inaudible. With progression, there was the occurrence or deterioration of cyanosis. If supportive or mechanical ventilation was given, the duration was short and the lungs were fully aerated again within 1–2.5 minutes, followed by the recovery of hypoxia and bradycardia sequentially. No sequelae can be found in the 3 months following up.
Discussion
TRAL is a rare but risk complication after ketamine sedation during cardiac catheterization in spontaneously breathing children. However, the mechanism is still unclear. As a previously unreported complication, TRAL shared distinct clinical and radiographic pictures. The hallmark of TRAL was the rapid and diffuse opacity change of bilateral lungs,which was the earliest sign of under X-ray fluoroscopy. It developed rapidly within 1 minute and lasted less than 2.5 minutes if supplemental oxygen was given. In this study, fluoroscopy allowed real time visualization of lungs in cardiac catheterization. Therefore, the detailed clinical course of TRAL became available. Accompanied by decrease in lung volume, TRAL manifested a picture characteristic of atelectasis rather than pulmonary edema/inflammation. The latter presented in POPE and ARDS [3–6].
Because no evidence of severe upper airway obstruction was identified in most children, it was possible that diffuse atelectasis of TRAL was peripheral and not the result of central obstruction.Considering very short duration, we speculated that TRAL might result from the spasm of peripheral airway and diffuse spasmic closure of distal airway can lead to widespread atelectasis and lowers lung volume. Many factors, as presented in this study,might have potential to precipitate the spasmic obstruction of peripheral airway, followed by the onset of TRAL. If it is true, the rapid opacity change of lungs becomes explainable. Further research is required to evaluate the mechanism.
Another distinct feature of TRAL is that it responds well to oxygen therapy. Whatever the etiology, most patients can be relieved quickly by 100% oxygen administered with bag and mask. If the mask fails to maintain adequate oxygenation, endotracheal intubation should follow immediately. Though most TRALs settled rapidly after the supportive oxygen therapy, one sick child died of TRAL in this study(despite the fact that mechanical ventilation was supported with the placement of the endotracheal tube). No consent was obtained to carry out the autopsy, and the cause remained unclear. The other 32 children recovered well and no sequelae were left. In our opinion, the earlier the diagnosis was made and supportive oxygen was given, the better the TRAL recovered. Because the earliest sign of TRAL was the rapid increase in opacity of lungs under X-ray fluoroscopy, it was important to recognize the phenomenon.
Several factors probably precipitated the onset of TRAL in this study. Acute reduction in pulmonary blood flow may play an important role in the onset of TRAL, which was triggered by selective injection into major aortopulmonary collateral arteries in ten children, and by PBPV in four patients. There is a direct anatomical and physiological relation between the lung vasculature and the airways, and small peribronchiolar vessels serve an erectile function and maintain airway patency [7, 8]. The cessation of pulmonary blood flow had a major impact on lung mechanics. It has been confirmed that there was an abrupt and important increase in airway resistance, a fall in lung compliance, and a decrease in tidal volume during pulmonary valvuloplasty[9]. In addition, the similar decrease of pulmonary blood flow may result from selective injection of contrast medium into collateral arteries in PAA or TOF, especially when stenosis occurred in the proximal segment of collateral arteries. It also should be responsible for the only death of the child with PAA whose oxygenation failed to be maintained though the mechanical ventilation with endotracheal intubation was given.
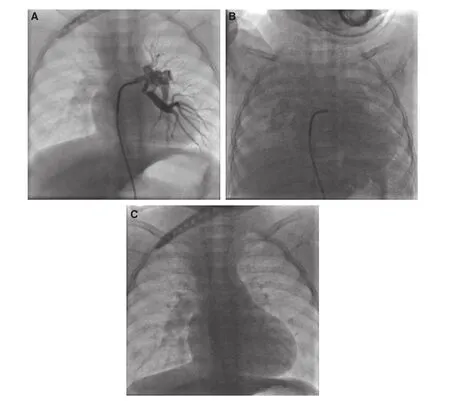
Figure 1 The Child was Diagnosed as PAA with VSD.Selective injection into collateral artery was performed (A), and precipitated the onset of TRAL (B). Supportive 100% oxygen therapy with bag and face mask was started immediately, and the lungs were fully aerated again in 2.5 minutes (C). The onset of TRAL was characterized by the marked opacity lung associated with decrease of lung volume.
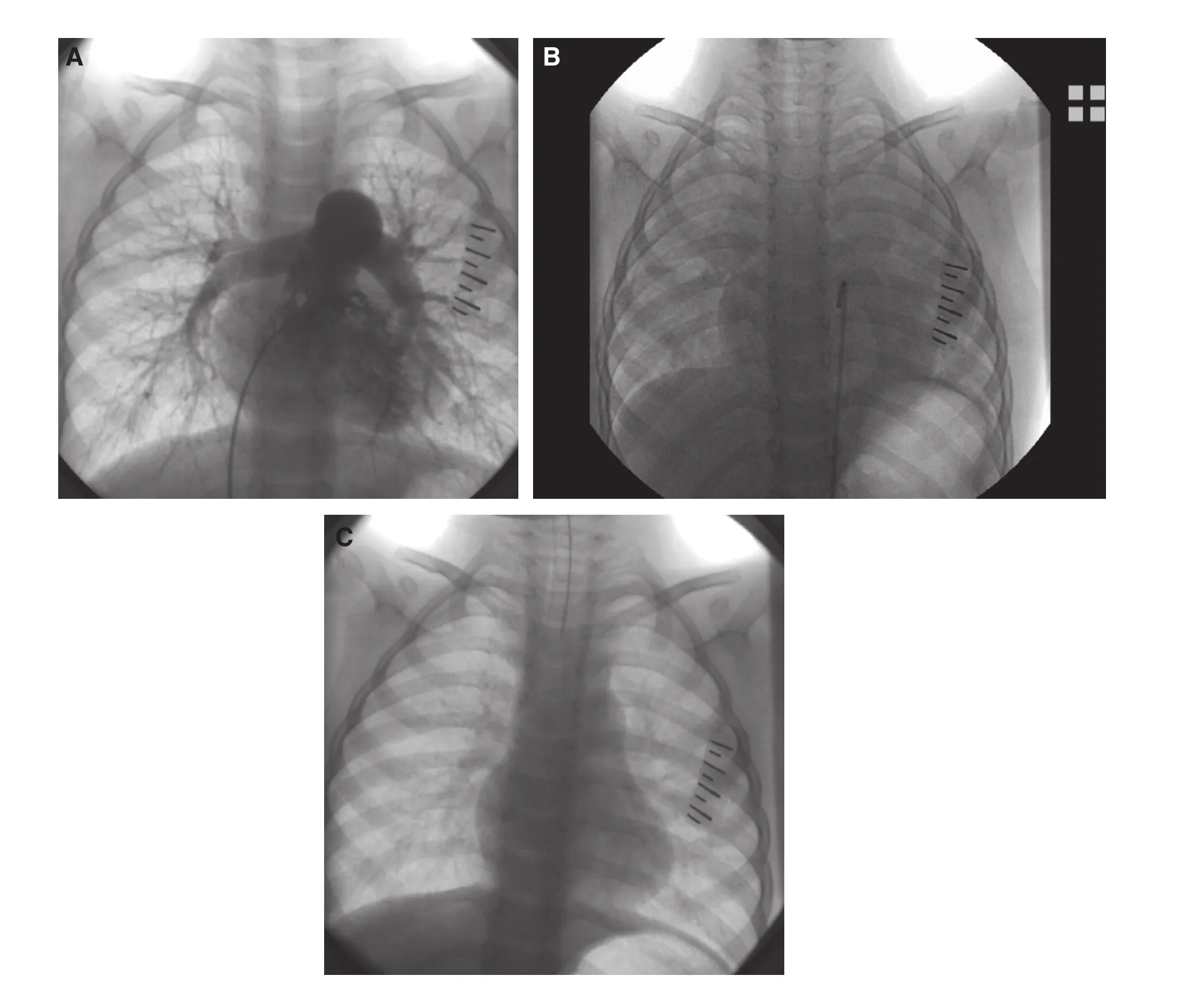
Figure 2 TRAL Occurred Immediately After PBPV.The X ray was taken before (A), during (B) and after TRAL (C). The lung opacity was accompanied with decrease in lung volume. After mechanical ventilation with the placement of the endotracheal tube, TRAL resolved quickly and the duration was about 1.5 minutes (C).
Angiocardiography was performed in 91% of patients in the study. It has been recognized that there were increases in airways and pulmonary vascular resistance after intravascular injection of contrast medium, though the mechanisms responsible for the effect remained unclear [10]. Other pulmonary adverse effects include pulmonary edema,bronchospasm and so on [11, 12]. It is still unclear whether contrast medium plays a role in the attack of TRAL. The same was to the excessive secretion of airway in three children. In addition, one fourth of patients had no obvious trigger factors, and it is unclear that TRAL of these patients occurred spontaneously or related with other unknown factors.
It was during general anaesthesia with ketamine that TRAL occurred in the study. Though atelectasis can develop as a side effect of most anaesthetics during general anaesthesia, ketamine was considered an exception [13]. In addition, not conventional chest radiography but computed tomography could show the atelectasis resulting from general anaesthesia.Ketamine is a dissociative anesthetic agent and provides adequate anesthesia for most catheterization procedures [14, 15]. It was the only anaesthetic so far tested that has not produced atelectasis unless the patients were paralysed. The major side effect of ketamine is airway secretion, which can result in aspiration and laryngospasm. To offset the increase in airway secretions, atropine was administered in this study. Nevertheless, excessive secretion of airway still occurred in three patients. However, no severe upper respiratory obstruction was identified in these children. It is unknown whether there are direct relation between TRAL and the side effect of ketamine.
Cyanotic CHD, mainly TOF and PAA, account for 70% in this study. Some clinical similarities present in both TRAL and paroxysmal hypoxemic spell, as seen in TOF and PAA. However, the differences are also obvious between them. TRAL responds well to supportive oxygen therapy and can also occur in acyanotic CHD, such as ASD and PDA. In addition, it is unclear about the radiographic features of hypoxemic spell. The mechanism of hypoxemic spell is still obscure [16,17]. Though the most widely accepted explanation is that the hypoxemic spell probably results from the spasm of right ventricular infundibulum,it fails to explain the spells that occur in patients with TOF and PAA where pulmonary blood flow does not cross the right ventricular outflow tract[14, 15].
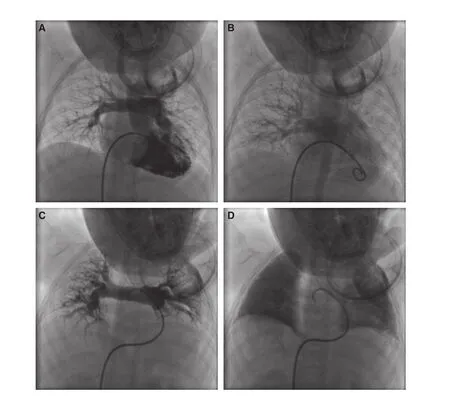
Figure 3 Before (A, B) and During (C, D) the Onset of TRAL, the Images of Pulmonary Arterial Phase (A, C) and Venous Phase (B, D) were Recorded.The branches of pulmonary arteries crowed, the lung volume decreased and the venous phase was obviously delayed (C, D).
Study Limitations
The research was a retrospective study and many measurements were not set before the study. The SpO2monitored by pulse oximetry may be inaccurate in cyanotic patients, and the duration of TRAL was only an approximate value. In addition, no detailed data were recorded about hemodynamics and lung mechanics in the attack,because TRAL was a life threatening event and all attentions were paid to the maintenance of adequate oxygenation.
Conclusions
TRAL is a distinct and risk respiratory complication characterized by transient and reversible pulmonary collapse in pediatric cardiac catheterization. Rapid and diffuse opacity of bilateral lungs is the earliest sign of TRAL, and the immediate supportive oxygen is crucial. However, further research is required to evaluate the mechanism.
Sources of Funding
This work was supported by Beijing Natural Science Foundation 7162160 and National Natural Science Foundation of China (81130029, 81341045).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
1. Mester R, Easley RB, Brady KM, Chilson K, Tobias JD.Monitored anesthesia care with a combination of ketamine and dexmedeto midine during cardiac catheterization. Am J Ther 2008;15(1):24–30.
2. Greene CA, Gillette PC, Fyfe DA.Frequency of respiratory compromise after ketamine sedation for cardiac catheterization in patients less than 21 years of age. Am J Cardiol 1991;68(10):1116–7.
3. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS.Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–24.
4. Heffner JE, Brown LK, BarbiericA, Harpel KS, DeLeo J.Prospective validation of an acute respiratory distress syndrome predictive score. Am J Respir Crit Care Med 1995;152:1518–26.
5. Stradling JR, Bolton P. Upper airways obstruction as cause of pulmonary oedema. Lancet 1982;1:1353–4.
6. Lee KW, Downes JJ. Pulmonary edema secondary to laryngospasm in children. Anesthesiology 1983;59:347–9.
7. Bancalari E, Jesse MJ, Gelband H,Garcia O. Lung mechanics in congenital heart disease with increased and decreased pulmonary blood flow. J Pediatr 1977;90:192–5.
8. Gray BA, McCaffree DR, Sivak ED, McCurdy HT. Effect of pulmonary vascular engorgement on respiratory mechanics in the dog. J Appl Physiol 1978;45:119–27.
9. Schulze-Neick I, Penny DJ, Derrick GP, Dhillon R, Rigby ML, Kelleher A, et al. Pulmonary vascular-bronchial interactions: acute reduction in pulmonary blood flow alters lung mechanics. Heart 2000;84:284–9.
10. Cipolla P, Castano M, Kirchin MA,de Haën C, Tirone P. Effects of iodinated contrast media on pulmonary airway resistance in anesthetized guinea pigs. Acad Radiol 1995;2:306–12.
11. Paul RE, George G. Fatal noncardiogenic pulmonary oedema after intravenous non-ionic contrast radiographic contrast. Lancet 2002;359:1037–8.
12. Lasser EC, Lyon SG, Berry CC.Reports on contrast media reactions: analysis of data from reports to the U.S. Food and Drug Administration. Radiology 1997;203:605–10.
13. Tokics L, Strandberg A, Brismar B, Lundquist H, Hedenstierna G.Computerized tomography of the chest and gas exchange measurements during ketamine anaesthesia. Acta Anaesthesiol Scand 1987;31:684–92.
14. Morray JP, Lynn AM, Stamm SJ, Herndon PS, Kawabori I,Stevenson JG. Hemodynamic effects of ketamine in children with congenital heart disease. Anesth Analg 1984;63:895–9.
15. Berman W Jr, Fripp RR, Rubler M,Alderete L. Hemodynamic effects of ketamine in children undergoing cardiac catheterization. Pediatr Cardiol 1990;11:72–6.
16. Faithfull NS, Haider R. Ketamine for cardiac catheterisation. An evaluation of its use in children.Anaesthesia 1971;26:318–23.
17. Coppel DL, Dundee JW. Ketamine anaesthesia for cardiac catheterisation. Anaesthesia 1972;27:25–31.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Carotid Artery Stenting: 2016 and Beyond
- Will Transcatheter Aortic Valve Replacement(TAVR) be the Primary Therapy for Aortic Stenosis?
- The Future of Transcatheter Therapy for Mitral Valve Disease
- The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
- Cardiovascular Abnormalities Among Patients with Spontaneous Subarachnoid Hemorrhage.A Single Center Experience
- Identification and Management of Iatrogenic Aortocoronary Dissection
