Will Transcatheter Aortic Valve Replacement(TAVR) be the Primary Therapy for Aortic Stenosis?
Jose F. Condado, MD, MS and Peter C. Block, MD, FACC, MSCAI
1Structural Heart and Valve Center, Division of Cardiology, Emory University School of Medicine, Atlanta, GA, USA
Introduction
Transcatheter aortic valve replacement (TAVR)has become the treatment of choice in selected patients with severe aortic stenosis (AS). The results from large randomized clinical trials in the US and Europe have established the safety and feasibility of this relatively new technique [1–4].Though TAVR is already Food and Drug Administration (FDA) approved for patients with high and very high surgical risk [5], clinical trials are currently enrolling lower risk patient populations.This review reports current practices and evidence for the use of TAVR, and its implications for the future treatment of AS.
Patient Selection and Procedural Planning
Currently, TAVR is FDA approved for patients considered high or very high surgical risk (essentially inoperable). But with clinical trials now enrolling patients with intermediate surgical risk, it is likely that TAVR will soon be approved for lower risk patients as well.
The first step in evaluating candidate patients for TAVR is confirming the diagnosis of AS and determining surgical risk. The indication for replacement, regardless of the technique used (transcatheter or surgical), is based on the presence of symptoms(angina, dyspnea or syncope) and/or left ventricular dysfunction in a patient with severe AS, usually, determined by transthoracic echocardiography(TTE) [5]. AS is considered severe when the TTE aortic valve mean gradient (AVMG) is ≥40 mmHg,peak jet velocity ≥4 m/s and area ≤1 cm2. Clinicians must be aware however, that some of these criteria may be decreased in patients with depressed ventricular function (low flow/low gradient AS). In such cases, a dobutamine stress echocardiogram is used to differentiate between severe and moderate AS, based on the degree of increase in the AVMG during dobutamine infusion [5].
Once the diagnosis is secure, replacement strategy is based on surgical risk, anatomy and preference, and is best determined by a heart team. A heart team is a multidisciplinary group that includes an interventional cardiologist, echocardiographer and cardiothoracic surgeon, all with experience in the management of valvular heart disease. This team determines surgical risk using conventional risk factors, incorporated in validated risk scores such as the STS predictive risk of mortality (PROM)score and the EuroScore [6, 7], but also considers non-conventional risk factors such as porcelain aorta, frailty, and other comorbidities not included in the usual risk profiles. The preprocedural evaluation also includes transesophageal echocardiography (TEE) for a more detailed characterization of the aortic valve, pulmonary function testing for the determination of lung disease severity (if any),and cardiac catheterization to evaluate coronary anatomy, and to identify underlying coronary artery disease (CAD).
TAVR Preprocedural Planning
Once a patient is considered a candidate for TAVR a more detailed evaluation of the patient’s anatomy is necessary. First, the size of the descending aorta,femoral and iliac arteries is determined using an abdomen/pelvis computed tomography with intravenous contrast, and corroborated with an angiogram. Introduction of the delivery system using a transfemoral (TF) access is preferred. In patients with small and/or heavily calcified and tortuous ileofemoral vessels this approach is not possible, in which case an alternative access must be used. Anatomical evaluation of the transcatheter heart valve(THV) landing zone is equally important. THV size selection is based on the size of the aortic annulus[8–10]. Annular sizing can be judged using TEE,3D-TEE, balloon sizing in the cardiac catheterization laboratory and Mutidetector Cardiac Computed Tomography (MDCT). Because of the elliptical morphology of the annulus, the MDCT area derived diameter has emerged as the gold standard for annular sizing due to its higher precision and consistency [8, 9] (Figure 1). For most patients, oversizing between 6–12% (depending on patient anatomy and selected THV) is recommended. While excessive oversizing of the THV can result in an increased risk of complications such as conduction disturbances and annular rupture [11–14], too small a THV can result in paravalvular leak and patient-prosthesis mismatch [15–17]. MDCT is also an important tool used to evaluate aortic angulation, LVOT and annular calcification, size of the Sinuses of Valsalva and sinotubular junction, and distance between the aortic annulus and the coronary artery ostia. Valve type selection (i.e. balloon vs. self-expandable) is in part based on these anatomical characteristics as well. New MDCT software designed for the evaluation of structural heart disease (i.e. 3mensio, Pie medical imaging, Maastricht, The Netherlands) is extremely helpful in sizing the aortic annulus and can even provide an image of the THV within the landing area (Figure 2) [18].
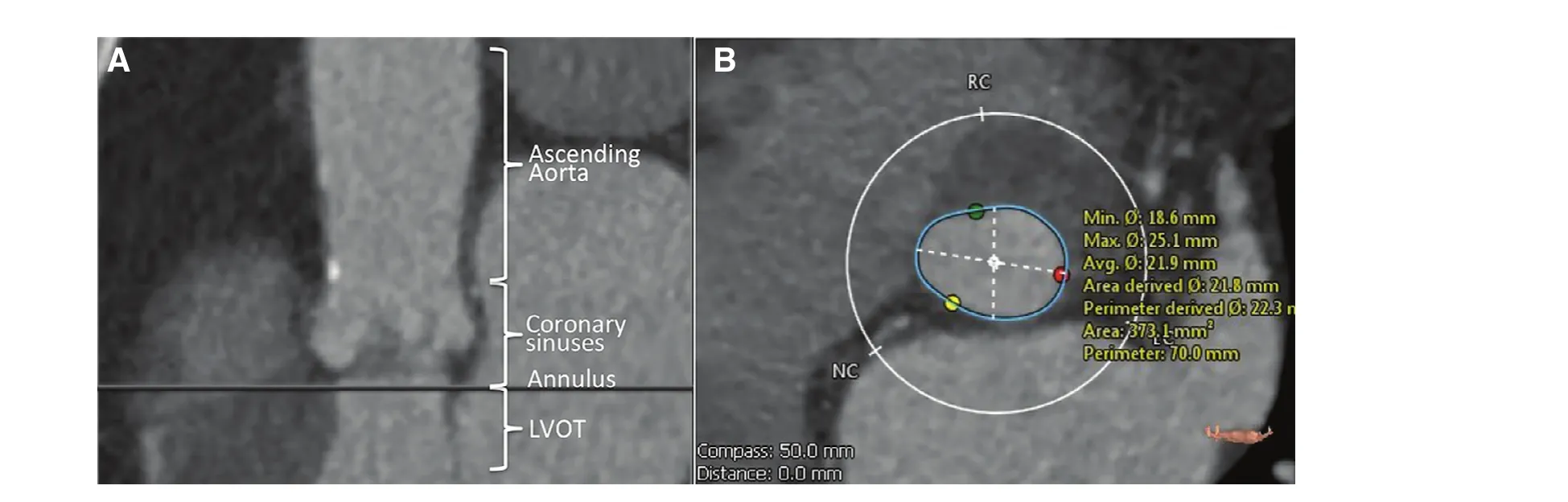
Figure 1 Evaluation of the Aortic Landing Zone Using Multidetector Cardiac Computed Tomography (MDCT).(A) Assessment of left ventricular outflow track (LVOT), aortic annulus, coronary sinuses and ascending aorta. (B) Aortic annular sizing, used for determination of transcatheter heart valve size.
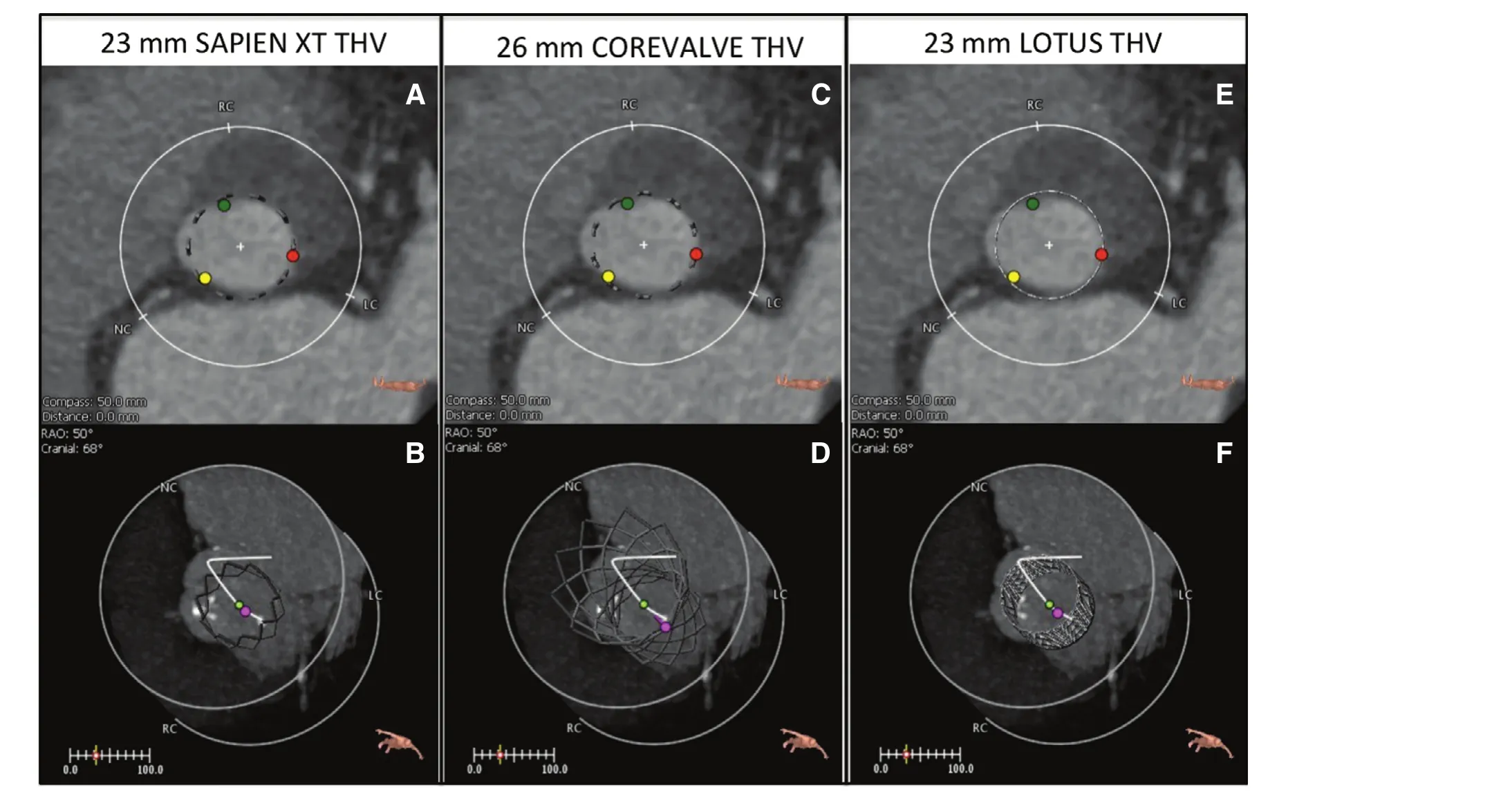
Figure 2 Valve Type and Size Determination Using Multidetector Ca rdiac Computed Tomography (MDCT) in a Patient With Aortic Annular Area of 480 mm3.Special software (3mensio, Pie Medical) for the evaluation of structural heart disease can be used to decide valve type and size based on the predicted sealing area (in this example), for a 23 mm SAPIEN XT (A, B) vs. 26 mm COREVALVE (C, D) vs.23 mm Lotus (E, F).
Progression of Transcatheter Heart Valve Technology
The initial implantation of a percutaneous aortic valve was performed by Cribier et al. in 2002 [19],and was followed by an exponential progress in valve technology and patient enrollment in Europe.The COREVALVE and SAPIEN valve received Conformité Européenne (CE) mark approval in 2007, based on early results of feasibility studies and local registries [20]. TAVR implementation increased over the following years across Europe[21], as encouraging results from European registries continued to emerge [22–24]. However, it was not until 2011 that the FDA commercially approved the SAPIEN valve in the US, based on the results of US clinical trials that showed a survival benefit with TAVR in patients considered inoperable [1, 3]or high-surgical risk [2, 4].
In the PARTNER I trial, Patients with severe AS that underwent TAVR with the balloon-expandable SAPIEN THV (Edwards Lifescience, Irvine,CA, USA) had a better 1-year (69.3% vs. 49.3%,P≤0.001), 2-year (56.7% vs. 32.0%, P≤0.001) and 5-year (28.2% vs. 6.4%, P≤0.001) survival than medical therapy in inoperable patients (Cohort B) [1,25, 26]; and better 30-day but similar 1-year (75.8%vs. 73.2%, P=0.44) and 2-year (66.1% vs. 65.0%,P=0.78) survival than high-risk patients treated with SAVR (Cohort A) [2, 27]. New iterations of the original valve, the SAPIEN XT and SAPIEN 3,are being studied in the PARTNER 2 trial, with encouraging preliminary reports. Compared to previous generations, the SAPIEN 3 valve is available in more sizes (20, 23, 26 and 29 mm), has greater leaflet overlap and an outer skirt to prevent paravalvar leak (PVL), and has a smaller delivery system diameter. The SAPIEN XT and SAPIEN 3 are now also CE mark and FDA approved for the treatment of native valve AS and for failing surgical bioprostheses (SAPIEN XT only) in the US.
In the COREVALVE US pivotal clinical trial,inoperable patients with severe AS that underwent TAVR with the self-expandable COREVALVE THV (Medtronic, Minneapolis, MN, USA) had a better 1-year survival than expected from comparable performance goals (91.6% vs. 75.4%, P≤0.001)[3]. In addition, high-risk patients had better 1-year mortality than similar patients treated with SAVR(85.8% vs. 80.9%, P≤0.001) [4]. This valve is now CE marked and FDA approved for the treatment of AS and also for insertion in patients with failing surgical bioprostheses. Its new generation,the COREVALVE Evolute R has the advantage of being partially repositionable and has less “flaring”of the proximal frame after deployment to decrease conduction disturbances.
Other THVs are available in Europe and are being studied in clinical trials in the US. The Lotus valve(Boston Scientific, Marlborough, MA, USA) is a fully repositionable and retrievable THV made with bovine pericardium on a braided nitinol frame that is deployed by decreasing the stent length, which results in a progressive increase of its diameter.After the initial REPRISE I and II trials [28, 29],the Lotus valve is currently being studied in a multicenter randomized clinical trial (REPRISE III). The Portico valve (St. Jude Medical, Inc. St. Paul, MN,USA) is a self-expandable and resheathable THV made with bovine and porcine pericardium over a nitinol stent. Compared to the COREVALVE, this valve has intra-annular leaflets, an important difference that affects its sizing algorithm. The Direct Flow valve (Direct Flow Medical, Santa Rosa,CA, USA) is a fully repositionable THV made with bovine pericardium mounted on a metal-free frame, which is inflatable. Once in proper position after inflation with pressurized saline, it is finally deployed by pressure injection of a polymer which solidifies. Finally, the JenaValve (JenaValve Technology, Inc. Wilmington, DE, USA), made with a porcine valve over a self-expandable nitinol stent,is a THV that has been used for the treatment of aortic regurgitation (AR) [30, 31]. This may have important implications for the expansion of TAVR in the treatment of AR.
TAVR Technique
In general, the “collapsed” THV is introduced within a delivery system that is advanced until the THV is deployed in the aortic annulus. Techniques may vary according the valve and access used. In transfemoral (TF) TAVR the delivery system is introduced using percutaneous femoral insertion or femoral cutdown, and is advanced retrograde through the aorta until the THV is coaxially aligned with the aortic annulus for deployment (Figure 3).TF access is preferred because it is less invasive and because of its association with a faster recovery and lower rates of adverse events than other approaches[32]. Institutions around the world are increasingly doing TF-TAVR using a “minimalist” approach, in which patients undergo the procedure under conscious sedation instead of general anesthesia. TTE is used for imaging instead of TEE in the minimalist approach [33, 34].
However, TF access may not be possible in up to one third of patients with AS [35, 36] due to small, heavily calcified and/or tortuous vessels. In such scenarios alternative access is needed. In the transapical (TA) approach, a small surgical incision in the 4th–5thintercostal space is used for the insertion of the THV delivery system through the left ventricular apex (Figure 2). This access requires anterograde deployment of the valve, and thus is only currently possible with the SAPIEN, Portico and Jenavalve. TA access allows a more coaxial deployment of the THV within the aortic annulus resulting in less PVL [37]. In a transaortic (TAo)approach, the delivery system is surgically inserted directly into the ascending aorta, using a 5–6 cm mini-sternotomy over the manubrium. This results in a retrograde deployment of the THV [38, 39],similar to the TF approach.
Finally, in patients who cannot undergo TF, TA or TAo approaches less commonly used alternative sites can be used such as transcarotid, subclavian/transinnominate and transcaval [38, 40, 41]. In the transcaval approach, the delivery system is inserted through the femoral vein and is crossed into the aorta by creating a fistula between the inferior vena cava and the aorta, which is closed at the end of the TAVR procedure with an Amplatzer device(St. Jude Medical) (Figure 4) [40, 42].
Regardless of the access used, confirmation of proper THV position and the degree of PVL is assessed with a combination of echocardiography and aortography after deployment.
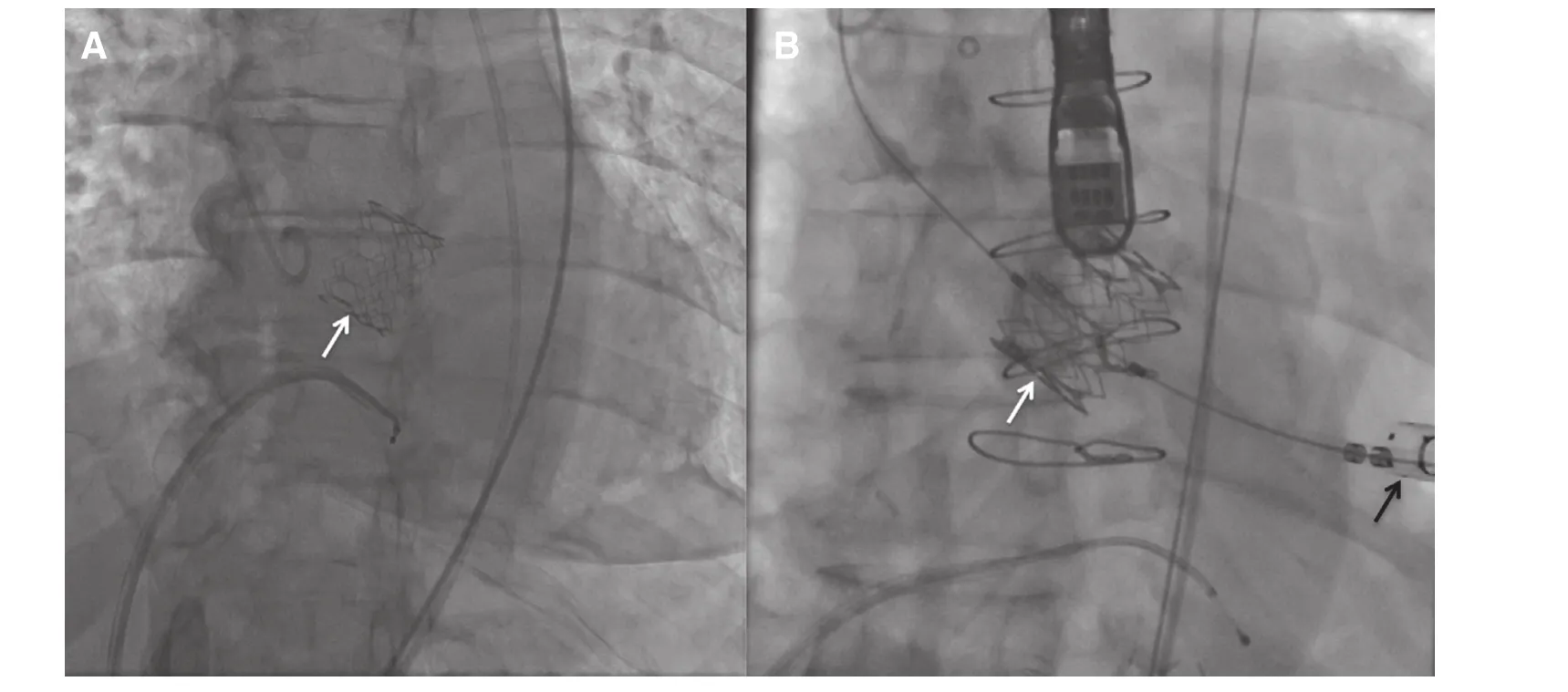
Figure 3 Fluoroscopy During Transcatheter Aortic Valve Replacement (TAVR) With a Balloon Expandable SAPIEN XT Valve (white arrow) Using a Transfemoral (TF, A) or Transapical (TA, B) Approach.Black arrow=TA access.
Complications
TAVR can be associated with procedural complications that can range from minor bleeding at the access site (minor vascular complication) to death. Improved patient selection, access and experience can reduce the likelihood of most of these complications [43–46].Additional common complications of TAVR include paravalvar leak, cerebrovascular events, conduction disturbances and major vascular complications.
Paravalvar Leak
Paravalvar leak (PVL) results from incomplete sealing between the deployed THV and the annular landing area. Risk factors for PVL include severe landing zone calcification [47, 48], device malposition and improper annular size ng [16, 17, 49]. The occurrence of PVL has important prognostic indications because even mild PVL has been associated with worse mid-term outcomes after TAVR [27, 50].Pre-procedural MDCT using software for the evaluation of structural heart disease (i.e. 3mensio, Pie medical imaging, Maastricht, The Netherlands) can quantify calcification, determine optimal deployment angle and size the annulus (Figures 1 and 2).THV type and size is then selected using this information. New generations of THV design will hopefully reduce this complication.
Cerebrovascular Events
Cerebrovascular events, specifically major strokes,occur in 4–8% of TAVR patients [1–4], and usually occur within 10 days of the procedure [51]. Current evidence suggests that most strokes associated with TAVR are embolic, caused by debris dislodgement during catheter manipulation while crossing the aorta and during valve deployment [52]. Initial results from the PARTNER IA trial raised concerns of higher stroke rates with TAVR than medical therapy or SAVR [1, 2], but results of the US pivotal COREVALVE clinical trial reported similar rates between TAVR and SAVR [4]. It is generally agreed that stroke is a possible complication regardless of the method for valve replacement (TAVR vs. SAVR) and the THV type used [46]. Embolic protection devices, percutaneously placed during TAVR, are being evaluated in clinical trials. These filter devices are designed to “catch” embolic debris before it reaches the cerebral circulation [53, 54].

Figure 4 Transcatheter Aortic Valve Replacement (TAVR) Using the Transcaval Approach.(A) An aortic snare is placed in the mid-aorta (dotted white arrow) and used as a target for a needle to cross from the inferior vena cava (IVC) to the aorta. (B) A wire guide is advanced through the needle and snared (white arrow). (C) The small 0.14 wire guide is then advanced to the aortic arch and replaced with a standard TAVR wire guide. The delivery system is then advanced using this a wire guide (C, bent white arrow). (D and E) TAVR is then performed in standard fashion: the valve is aligned with aortic annulus (D, notched white arrow) and deployed (E, notched white arrow). (F) After TAVR, the veno-aortic fistula is closed with an Amplatzer closure device (white arrow callout). Final aortography (F, black arrow) reveals trace leak into the IVC (F, stripped white arrow), which closes over time.
Conduction Disturbances
Injury of the cardiac conduction pathways adjacent to the aortic annulus after THV deployment can result in conduction abnormalities that range from transient atrioventricular (AV) and bundle branch blocks to complete AV block requiring implantation of a permanent pacemaker (PPM). Conduction disturbances requiring implantation of a new PPM vary widely among devices. They occur within 30-days in 4–38% of TAVR patients [1, 2, 4, 55], and are more frequent in patients that undergo TAVR with a self-expandable THV than with a balloon expandable THV [55]. However, the severity of the clinical impact of conduction disturbances after TAVR is controversial. Initial studies reported that the implantation of a new PPM was associated with later decrease in left ventricular function but with no adverse outcomes [56–58]. In contrast, more recent studies have reported that conduction disturbances(even isolated new left bundle branch block) are associated with increased length of stay, rehospitalization or 1-year mortality [13, 59], probably due to ventricular dyssynchrony. The full significance of these conduction abnormalities has yet to be determined in contemporary clinical trials and registries.
Vascular Complications
Vascular complications are more common with TAVR than with SAVR [1, 2, 4]; usually in the form of vascular injury caused by insertion of the delivery system or annular rupture during valve deployment.Not surprisingly, major vascular complications are associated with worse 30-day mortality after TAVR[60] but can often be prevented by using careful procedural planning, selection of access, annular sizing and percutaneous closure devices (i.e. Perclose device, Abbott vascular, Abbott Park, IL, USA).Operators must have a low threshold of suspicion for vascular complications in patients that develop hemodynamic instability after THV deployment.
Valve-in-Valve Transcatheter Aortic Valve Replacement
Valve-in-valve (VIV) TAVR has now emerged as an alternative to surgery in patients with failing aortic surgical bioprostheses [61] (Figure 5). Though patient selection, procedural planning and technique are similar to those described for patients with native valve AS, VIV-TAVR has unique challenges worth mentioning: malposition, coronary obstruction and suboptimal gradients [61]. The first two complications may be the result of distortion of the landing zone anatomy observed after surgical replacement with a bioprosthesis. Thus MDCT and TEE assessment are particularly important before VIV-TAVR. Increased post-VIV-TAVR procedural gradients are more common in patients with previously placed, small surgical bioprostheses. Patientprosthesis mismatch also can occur because the THV must necessarily be smaller than the original surgical bioprosthesis (“Russian doll model”).Use of MDCT sizing and widely available apps for VIV-TAVR can aid in determining the THV type,size and deployment location based on the specific surgical bioprosthesis. Recent in-vitro studies suggest that a supra-annular deployment of a SAPIEN XT valve can result in lower transvalvular pressure gradients, with the drawback of a decrease in radial force [62]. Similarly, the COREVALVE valve currently approved for VIV-TARV in the US may provide lower gradients in its normal position due to its supra-annular leaflet design. The best technical strategy for VIV-TAVR needs further study.
Will TAVR be Used for All Patients With Aortic Valve Disease?
TAVR is currently the standard of care for high and very high surgical risk patients with symptomatic AS [5]. Pending results of clinical trials, the benefit of TAVR almost certainly will extend into lower surgical risk populations. Proper patient selection will continue to be critical and require a comprehensive heart team evaluation that incorporates each patient’s surgical risk and anatomical characteristics [63].
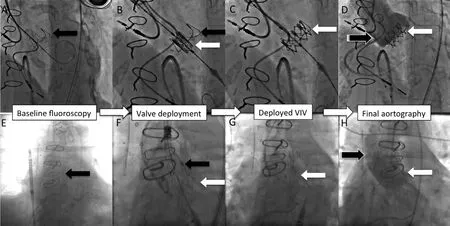
Figure 5 Valve-in-Valve Transcatheter Aortic Valve Replacement (VIV-TAVR) With a SAPIEN XT (A–D) and With a COREVALVE (E–H).Black arrow=surgical bioprosthesis. White arrow=transcatheter heart valve within the surgical bioprosthesis. Black arrow(white borders)=final aortography.
The better technology of the newer THVs can potentially improve TAVR procedural success.New generations of THV’s have more THV sizes and their delivery systems have smaller diameters.Hopefully these advantages will decrease complications and increase the inclusion criteria for TAVR. Whether delivery system diameters can be further decreased to allow a transbrachial and even transradial TAVR is speculative, but theoretically possible. Collaboration between clinicians and bioengineers will be critical to produce such innovations.
Technical improvements have helped make so called “minimalist” TF-TAVR possible in selected cases. Minimalist TF-TAVR is associated with shorter length of stay and lower costs with similar outcomes compared to standard TF-TAVR [33].In a high volume TAVR center, transition to minimalist TF-TAVR is possible without the need of a learning curve [34]. This suggests that a wider use of this strategy should be expected. However, validation and determination of inclusion criteria for minimalist TAVR are still needed. In addition, there are safety concerns for complex cases (i.e. patients with multiple comorbidities or complex anatomy),which may make universal adoption of minimalist TAVR difficult. However, this minimalist approach represents the first step to decrease cost and transition TAVR into a truly outpatient procedure.
Another important technical advance of TAVR has been the increasing options of alternative approaches.Patients that, in the past, could not be candidates for TF- or TA TAVR now may have multiple other options for transcatheter access. Alternative access sites now include trans-aortic, trans-carotid [38],subclavian, and more recently a transcaval approach[40, 42]. The transcaval approach deserves special mentioning. In this approach, the delivery system is introduced into the femoral vein and then in the mid-abdomen is crossed into the aorta by creating a fistula between the inferior vena cava (IVC) and the aorta. The shunt is closed with an Amplatzer device at the end of procedure (Figure 4). The transcaval approach has been shown to be feasible in patients with no other access option [40, 42], and is being studied in a clinical trial in the US. More evidence of safety, ideal inclusion criteria, and experience in multiple centers are needed. But adoption of the transcaval approach may make many patients with severe peripheral vascular disease candidates for a far simpler femoral venous approach for TAVR.Rather than having operative access through a TA or TAo approach, such patients could potentially have a minimalist transcaval TAVR, and be discharged early.
Reduction of complications is still a priority for all TAVR operators. Compared to initial studies, rates of TAVR related complications continue to decrease as the indications for TAVR expand to lower surgical risk patients [45, 46, 60, 64]. Outcomes will also improve with the use of better technology. For example, the use of cerebrovascular embolic protection devices (i.e. Embrella Embolic Deflector System [Edwards Lifesciences]), some of which have already CE mark approval in Europe,may help reduce the number and/or size of cerebrovascular events [54, 65].
It appears that complications are already decreasing as experience increases. In this respect, recently published results from the GARY registry are reassuring. Walther et al. have shown a decrease in the rate of complications over time in an all-comer registry of high risk patients that underwent TAVR in Germany [64].
The long-term durability of TAVR remains unknown. Initial fears that bioprosthesic valve tissue would be damaged by compression, become more fragile, and hence have shorter long term function, have given way to promising 5-year follow up data reported from clinical trials and registries [25, 66]. But only time will tell the average life expectancy of these valves. Recently reported subclinical leaflet thrombosis detected using MDCT has raised concerns on the future performance of some of these valves [67]. This observation, which needs to be validated in larger studies, raises the question of whether dual antiplatelet strategy after TAVR may be suboptimal in some patients. Trials are now under way to compare antiplatelet and anticoagulant treatment strategies after TAVR, and may well change post-TAVR therapies. THV long term durability and the most effective post-TAVR treatment will have to be proven before TAVR becomes the standard of care for low surgical risk patients with AS.
VIV-TAVR will increasingly be used in patients with prior surgical or transcatheter bioprostheses to avoid repeat open-heart surgery, especially in patients with elevated surgical risk. However, more data and experience is needed to determine the optimal THV type, size and deployment location. In the future, the technology of both surgical and transcatheter heart valves will perhaps be redesigned to make the VIV-TAVR strategy the standard of care of patients with degenerative bioprostheses.
As TAVR becomes an increasingly common treatment strategy for AS and bioprosthesis degeneration, more experience will be needed to determine the benefit of its use of the treatment of AR. Some of the unique challenges associated with AR include a more insidious disease onset,younger patient population, less clear indication for early valve replacement and concomitant aortic root dilation, which may complicate anchoring of a THV. Experience from case reports and small registries show that TAVR in AR is possible using existing valves (COREVALVE, Lotus,DirectFlow) [31, 68–70], new valves (Jenavalve,ACURATE TA, Enganger) [30, 71–73] or docking devices (Helio dock, Edwards Lifescience)designed to assist the deployment of a SAPIEN XT valve [74]. Larger controlled studies will be needed in the future.
TAVR in patients with bicuspid aortic valve disease is possible. A multi-center study by Mylotte et al. showed that in high-risk patients with bicuspid valve disease (AS, AR or mixed) that underwent TAVR, the 30-day device success was 89.9%,procedural mortality was 3.6% and 1-year mortality was 17.5% [75]. However, these patients had higher rates of post implantation PVL (28.4%) than observed in patients with senile AS [75]. Valve deployment in a congenital bicuspid aortic valve might also need to be different than in a functional bicuspid valve. The advantages of TAVR in young patients with congenital aortic valve disease remain to be determined. TAVR can be used as a means to prolong time between open-heart surgeries. However, a patient who is growing will ultimately have patient-prosthesis mismatch. Perhaps future innovation would allow us to implant a THV that can grow over time with the patient.
Conclusions
TAVR is the primary therapy for AS in patients considered inoperable and is increasingly used in intermediate and high surgical risk patients with native valve AS and in patients with a failing surgical bioprosthesis. The relatively rapid acceptance and successes observed with TAVR have been made possible through careful patient selection by heart teams,preprocedural imaging (i.e. MDCT annular sizing),THV technology (i.e. new generation valves), and procedural techniques (i.e. minimalist TF-TAVR and alternative percutaneous access options), as well as a decrease in complications as TAVR experience grows. When TAVR expands into patients with AR and AS at lower surgical risk, more studies will be needed regarding safety, long-term durability and antithrombotic medical therapy compared to surgical valves, but the future of TAVR is a bright one.
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
1. Leon MB, Smith CR, Mack M,Miller DC, Moses JW, Svensson LG, et al. Transcatheter aorticvalve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363(17):1597–607.
2. Smith CR, Leon MB, Mack MJ,Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364(23):2187–98.
3. Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS,Heimansohn D, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014;63(19):1972–81.
4. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS,Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370(19):1790–8.
5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd,Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(22):2438–88.
6. O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB,et al. The Society of thoracic surgeons 2008 cardiac surgery risk models: part 2–isolated valve surgery. Ann Thorac Surg 2009;88(1 Suppl):S23–42.
7. Biancari F, Juvonen T, Onorati F,Faggian G, Heikkinen J, Airaksinen J, et al. Meta-analysis on the performance of the EuroSCORE II and the Society of Thoracic Surgeons Scores in patients undergoing aortic valve replacement. J Cardiothor Vasc An 2014;28(6):1533–9.
8. Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59(14):1275–86.
9. Webb J, Gerosa G, Lefevre T, Leipsic J, Spence M, Thomas M, et al.Multicenter evaluation of a nextgeneration balloon-expandable transcatheter aortic valve. J Am Coll Cardiol 2014;64(21):2235–43.
10. Babaliaros VC, Junagadhwalla Z, Lerakis S, Thourani V, Liff D, Chen E, et al. Use of balloon aortic valvuloplasty to size the aortic annulus before implantation of a balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv 2010;3(1):114–8.
11. Pasic M, Unbehaun A, Buz S,Drews T, Hetzer R. Annular rupture during transcatheter aortic valve replacement: classification,pathophysiology, diagnostics, treatment approaches, and prevention.JACC Cardiovasc Interv 2015;8(1 Pt A):1–9.
12. Steinberg BA, Harrison JK,Frazier- Mills C, Hughes GC, Piccini JP. Cardiac conduction system disease after transcatheter aortic valve replacement. American heart journal. 2012;164(5):664–71.
13. Nazif TM, Dizon JM, Hahn RT, Xu K, Babaliaros V, Douglas P, et al.Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER(Placement of AoRtic TraNscathetER Valves) trial and registry.JACC Cardiovasc Interv 2015;8(1 Pt A):60–9.
14. Blanke P, Reinohl J, Schlensak C, Siepe M, Pache G, Euringer W, et al. Prosthesis oversizing in balloon-expandable transcatheter aortic valve implantation is associated with contained rupture of the aortic root. Circ Cardiov Interv 2012;5(4):540–8.
15. Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R,Wheeler M, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol 2012;59(14):1287–94.
16. Buzzatti N, Maisano F, Latib A,Cioni M, Taramasso M, Mussardo M, et al. Computed tomographybased evaluation of aortic annulus,prosthesis size and impact on early residual aortic regurgitation after transcatheter aortic valve implantation. Eur J Cardiothorac Surg 2013;43(1):43–50; discussion–1.
17. Schultz CJ, Tzikas A, Moelker A,Rossi A, Nuis RJ, Geleijnse MM,et al. Correlates on MSCT of paravalvular aortic regurgitation after transcatheter aortic valve implantation using the Medtronic CoreValve prosthesis. Catheter Cardiovasc Interv 2011;78(3):446–55.
18. Stortecky S, Heg D, Gloekler S, Wenaweser P, Windecker S,Buellesfeld L. Accuracy and reproducibility of aortic annulus sizing using a dedicated threedimensional computed tomography reconstruction tool in patients evaluated for transcatheter aortic valve replacement. EuroIntervention 2014;10(3):339–46.
19. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description.Circulation 2002;106(24):3006–8.
20. Cribier A. Development of transcatheter aortic valve implantation(TAVI): a 20-year odyssey. Arch Cardiovasc Dis 2012;105(3):146–52.
21. Mylotte D, Osnabrugge RL, Windecker S, Lefèvre T, de Jaegere P,Jeger R, et al. Transcatheter aortic valve replacement in Europe: adoption trends and factors influencing device utilization. J Am Coll Cardiol 2013;62(3):210–9.
2 2. Dvir D, Barbash IM, Ben-Dor I,Okubagzi P, Satler LF, Waksman R, et al. The development of transcatheter aortic valve replacement in the USA. Arch Cardiovasc Dis 2012;105(3):160–4.
2 3. Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis:the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58(20):2130–8.
2 4. Gilard M, Eltchaninoff H, Iung B,Donzeau-Gouge P, Chevreul K,Fajadet J, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366(18):1705–15.
2 5. Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG,Kodali S, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385(9986):2485–91.
2 6. Makkar RR, Fontana GP,Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis.N Engl J Med 2012;366(18):1696–704.
2 7. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG,Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366(18):1686–95.
2 8. Meredith Am IT, Walters DL,Dumonteil N, Worthley SG, Tchétché D, Manoharan G, et al. Transcatheter aortic valve replacement for severe symptomatic aortic stenosis using a repositionable valve system: 30-day primary endpoint results from the REPRISE II study. J Am Coll Cardiol 2014;64(13):1339–48.
29. Meredith IT, Worthley SG, Whitbourn RJ, Antonis P, Montarello JK, Newcomb AE, et al. Transfemoral aortic valve replacement with the repositionable Lotus Valve System in high surgical risk patients:the REPRISE I study. EuroIntervention 2014;9(11):1264–70.
30. Seiffert M, Bader R, Kappert U,Rastan A, Krapf S, Bleiziffer S,et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014;7(10):1168–74.
31. Schofer J, Nietlispach F, Bijuklic K, Colombo A, Gatto F, De Marco F, et al. Transfemoral Implantation of a Fully Repositionable and Retrievable Transcatheter Valve for Noncalcified Pure Aortic Regurgitation. JACC Cardiovasc Interv 2015;8(14):1842–9.
32. Blackstone EH, Suri RM,Rajeswaran J, Babaliaros V,Douglas PS, Fearon WF, et al.Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves(PARTNER)-I trial substudy.Circulation 2015;131(22):1989–2000.
33. Babaliaros V, Devireddy C, Lerakis S, Leonardi R, Iturra SA, Mavromatis K, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv 2014;7(8):898–904.
34. Jensen HA, Condado JF, Devireddy C, Binongo J, Leshnower BG,Babaliaros V, et al. Minimalist transcatheter aortic valve replacement:the new standard for surgeons and cardiologists using transfemoral access? J Thorac Cardiovasc Surg 2015;150(4):833–9.
35. Descoutures F, Himbert D, Lepage L, Iung B, Détaint D, Tchetche D,et al. Contemporary surgical or percutaneous management of severe aortic stenosis in the elderly. Eur Heart J 2008;29(11):1410–7.
36. R odes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM,et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55(11):1080–90.
37. N ombela-Franco L, Rodes-Cabau J, DeLarochelliere R, Larose E,Doyle D, Villeneuve J, et al. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv 2012;5(5):499–512.
38. T hourani VH, Gunter RL, Neravetla S, Block P, Guyton RA,Kilgo P, et al. Use of transaortic,transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surgery. 2013;96(4):1349–57.
39. L ardizabal JA, O’Neill BP, Desai HV, Macon CJ, Rodriguez AP,Martinez CA, et al. The transaortic approach for transcatheter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013;61(23):2341–5.
40. G reenbaum AB, O’Neill WW,Paone G, Guerrero ME, Wyman JF, Cooper RL, et al. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014;63(25 Pt A):2795–804.
41. P etronio AS, De Carlo M, Bedogni F, Marzocchi A, Klugmann S, Maisano F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010;3(4):359–66.
42. L ederman RJ, Babaliaros VC,Greenbaum AB. How to perform transcaval access and closure for transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2015;86(7):1242–54.
43. A lli O, Rihal C, Suri R, Greason KL, Waksman R, Minha S, et al.TCT–747 Transcatheter aortic valve replacement: assessment of the learning curve based on the PARTNER trial. J Am Coll Cardiol 2013;62(18_S1):B227–B8.
44. A guirre J, Waskowski R, Poddar K, Kapadia S, Krishnaswamy A,McCullough R, et al. Transcatheter aortic valve replacement: Experience with the transapical approach,alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014;148(4):1417–22.
45. A lli O, Rihal CS, Suri RM, Greason KL, Waksman R, Minha S,et al. Learning curves for transfemoral transcatheter aortic valve replacement in the PARTNER-I trial: technical performance. Catheter Cardiovasc Interv 2015. Pub-Med PMID: 26256280.
46. A thappan G, Gajulapalli RD, Sengodan P, Bhardwaj A, Ellis SG,Svensson L, et al. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a metaanalysis and systematic review of literature. J Am Coll Cardiol 2014;63(20):2101–10.
47. K oos R, Altiok E, Mahnken AH,Neizel M, Dohmen G, Marx N,et al. Evaluation of aortic root for definition of prosthesis size by magnetic resonance imaging and cardiac computed tomography:implications for transcatheter aortic valve implantation. Int J Cardiol 2012;158(3):353–8.
48. K halique OK, Hahn RT, Gada H,Nazif TM, Vahl TP, George I, et al.Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv 2014;7(8):885–94.
49. D etaint D, Lepage L, Himbert D,Brochet E, Messika-Zeitoun D,Iung B, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve:implantation impact of device and annulus discongruence. JACC Cardiovasc Interv 2009;2(9):821–7.
50. T amburino C, Capodanno D,Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation.2011;123(3):299–308.
51. M iller DC, Blackstone EH, Mack MJ, Svensson LG, Kodali SK,Kapadia S, et al. Transcatheter(TAVR) versus surgical (AVR) aortic valve replacement: occurrence,hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Surg 2012;143(4):832–43.e13.
52. F airbairn TA, Mather AN, Bijsterveld P, Worthy G, Currie S,Goddard AJ, et al. Diffusionweighted MRI determined cerebral embolic infarction following transcatheter aortic valve implantation: assessment of predictive risk factors and the relationship to subsequent health status. Heart 2012;98(1):18–23.
53. N ietlispach F, Wijesinghe N, Gurvitch R, Tay E, Carpenter JP, Burns C, et al. An embolic deflection device for aortic valve interventions. JACC Cardiovasc Interv 2010;3(11):1133–8.
54. N aber CK, Ghanem A, Abizaid AA, Wolf A, Sinning JM, Werner N, et al. First-in-man use of a novel embolic protection device for patients undergoing transcatheter aortic valve implantation. Euro-Intervention 2012;8(1):43–50.
55. A bdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tölg R, et al. Comparison of balloonexpandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. J Am Med Assoc 2014;311(15):1503–14.
56. Bu ellesfeld L, Stortecky S, Heg D,Hausen S, Mueller R, Wenaweser P, et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol 2012;60(6):493–501.
57. De Carlo M, Gianninic, Bedogni F, Klugmann S, Brambilla N, De Marco F, et al. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation.Am Heart J 2012;163(3):492–9.
58. Ur ena M, Webb JG, Tamburino C, Muñoz-García AJ, Cheema A,Dager AE, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation:impact on late clinical outcomes and left ventricular function. Circulation 2014;129(11):1233–43.
59. Dizo n JM, Nazif TM, Hess PL,Biviano A, Garan H, Douglas PS,et al. Chronic pacing and adverse outcomes after transcatheter aortic valve implantation. Heart 2015;101(20):1665–71.
60. Genereux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial.J Am Coll Cardiol 2012;60(12):1043–52.
61. Dvir D, Webb JG, Bleiziffer S,Pasic M, Waksman R, Kodali S,et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. J Am Med Assoc 2014;312(2):162–70.
62. Midha PA, Raghav V, Condado JF,et al. How can we help a patient with a small failing bioprosthesis?JACC Cardiovasc Interv 2015.
63. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM,Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33(19):2403–18.
64. Walther T, Hamm CW, Schuler G,Berkowitsch A, Kötting J, Mangner N, et al. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the gary registry. J Am Coll Cardiol 2015;65(20):2173–80.
6 5. Samim M, Agostoni P, Hendrikse J, Budde RP, Nijhoff F,Kluin J, et al. Embrella embolic deflection device for cerebral protection during transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2015;149(3):799–805.e1–2.
6 6. Duncan A, Ludman P, Banya W,Cunningham D, Marlee D, Davies S, et al. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K.Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv 2015;8(5):645–53.
6 7. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, de Backer O, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015;373(21):2015–24.
6 8. Roy DA, Schaefer U, Guetta V,Hildick-Smith D, Möllmann H,Dumonteil N, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol 2013;61(15):1577–84.
69. Testa L, Latib A, Rossi ML, De Marco F, De Carlo M, Fiorina C,et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention 2014;10(6):739–45.
70. Wohrle J, Rodewald C, Rottbauer W. Transfemoral aortic valve implantation in pure native aortic valve insufficiency using the repositionable and retrievable lotus valve. Catheter Cardiovasc Interv 2015;87(5):993–5.
71. Wendt D, Kahlert P, Pasa S, El-Chilali K, Al-Rashid F, Tsagakis K,et al. Transapical transcatheter aortic valve for severe aortic regurgitation: expanding the limits. JACC Cardiovasc Interv 2014;7(10):1159–67.
72. Kiefer P, Seeburger J, Mohr FW,Holzhey DM. Transcatheter aortic valve replacement for isolated aortic valve insufficiency: experience with the Engager valve. J Thorac Cardiovasc Surg 2014;147(4):e37–8.
73. Wei L, Liu H, Zhu L, Yang Y, Zheng J, Guo K, et al. A new transcatheter aortic valve replacement system for predominant aortic regurgitation implantation of the J-valve and early outcome. JACC Cardiovasc Interv 2015;8(14):1831–41.
74. Barbanti M, Ye J, Pasupati S,El-Gamel A, Webb JG. The Helio transcatheter aortic dock for patients with aortic regurgitation. EuroIntervention 2013;9(9 Suppl):S91–4.
75. Mylotte D, Lefevre T, Sondergaard L, Watanabe Y, Modine T, Dvir D,et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014;64(22):2330–9.
 Cardiovascular Innovations and Applications2016年2期
Cardiovascular Innovations and Applications2016年2期
- Cardiovascular Innovations and Applications的其它文章
- Transient Pulmonary Atelectasis after Ketamine Sedation during Cardiac Catheterization in Spontaneously Breathing Children with Congenital Heart Disease
- Identification and Management of Iatrogenic Aortocoronary Dissection
- Cardiovascular Abnormalities Among Patients with Spontaneous Subarachnoid Hemorrhage.A Single Center Experience
- Coronary Artery Chronic Total Occlusion
- Carotid Artery Stenting: 2016 and Beyond
- The Transradial Approach for Cardiac Catheterization and Percutaneous Coronary Intervention: A Review
