添加Y(NO3)3电解液体系MAO陶瓷层组织和隔热性能研究*
赵玉厚,韩 婧,王 萍(西安工业大学材料与化工学院,西安710021)
添加Y(NO3)3电解液体系MAO陶瓷层组织和隔热性能研究*
赵玉厚,韩婧,王萍
(西安工业大学材料与化工学院,西安710021)
摘 要:为研究电解液中Y(NO3)3对微弧氧化陶瓷层组织、生长速率及隔热性能的影响,通过微弧氧化技术在锆盐体系和锆钇盐体系电解液中于Al-Si合金表面制备ZrO2-Al2O3陶瓷层和Y2O3-ZrO2-Al2O3陶瓷层.采用环境扫描电子显微镜和X射线衍射仪分别对陶瓷层进行了表面、截面形貌分析,以及物相组成分析.利用涡流测厚仪测量不同反应时间段的膜厚,分析两种体系陶瓷层生长速率.通过自制隔热测试装置对两种不同体系陶瓷层进行了隔热性能测试.结果表明:ZrO2-Al2O3陶瓷层表面由胞状熔融物烧结而成,粗糙度较大,并分布着孔径较大的放电通道,膜厚约20μm;而Y2O3-ZrO2-Al2O3陶瓷层表面由细小颗粒组成,粗糙度较小,且陶瓷层更加致密,厚度增大到28μm.两种体系陶瓷层均形成了ZrO2及Al2O3相,在约20°~30°范围之间出现明显的“馒头包”现象,说明陶瓷层中均含有非晶成分;但锆钇盐体系陶瓷层中形成了钇部分稳定锆的固溶体(Y2O3和Y0.15Zr0.85O1.93),且Y2O3-ZrO2-Al2O3陶瓷层的衍射峰“馒头包”现象更为严重,非晶成分含量更高,说明电解液中Y(NO3)3的加入提高了反应温度.Y2O3-ZrO2-Al2O3陶瓷层生长速度大于ZrO2-Al2O3陶瓷层,主要表现为向外生长厚度明显增大.ZrO2-Al2O3陶瓷层与Y2O3-ZrO2-Al2O3陶瓷层的隔热温度分别为45.9℃和53.4℃,说明后者具有更优的隔热效果.
关键词:微弧氧化;Al-Si合金;陶瓷层;电解版;隔热性能
铸造Al-Si合金因力学性能和加工性能良好,具有广阔的应用前景.但其硬度低,耐磨性差等缺点,又常限制其广泛应用[1].微弧氧化(Micro Arc Oxidation,MAO)技术作为一种金属表面非常有效的陶瓷层原位生长技术备受人们重视[2-4].它是利用溶液中微弧放电直接在Al、Mg和Ti等金属表面生成陶瓷层,由于陶瓷层与基体形成冶金结合,可显著提高基体合金的耐磨、耐腐蚀及耐热性能[5-9],对改善Al-Si合金表面性能、扩大其应用范围具有重要的现实意义.
陶瓷材料具有耐腐蚀、耐磨、硬度大和强度高等优点,但由于陶瓷是由离子键或共价键的晶粒构成的多晶烧结材料,在室温下难以产生滑移或位错运动,一旦处于受力状态就难以通过滑移或位错所引起的塑性变形来松弛应力,而且裂纹的生成及扩张所需能量较小,所以陶瓷材料作为金属表面膜层首要克服的就是脆性[10-12].ZrO2相变增韧陶瓷利用相变特性来提高陶瓷材料的断裂韧性和抗弯强度,使其具有优良的力学性能,低的热导率和良好的抗热性.但是,由于温度的改变,ZrO2的体积会发生膨胀和收缩,从而导致膜层开裂.可以在ZrO2中加入CaO、MgO和Y2O3等氧化物作为稳定剂,在保持主相结构的同时,使之与ZrO2形成固溶体或者复合体,改变晶体内部结构,形成亚稳态的四方相或立方相,使其由单一的单斜相转变成双晶结构的四方和立方相,从而减少ZrO2陶瓷层由于脆性导致的开裂[13-16].目前,对于Y2O3-ZrO2陶瓷层的研究已有不少,但多是建立在铝合金以外的基体表面,如镁合金[9]、不锈钢[17],或者是对性能方面的探讨,如耐腐蚀性能、抗高温氧化及耐磨性能等[18-19],而针对铝合金表面Y2O3-ZrO2-Al2O3陶瓷层隔热性能的文献还甚少.
文中利用微弧氧化制备陶瓷层过程中以Y2O3作为稳定剂掺入ZrO2-Al2O3陶瓷层中形成Y2O3-ZrO2-Al2O3陶瓷层,将其与ZrO2-Al2O3陶瓷层进行对比,研究Y2O3-ZrO2-Al2O3陶瓷层微观组织形貌,生长方式及隔热性能.
1 实验材料及方法
试验中所用的基体材料为铸造Al-12.5%Si合金,加工后制得规格为Ø40 mm×10 mm的圆柱形试样.使用240#、400#、600#、800#的砂纸依次打磨试样,将磨好的试样用丙酮擦拭,清除表面油污,吹干待用.使用MAO-10C型微弧氧化电源,不锈钢板作为阴极电极,在K2Zr F6、KOH(加或不加0.05 g·L—1Y(NO3)3)体系电解液中进行30 min微弧氧化工艺.分别使用FEI quanta 400环境扫描电镜(Scanning Electron Microscopy,SEM)和XRD-6000型X射线衍射(X-Ray Diffraction,XRD)仪对陶瓷层表面、截面显微形貌及物相组成进行分析.采用TT260型涡流测厚仪测量陶瓷层厚度,对试样的10个不同部位厚度求平均值作为陶瓷层的最终厚度.使用自制隔热测试装置进行隔热性能测试.
2 实验结果与分析
2.1陶瓷层表面形貌分析
图1和图2分别为ZrO2-Al2O3陶瓷层和Y2O3-ZrO2-Al2O3陶瓷层微观组织形貌.从图1 (a)可看出,ZrO2-Al2O3陶瓷层表面粗糙度较大,由许多胞状熔融物烧结在微孔周围组成.这些微孔是当应用电压达到击穿电压时,陶瓷层表面在高温高压作用下局部微区被击穿放电,基体表面熔融氧化物喷出而留下的通道[20].由图2(a)中Y2O3-ZrO2-Al2O3陶瓷层低倍扫描照片可看到,表面呈较平坦片层状.图2(a)右上角部分为标注区域的放大照片,可观察到陶瓷层表面是由均匀分布的小颗粒状沉积物组成的,没有明显的放电通道或已被熔融氧化物填埋.这种形貌的形成是因为在锆钇盐电解液体系中,稀土元素Y的电负性强,离子半径较大,容易被极化和变形,所以溶液中的Y3+会优先在基体表面的缺陷处吸附形成活性点[21],这些活性点的存在给陶瓷层晶粒的形成提供了异质的形核中心,能有效地降低形核的活化能,进一步提高晶粒的形成速度,从而使得陶瓷层晶粒细小、质地致密[22].
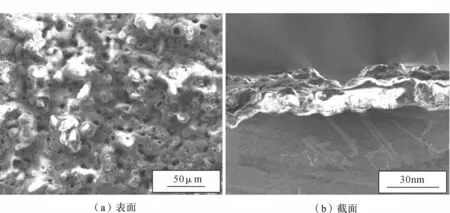
图1 ZrO2-Al2O3陶瓷层微观形貌Fig.1 Morphology of ZrO2-Al2O3ceramic coating
图1(b)和图2(b)分别为两种电解液体系陶瓷层的截面.由图1和图2可看到,ZrO2-Al2O3陶瓷层疏松多孔,与基体之间的界面呈啮齿状结合,而Y2O3-ZrO2-Al2O3陶瓷层则致密均匀,与基体合金的界面呈过渡性的紧密结合,其厚度比ZrO2-Al2O3陶瓷层稍厚,约为28μm,厚度比值为7∶5.
2.2陶瓷层物相分析
图3~4为ZrO2-Al2O3陶瓷层和Y2O3-ZrO2-Al2O3陶瓷层X射线衍射图.由图3和图4可看出,ZrO2-Al2O3陶瓷层的主要物相为Al2O3、ZrO2和少量Al,而Y2O3-ZrO2-Al2O3陶瓷层中除这些物相外,还形成了钇部分稳定锆的固溶体(Y2O3和Y0.15Zr0.85O1.93).有研究表明,当Y2O3含量在1.5%~7.5%之间,即氧空位浓度为1.5%~7.5%时,四方相可以保留至室温,其锆-氧关系为Y0.03Zr0.97O1.97~Y0.15Zr0.85O1.85[23].两种体系陶瓷层的X射线衍射图谱在约20°~30°范围出现“馒头包”现象,并且Y2O3-ZrO2-Al2O3陶瓷层的“馒头包”现象更明显,说明陶瓷层中均含有一定非晶成分,如γ-Al2O3、SiO2等,并且后者含量更高.
由于亚稳相γ-Al2O3与高温稳定相α-Al2O3的晶型转变温度为1 370℃左右,而m-ZrO2(单斜相)向t-ZrO2(四方相)的相转变温度也较为接近,在1 050~1 200℃之间[21],所以ZrO2-Al2O3陶瓷层的反应温度可达到1 370℃以上.又由于Y2O3-ZrO2-Al2O3陶瓷层中的非晶成分含量高于ZrO2-Al2O3陶瓷层,所以可以判断其反应温度也在ZrO2-Al2O3陶瓷层之上.

图2 Y2O3-ZrO2-Al2O3陶瓷层微观形貌Fig.2 Morphology of Y2O3-ZrO2-Al2O3ceramic coating
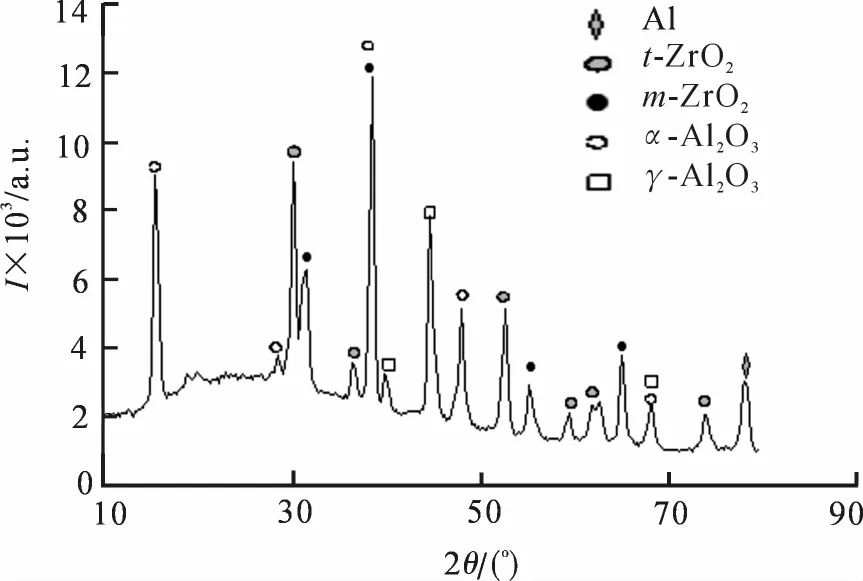
图3 ZrO2-Al2O3陶瓷层XRD衍射图谱Fig.3 XRD patterns of ZrO2-Al2O3ceramic coating

图4 Y2O3-ZrO2-Al2O3陶瓷层XRD衍射图谱Fig.4 XRD patterns of Y2O3-ZrO2-Al2O3ceramic coating
2.3陶瓷层的生长速度对比
图5为ZrO2-Al2O3陶瓷层及Y2O3-ZrO2-Al2O3陶瓷层厚度随时间的增长曲线对比.由图5可以看出,在初始阶段,二者的膜层增长速率相差不大.随后,ZrO2-Al2O3陶瓷层厚度增长缓慢,Y2O3-ZrO2-Al2O3陶瓷层厚度以基本不变速率增长,15 min后,二者厚度的差值基本保持恒定,这一阶段陶瓷层反应速度主要受电解质浓度的影响.

图5 两种体系陶瓷层生长速度曲线对比Fig.5 Comparison of growth rate curves of the ceramic coatings formed in the two different solutions
当在电解液体系中添加钇盐后,由于Y3+的引入提高了陶瓷层中ZrO2的氧空位,致使在微弧氧化过程中氧空位增多,扩散通道增多,使得氧向陶瓷层中甚至基体表面的扩散速率增大,从而加剧反应速度.同时,氧空位使得基体表面在进行微弧氧化过程中更容易击穿陶瓷层产生更多的放电通道,使得放电火花密集而迅速反应,促使已经形成的陶瓷层不断被熔化、喷出及烧结,这也说明了反应速度的提高.综上所述,Y2O3-ZrO2-Al2O3陶瓷层较ZrO2-Al2O3陶瓷层生长速度快.
2.4陶瓷层的生长方式对比
微弧氧化陶瓷层成膜过程中发生了热化学、电化学、等离子体化学及界面化学等一系列反应,因而有着复杂的成膜机理.随着氧化时间的延长,其膜层的主要生长方式也会发生相应的变化,膜层的生长方式主要有向内生长和向外生长两种.
图6为两种电解液体系陶瓷层的生长方式曲线.从图6(a)可得知,ZrO2-Al2O3陶瓷层在初始阶段约1 min内,膜层增长主要靠向外生长.而2~25 min之间,随着陶瓷层向外生长的厚度逐渐降低,向内生长的厚度不断增大,直至25 min后陶瓷层不再向外生长,而全部由向内生长所代替.
从图6(b)中Y2O3-ZrO2-Al2O3陶瓷层的生长方式曲线可看出,基本在整个微弧氧化过程中,陶瓷层的增厚均由向内生长起主导作用.在前20 min内,陶瓷层向外生长厚度与向内生长一样逐渐增大,只是向外增长的速度较慢;而当达到20 min后,陶瓷层的向外生长厚度开始下降,直至30 min时为零;其后的时间段又由向内生长主导陶瓷层的增厚.
两种电解液体系陶瓷层在前25~30 min均存在向内生长与向外生长同时影响厚度,而后向外生长厚度变为零,陶瓷层厚度全部由向内生长控制.这是因为在微弧氧化初期,基体表面所发生的反应类似于传统的阳极氧化反应,生成疏松的氧化铝薄膜.随着电压的升高,突破法拉弟区,又由于此时基体表面已形成一定厚度的阻挡膜,为微弧氧化的电击穿提供了失稳表面,从而进入到微弧氧化阶段.氧化膜的某些薄弱部分首先被击穿,发生微区弧光放电现象.在微弧放电阶段,电解液中带负电的Zr (OH)4溶胶粒子不断向阳极表面迁移,或进入放电通道中参与反应,并且陶瓷层内层靠近基体表面受高温高压影响不断将熔融氧化物喷出,烧结,使得向内生长厚度不断增大,并逐渐取代向外生长. 但Y2O3-ZrO2-Al2O3陶瓷层的向内生长增厚速度明显较ZrO2-Al2O3陶瓷层快,而且Y2O3-ZrO2-Al2O3陶瓷层向外生长的时间较长,厚度也较大.这主要是由于Y3+的引入提高了陶瓷层中ZrO2的氧空位,不仅促使反应加剧,而且导致陶瓷层放电通道密度增大.放电通道作为陶瓷层向内生长的主要方式,自然促进陶瓷层向内生长厚度.并且当在碱性锆盐溶液中加入钇盐,生成的Y(OH)3也会向陶瓷层表面沉积,所以使得陶瓷层向外生长的厚度增大.
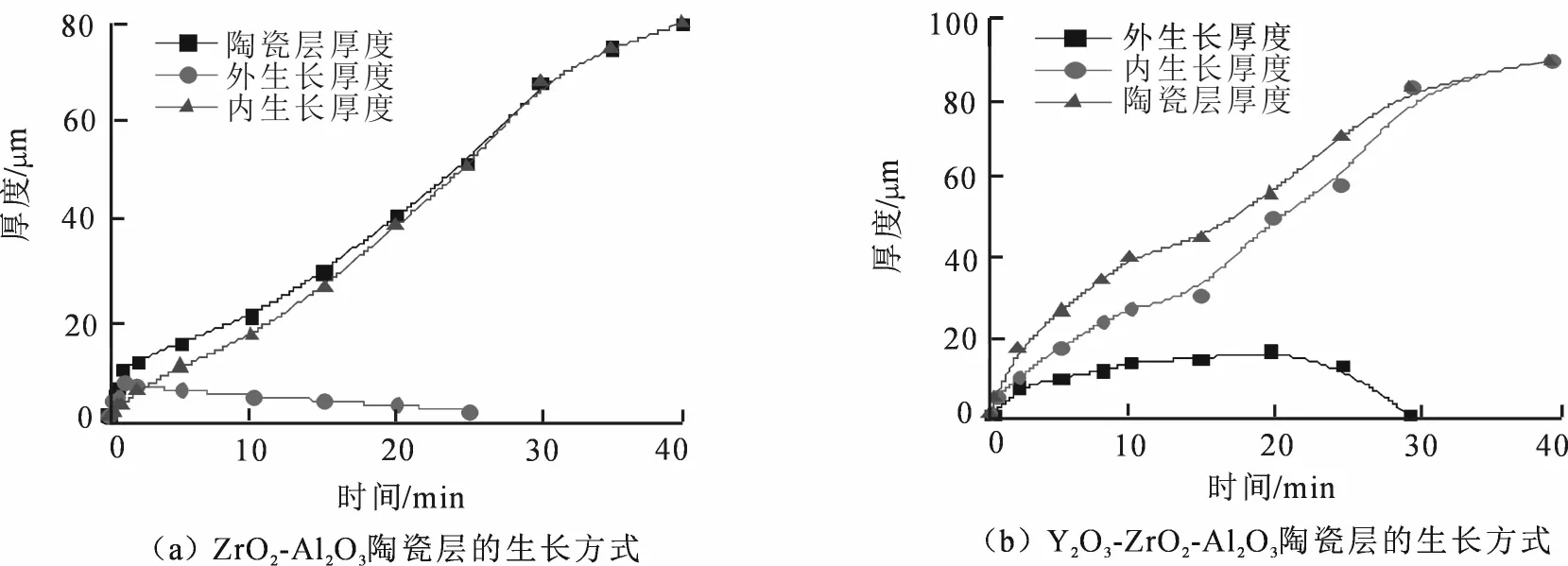
图6 两种体系陶瓷层的生长方式曲线Fig.6 Growth mode curves of the ceramic coatings
2.5陶瓷层隔热性能对比
图7为两种不同电解液体系陶瓷层的隔热性能测试结果.相同时间内,Y2O3-ZrO2-Al2O3陶瓷层的隔热温度(53.4℃)较高于ZrO2-Al2O3陶瓷层(45.9℃),说明钇盐的加入显著提高了陶瓷层的隔热效果,主要原因在于微弧氧化成膜过程中熔融氧化物由于遇到电解液快冷而形成的非晶成分,以及其组织中形成的细小晶粒[24].研究表明:单晶的热导率为3.5×104W·K—1,而非晶的热导率仅为1.0×102W·K—1[24].通过X射线衍射结果得知在Y2O3-ZrO2-Al2O3陶瓷层中SiO2等的非晶成分含量高于ZrO2-Al2O3陶瓷层,并且由陶瓷层表面扫描结果可知,Y2O3-ZrO2-Al2O3陶瓷层表面由细小晶粒组成.其次,由于Y2O3-ZrO2-Al2O3陶瓷层晶格中的氧空位与散射声子的作用会增加声子在晶格中的离散度,随着离散度的增加,声子与声子发生相互作用的可能性变大,因而会进一步降低声子的平均自由程[25],从而提高了隔热效果. ZrO2-Al2O3陶瓷层因孔隙率较高,声子的散射加强并额外消耗了能量[25],使得其隔热效果也不错.最后,Y2O3-ZrO2-Al2O3陶瓷层在厚度上稍占优势,使得隔热性能有所提高,但与其它因素相比,其对隔热效果的影响甚微[24].
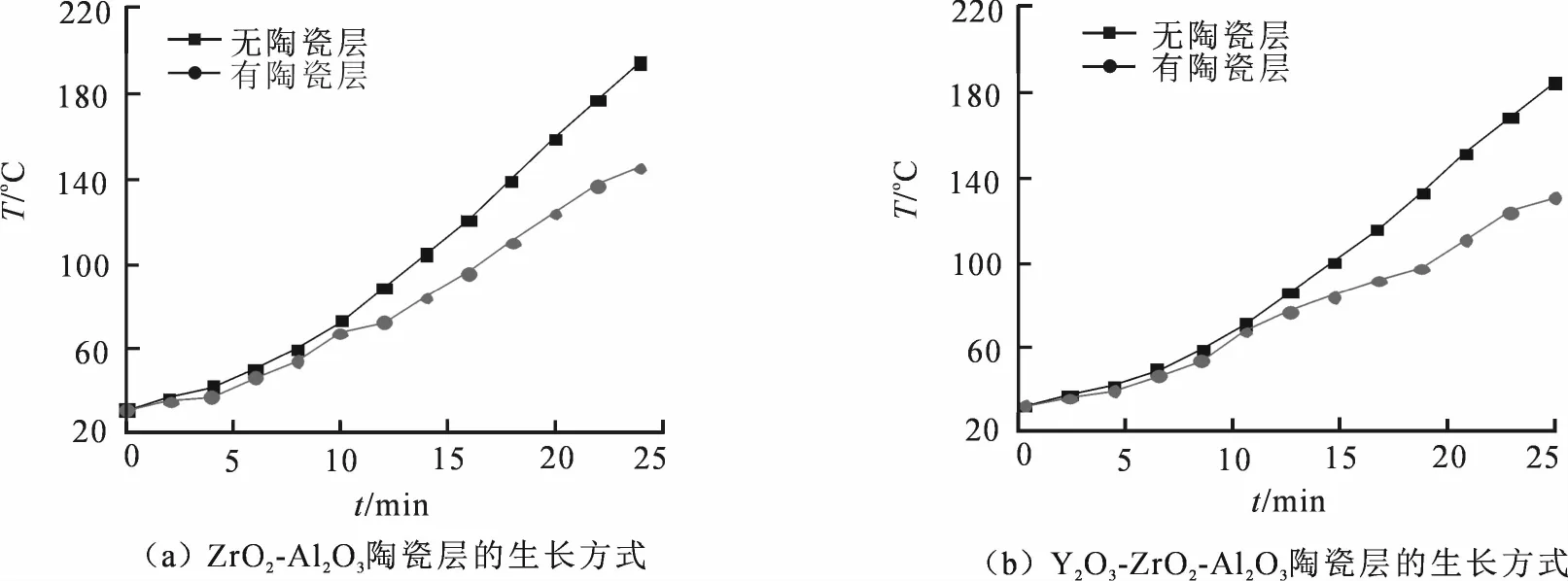
图7 两种体系陶瓷层的隔热性能测试Fig.7 Heat insulation properties test of the ceramic coatings formed in two different solutions
3 结论
1)ZrO2-Al2O3陶瓷层表面由许多胞状熔融物烧结在微孔周围组成,粗糙度较大,较薄且疏松,而电解液中加入Y(NO3)3使得陶瓷层表面形成细小晶粒,粗糙度降低,厚度有所增大,且更致密.两种体系陶瓷层主要物相都含有ZrO2和Al2O3,Y2O3-ZrO2-Al2O3陶瓷层还形成了钇部分稳定锆的固溶体(Y2O3和Y0.15Zr0.85O1.93),证明增加了ZrO2的氧空位;并且其非晶成分含量高于ZrO2-Al2O3陶瓷层,说明反应温度有所提高.
2)由于Y3+的引入提高了陶瓷层中氧空位,使得在微弧氧化过程中扩散通道增多,从而提高了陶瓷层的生长速度,并促进了向内生长,故而Y2O3-ZrO2-Al2O3陶瓷层的生长速率高于ZrO2-Al2O3陶瓷层.
3)Y2O3-ZrO2-Al2O3陶瓷层中大量的微小晶粒、非晶成分以及氧空位等因素都使得陶瓷层的隔热性能好于ZrO2-Al2O3陶瓷层.
参考文献:
[1] 余磊,蔡启舟.ZL101等离子体电解氧化工艺及膜层形成机理的研究[D].武汉:华中科技大学,2007. YU Lei,CAI Qizhou.Research on Process and Coating Formation Mechanism in Plasma Electrolytic Oxidation of ZL101[D].Wuhan:Huazhong University of Science and Technology,2007.(in Chinese)
[2] VOEVODIN A A,YEROKHIN A L,LYUBIMOV V V,et al.Characterization of Wear Protective Al-Si-O Coatings Formed on Al-Based Alloys by Microarc Discharge Treatment[J].Surface and Coatings Technology,1996,86(1):516.
[3] YEROKHIN A L,NIE X,LEYLAND A,et al.Plasma Electrolysis for Surface Engineering[J].Surface and Coatings Technology,1999,122(2/3):73.
[4] CHEN Z T,LI G,WU Z Q,et al.The Crack Propagating Behavior of Composite Coatings Prepared by PEO on Aluminized Steel during in Situ Tensile Processing[J].Materials Science and Engineering:A,2011,528(3):1409.
[5] HU C J,HSIEH M H.Preparation of Ceramic Coatings on an Al-Si Alloy by the Incorporation of ZrO2Particles in Microarc Oxidation[J].Surface and Coatings Technology,2014,258(15):275.
[6] LIU F,SHAN D Y,SONG Y W,et al.Corrosion Behavior of the Composite Ceramic Coating Containing Zirconium Oxides on AM30 Magnesium Alloy by Plasma Electrolytic Oxidation[J].Corrosion Science,2011,53(11):3845.
[7] HUSSEIN R O,NORTHWOOD D O,NIE X.The Effect of Processing Parameters and Substrate Composition on the Corrosion Resistance of Plasma Electrolytic Oxidation(PEO)Coated Magnesium Alloys [J].Surface&Coatings Technology,2013,237(12):357.
[8] HUSSEIN R O,NORTHWOOD D O,SU J F,et al. A Study of the Interactive Effects of Hybrid Current Modes on the Tribological Properties of a PEO(Plasma Electrolytic Oxidation)Coated AM60B Mg-Alloy [J].Surfce and Coatings Technology,2013,215(2):421.
[9] 蔡启舟,刘峰,严青松,等.镁合金微弧氧化Y2O3-ZrO2-MgO膜制备及性能[J].华中科技大学学报(自然科学版),2011,39(8):27. CAI Qizhou,LIU Feng,YAN Qingsong,et al.Preparation and Performance of Y2O3-ZrO2-MgO Composite Coating on Magnesium Alloy by Microarc Oxidation[J].Journal of Huazhong University of Science and Technology(Natural Science Edition),2011,39 (8):27.(in Chinese)
[10] 熊炳昆,林振汉,杨新民,等.二氧化锆制备工艺与应用[M].北京:冶金工业出版社,2009. XIONG Bingkun,LIN Zhenhan,YANG Xinmin,et al.Preparation Process and Application of Zirconium Dioxide[M].Beijing:Metallurgical Industry Press,2009.(in Chinese)
[11] 金志浩,高积强,乔冠军.工程陶瓷材料[M].西安:西安交通大学出版社,2000. JIN Zhihao,GAO Jiqiang,QIAO Guanjun.Engineering Ceramics[M].Xi’an:Xi’an Jiaotong University Press,2000.(in Chinese)
[12] 徐进,宋玉泉.结构陶瓷超塑性的研究[D].长春:吉林大学,2005. XU Jin,SONG Yuquan.A Study of Superplasticity of Structural Ceramics[D].Changchun:Jilin University,2005.(in Chinese)
[13] 宋玉泉,刘颖,徐进,等.结构陶瓷及其超塑性[J].金属学报,2009,45(1):6. SONG Yuquan,LIU Ying,XU Jin,et al.Structural Ceramics and Their Superplasticity[J].Acta Metallurgica Sinica,2009,45(1):6.(in Chinese)
[14] 林振汉,张玲秀,陆芝华.氧化锆基系的相变和稳定化作用的研究[J].上海金属:有色分册,1988,9(2):27. LIN Zhenhan,ZHANG Lingxiu,LU Zhihua.Study of Phase Change and Stabilization in Zirconia Base Systems[J].Shanghai Metal:Nonferrous Volume,1988,9(2):27.(in Chinese)
[15] 尹衍升,陈守钢,刘英才.氧化锆陶瓷的掺杂稳定及生长动力学[M].北京:化学工业出版社,2004. YIN Yansheng,CHEN Shougang,LIU Yingcai.Stability and Growth Kinetics of Zirconia Ceramics [M].Beijing:Chemical Industry Press,2004. (in Chinese)
[16] 林振汉.氧化锆材料的特性及在结构陶瓷中的应用和发展[J].稀有金属快报,2004,23(6):6. LIN Zhenhan.Characteristics of Zirconia Materials and the Application and Development of Structural Ceramics[J].Rare Metal Letters,2004,23(6):6. (in Chinese)
[17] 于维平,李凡,孟琳.双等离子体微弧沉积ZrO2和Y2O3-ZrO2陶瓷涂层[J].材料通报,2001,46(20):1754. YU Weiping,LI Fan,MENG Lin.Ceramic Coatings of ZrO2and Y2O3-ZrO2by Double Plasma Micro-arc Deposition[J].Material Bulletin,2001,46(20):1754.(in Chinese)
[18] 骆海贺.AZ91D镁合金微弧氧化Y2O3-ZrO2复合陶瓷膜层的制备、表征及性能研究[D].武汉:华中科技大学,2009. LUO Haihe.Preparation,Characterization and Performances of Y2O3-ZrO2Composite Ceramic Coatings on AZ91D Magnesium Alloy by Microarc Oxidation[D].Wuhan:Huazhong University of Science and Technology,2009.(in Chinese)
[19] 刘峰,蔡启舟.AZ91D镁合金微弧氧化Y2O3-ZrO2-MgO复合膜的制备及性能研究[D].武汉:华中科技大学,2012. LIU Feng,CAI Qizhou.Preparation and Performances of Y2O3-ZrO2-MgO Composite Ceramic Coatings on AZ91D Magnesium Alloy by Micro-arc Oxidation[D]. Wuhan:Huazhong University of Science and Technology,2012.(in Chinese)
[20] LIU X H,ZHU L Q,LIU H C,et al.Investigation of MAO Coating Growth Mechanism on Al Alloy by Two-step Oxidation Method[J].Applied Surface Science,2014,293:12.
[21] 张静,刘向东,刘晓丽,等.稀土对ZAlSi12Cu2Mg1陶瓷层性能的影响[J].特种铸造及有色合金,2007,27 (12):966. ZHANG Jing,LIU Xiangdong,LIU Xiaoli,et al.The Influence of Rare Earth on Performance of ZAlSi12Cu2Mg1Ceramic Layer[J].Special Casting and Nonferrous Alloys,2007,27(12):966.(in Chinese)
[22] 张圣麟,岳小钦,陈燕燕.氧化钇对6061铝合金磷化膜性能的影响[J].腐蚀与防护,2009,30(6):398. ZHANG Shenglin,YUE Xiaoqin,CHEN Yanyan. Effect of Yttrium Oxide on Phosphate Coating Per-formance on 6061 Al Alloy[J].Corrosion&Protection,2009,30(6):398.(in Chinese)
[23] 路新瀛,梁开明,顾守仁,等.氧空位对氧化锆相结构稳定性及相变过程的影响[J].硅酸盐学报,1996,24 (6):670. LU Xinying,LIANG Kaiming,GU Shouren,et al. Influence of Oxygen Vacancy on Phase Structure and Transformation of Zirconia[J].Journal of the Chinese Ceramic Society,1996,24(6):670. (in Chinese)
[24] CURRAN J A,CLYNE T W.The Thermal Conductivity of Plasma Electrolytic Oxide Coatings on Aluminium and Magnesium[J].Surface and Coatings Technology,2005,199(2/3):177.
[25] 牟仁德,陶春虎,陆峰.热障涂层隔热性能研究[D].北京:北京航空材料研究院,2007. MOU Rende,TAO Chunhu,LU Feng.Investigation of Thermal Insulation Effect on Thermal Barrier Coatings[D].Beijing:Beijing Institute of Aeronautical Materials,2007.(in Chinese)
(责任编辑、校对 张 超)
简 讯
ELID超精密镜面磨削设备
在线电解砂轮修整(Electrolytic In-process Dressing,ELID)磨削技术是适应现代化高技术发展需要而发展起来的一种机械加工新工艺,其集成了现代机械、液压、光学、电子、计算机、计量及材料等先进技术成就,被国际上公认为是最有前途的超精密镜面磨削方法。
西安工业大学在对氧化膜产生机理和电解参数对氧化膜生成影响规律的研究积累基础上,提出利用现代测试和计算机控制技术,对磨削过程中的氧化膜厚度进行在线测量,并通过计算机比较分析后对电解参数进行在线控制,使得氧化膜在磨削过程中保持恒定,研制出了ELID超精密镜面磨削设备,该设备成功实现了镜面磨削,提高了磨削过程的稳定性,具有磨削效率高、工艺过程简单等优点,从而大大缩短达到纳米表面的加工时间,提高了纳米表面生成效率,突破了该项技术在生产实践中的效率瓶颈,填补了国内空白,对提高我国的超精密加工技术起到重要的作用。
该设备具有良好的性价比,可广泛应用于光学玻璃、工程陶瓷、半导体材料、单晶材料及硬质合金等先进材料的精密与超精密加工,促进这些材料在高技术领域的应用,其在工程实际中的应用极大地改善了相关产品性能与可靠性。由西安工业大学开发的ELID超精密镜面磨削设备,形成批量生产后预计售价为40万元人民币左右,而在实现同等功能的情况下,国外公司的产品价格却非常昂贵,基本售价在9~18万美元之间。如日本THE NEXSYS CORPORATION生产的UVG-380超精密磨削设备售价为18万美元。该型设备的研制成功必将促进我国军工行业加工技术水平的进步,对提高我国高技术产品的制造能力,降低制造成本,提高产品在国际市场的竞争力都具有极其重要的意义。
(张立新)
Study of Microstructure and Heat Insulation Properties of PEO Ceramic Coatings Formed in Electrolyte With Y(NO3)3
ZHAO Yuhou,HAN Jing,WANG Ping
(School of Materials and Chemical Engineering,Xi’an Technological University,Xi’an 710021,China)
Abstract:Effects of Y(NO3)3in electrolyte on the microstructure,growth rate and heat insulation properties of PEO ceramic coatings were investigated by preparing ZrO2-Al2O3ceramic coating and Y2O3-ZrO2-Al2O3ceramic coating on Al-Si alloys through plasma electrolytic oxidation(PEO)technique in zirconium salt solution and zirconium yttrium salt solution,respectively.The surface and cross-sectionbook=132,ebook=49morphologies and the phase composition of the coatings were characterized by environmental scanning electron microscope(ESEM)and X-ray diffraction(XRD).The thicknesses in different stages of reaction were measured by eddy current thickness meter to study the growth rate of the coatings formed in the two different solution systems.Last,the heat insulation properties were tested through self-made heat insulation testing device.The result indicated:The surface of the ZrO2-Al2O3ceramic coating was composed of cystiform sintered melts,and was rather coarse with many discharge channels of large diameter scattered on it evenly.Its thickness was about 20μm.However,the surface of the Y2O3-ZrO2-Al2O3ceramic coating was smooth and dense,which was consisted of fine particles.And the thickness of it increased to around 28μm.Both of the two kinds of ceramic coatings included phases of ZrO2and Al2O3,and the diffraction peaks appeared the phenomenon of“steamed bun peak”in 2θrange of about 20°~30°,which indicated apparently a certain proportion of amorphous components.Moreover,solid solution of yttrium partially stabilized zirconium(Y2O3and Y0.15Zr0.85O1.93)also was found in the coating treated in zirconium yttrium salt solution.The Y2O3-ZrO2-Al2O3ceramic coating displayed heavier" steamed bun peak"phenomenon,meant higher content of amorphous components in it,also suggested that the addition of Y(NO3)3in the electrolyte improved the reaction temperature.The result of growth rate and mode showed that the Y2O3-ZrO2-Al2O3ceramic coating growed faster than ZrO2-Al2O3ceramic coating,mainly due to the obviously increased thickness of the outward growth.Finally,the heat insulation property test concluded that the heat insulation temperature of ZrO2-Al2O3ceramic coating and Y2O3-ZrO2-Al2O3ceramic coating was 45.9℃and 53.4℃,respectively,which illustrated the latter one had a better heat insulation effect.
Key Words:MAO;Al-Si alloys;ceramic coating;electrolyte;heat insulation properties
作者简介:赵玉厚(1961—),男,西安工业大学教授,主要研究方向为铝基复合材料.E-mail:zhyh01@163.com.
基金资助:国家自然科学基金项目(51401155;51201120);陕西省自然科学基础研究计划项目(2015JQ5175);国家重点基础研究发展计划(2012CB619602-3;2012CB619606-2);陕西省教育厅项目(2012JC13)
*收稿日期:2015-07-24
DOI:10.16185/j.jxatu.edu.cn.2016.02.008
文献标志码:中图号: TG174.4 A
文章编号:1673-9965(2016)02-0131-08

