Potent water extracts of Indonesian medicinal plants against PTP1B
Potent water extracts of Indonesian medicinal plants against PTP1B
Tel: +62 271 717417x167
Fax: +62 271 715448
E-mail: azis.saifudin@ums.ac.id
Peer review under responsibility of Hainan Medical University.
Foundation Project: Supported by Faculty of Pharmacy, Universitas Muhammadiyah Surakarta (Ref.001/DM-I /FF/2014).
Azis Saifudin1*, Tepy Usia2, Subehan AbLallo3, Hiroyuki Morita4, Ken Tanaka5, Yasuhiro Tezuka61Faculty of Pharmacy, Universitas Muhammadiyah Surakarta, Pabelan, KTS Solo, Jawa Tengah 57102, Indonesia
2Research Center of National Drug and Food Control, Jl. Percetakan Negara No. 23, Jakarta 10560, Indonesia
3Faculty of Pharmacy, Hasanuddin University, Tamalanrea No. 9, Makassar, Sulawesi Selatan 90245, Indonesia
4Division of Natural Products Chemistry, Institute of Natural Medicine, University of Toyama, Toyama, 2630 Sugitani, Toyama 930-0194, Japan
5Department of Pharmacognosy, College of Pharmaceutical Science, Ritsumeikan University, 1-1-1 Nojihigashi,
Kusatsu, Shiga 525-8577, Japan
6Faculty of Pharmaceutical Sciences, Hokuriku University, Ho-3, Kanagawa machi, Kanazawa 920-1181, Japan
ARTICLE INFO
Article history:
Received 30 Jul 2015
Received in revised form 17 Aug 2015
Accepted 28 Sep 2015
Available online 10 Nov 2015
Keywords:
Water extract
Indonesian medicinal plants
PTP1B inhibitor
Diabetes
Carbohydrate
Nuclear magnetic resonance
ABSTRACT
Objective: To examine the potent of water as a solvent agent in the preparation of traditional herbal medicine.
Methods: Water extracts of 18 plants were prepared through reflux and examined (25 μg/mL) to evaluate their possibility for inhibiting protein tyrosine phosphatase 1B (PTP1B). The determination of IC50values was performed for the samples possessing more than 80% inhibition. Meanwhile, those exhibiting IC50values more than 7.0 μg/mL were further profiled for their chemical constituents through nuclear magnetic resonance (NMR) measurement.
Results: About 44% (8) of the examined samples showed more than 80% inhibition against PTP1B. The water extracts of Elephantopus scaber, Helicteres isora aerial parts, Elaeocarpus grandiflorus (E. grandiflorus) fruits, Melaleuca leucadendron leaves, and Quercus infectoria gum had IC50values ranging from 2.05 to 6.90 μg/mL. Meanwhile, Andropogon nardus and Centella asiatica were at the area of δ 3.0–4.0 ppm. Further, the13C NMR observation of samples possessing the most intensive signals in their proton NMR Cinnamomum burmannii and E. grandiflorus showed the peaks at the area of δ 60–90 ppm as the supportive evidence for sugar group signals. Intriguingly, a disaccharide from E. grandiflorus could be an active inhibitor towards PTB1B.
Conclusions: In contrast to the mainstream solvents currently used in modern herbal manufactures especially Jamu medicine in Indonesia, pure-water-extracted materials should be reconsidered and could be reemerged for future studies and for the manufacture of herbal medicines. In addition, the activity of Jamu components should be confirmed that their antidiabetes and antiobesity activities could be through the inhibition of PTP1B.
1. Introduction
Traditional medicines e.g. medicinal plants have been embedded in human civilization. Indonesian herbal preparation, locally called Jamu medicine, remains popular to date. Its preparation was originally done by crushing or squeezing or boiling herbal materials in water. In the past, people and traditional societies mainly employed water as the extracting solvent [1]. In this way, water soluble matters became the main constituent of traditional medicines. In two decades, Jamu has been formulated and prepared in modern dosage forms. Jamu industries have also recently been thriving very well. At the main time, Jamu medicines industries have been thriving very well in two decades. As revealed by Indonesian Ministry ofHealth through health basic research report, 49% of 240 million Indonesian population is Jamu users[2].
The introduction of organic solvents for herbal extraction and support of official guiding monographs [3,4], however, has indirectly encouraged the ethanol usage in industrial field as well as in academic activities. Indeed, this became a hall mark of an era where a vast majority of registered Jamu products in the market are prepared from ethanol extract (ethanol 50%–96%). The supportive advantages of using ethanol are in view of its fast drying process, less toxic content, and simplicity to avoid microbial growth in comparison to water. This phenomenon, as a consequence, has marginalized the water utility in herbal extraction in the last two decades. Previously, Jamu was prepared mainly as recenter paratus by infusion or decoction [5], in which water soluble matters were the main component. In other words, representing herbal medicines with organic solvent extracts is most likely not factual and could be biased from its historical traditions.
To confirm the nature of traditional preparation of herbal medicines through water solvent extraction, 18 water extracts referred in traditional formulation records of Jamu components for antidiabetes or antiobesity: Abrus precatorius L. (A. precatorius), Acarus calamus L. (A. calamus), Andropogon nardus L. (A. nardus), Centella asiatica L. (C. asiatica), Coriandrum sativum L. (C. sativum), Elaeocarpus grandiflorus Smith (E. grandiflorus), Elephantopus scaber L. (E. scaber), Helicteres isora L. (H. isora), Imperata cylindrica (L.) P. Beauv (I. cylindrica), Kaempferia galanga L. (K. galanga), Melaleuca leudendron L. (M. leudendron), Myristica fragrans (Houtt.) (M. fragrans), Nigella sativa L. (N. sativa), Pandanus amaryllifolius Roxb. (P. amaryllifolius), Parkia roxburghii G. Don. (P. roxburghii), Phyllanthus niruri L. (P. niruri), Quercus infectoria (Oliver) (Q. infectoria), and Trigonella foenum-graecum L. (T. foenum-graecum) were submitted for test against protein tyrosine phosphatase 1B (PTP1B) as a molecular target model. PTP1B is a validated negative insulin regulator in its main targeted organs, i.e. lever, kidney, and skin [6–8]. Meanwhile, excessive PTP1B in brain will impair the signaling of leptin, a major appetite regulator [9,10]. Here, the samples showing more than 80% inhibitory activities were further determined for their IC50values. To observe chemical constituent groups which could be possible corresponding for this bioactivity, a chemical profiling was conducted by using proton and carbon nuclear magnetic resonance [1H/13C nuclear magnetic resonance (NMR)]. NMR spectrometry is a reliable and comprehensive method for being able to provide a“holistic view”[11] compared to the other customized analytical methods such as liquid or gas chromatography, infrared spectrometry, and mass spectrometry. The area of diagnostics, i.e. amino acids (δ 2–2.8 ppm), sugars (δ 3–4 ppm), and aromatic regions (δ 6–8 ppm) that NMR allows us to specifically distinguish the peaks of interest. It is robust, no need for calibration, indestructible towards analyses, and efficiency e.g. 10 min for data acquisition. In a course of studies for finding PTP1B inhibitors, water extract of Blumea balsmifera leaves, Cinnamomum burmannii (C. burmannii) barks, Syzygium aromaticum fruits, and Syzygium polyanthum leaves exhibited some potent activities with IC50values of 2.68, 6.25, 4.07, and 3.14 μg/mL, respectively [12]. Moreover, they have been recorded in Jamu formulations for the treatment of diabetes and obesity as well [13,14]. In this paper, we also report their chemical constituent profiles based on NMR spectra.
2. Materials and methods
2.1. Herbal materials
The herbal materials used in this study (Table 1) were purchased from Pasar Genteng Surabaya and authenticated by one of the authors (Dr. Subehan). The voucher specimens were preserved in Museum of Materia Medica, Analytical Research Center for Ethnomedicines, Institute of Natural Medicine, University of Toyama, Toyama, Japan.
2.2. Preparation of test solutions
Each medicinal herb (25–150 g) was cut into small pieces and extracted with water (150–400 mL, 1-h reflux, 100°C,×2). After filtration, the water solution was concentrated under a reduced pressure, followed by lyophilization, to result in a water extract. All extracts were stored under−80°C before being in use. For the PTP1B inhibitory assay, the extracts were dissolved in distilled water at the concentration of 1 mg/mL and aliquots of the solution were added to each well. The final concentration for each sample was 25 μg/mL.
2.3. PTP1B inhibitory assay
The assay was performed in 96-well clear polystyrene microplate (Corning, NY, USA) according to Cui et al. with slight modifications [21]. Each well contained 0.05 μg PTP1B (Enzo Life Sciences, Farmingdale, NY), 2 mmol/L pnitrophenyl phosphate (pNPP; Wako Pure Chemical Industries, Osaka, Japan), and 50 mmol/L citrate buffer containing 0.1 mmol/L NaCl, 1 mmol/L dithiothreitol, and 1 mmol/L ethylenediamine-N,N,N',N'-tetraacetic acid. The final volume of the mixture was 200 μL. The reaction was initiated by the addition of pNPP, incubated at 37°C for 30 min, and terminated with the addition of a stop solution (10 mol/L NaOH). The amount of p-nitrophenol produced was estimated by measuring the absorbance at 405 nm using a Perkin-Elmer HTS 7000 bioassay reader. To identify the level of nonenzymatic substrate hydrolysis, the absorbance of wells only containing buffer and pNPP were measured for correction. The difference between full enzymatic activity and the correction was set arbitrarily to 100% activity. The percent residual activity was calculated using the following formula:

where Abs(full enzymatic) was the absorbance of p-nitrophenol liberated by the enzyme in the system without a test sample in contrast to Abs(test sample). The assays were performed in triplicate for all samples. The recognized phosphatase inhibitors, RK-682 (purity 98%; Enzo Life Sciences) [22] and ursolic acid (purity 90%; Tokyo Chemical Industry, Tokyo, Japan) [23] were used as the positive controls.
2.4. Statistical analyses
IC50values were obtained by calculating the linear regression using MS Excel. A number of statistical differences were thencompared using ANOVA with Tukey's post-hoc test. P values<0.05 were considered to be significant.
2.5. NMR measurement
NMR spectra were measured using a JEOL JNM-LA400 spectrometer. Chemical shifts were given in the δ scale. Sample for measurement was 15–20 mg and dissolved up to 0.8 mL with D2O (Cambridge Isotope Lab, UK). The NMR chemical shifts in the spectra were referenced to 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid at 0.00 ppm. For1H NMR measurement, each spectrum consisted of 96 scans of 32768 points. On the other hand,13C NMR of Eucalyptus floribunda and C. burmannii measurements consisted of 43478 and 28553 scans, respectively.
3. Results
3.1. PTP1B inhibitory activity
Among the extracts listed in Figure 1, A. nardus (85.2%), C. asiatica aerial parts (83.3%), E. grandiflorus fruits (96.4%), E. scaber leaves (96.4%), H. isora gum (95.9%), Melaleuca leucadendron (M. leucadendron) leaves (96.0%), P. niruri (83.3%), and Q. infectoria (94.7%) exhibited more than 80% inhibition at the concentration of 25 μg/mL. Meanwhile, the positive controls RK-682 and ursolic acid exhibited 98.8% inhibition.
In contrast, P. roxburghii (65.4%) extract showed a moderate activity. The extracts of A. precatorius (6.6%), A. calamus (21.6%), C. sativum (12.1%), I. cylindrical (18.1%), and P. amaryllifolius (17.6%), meanwhile, exhibited mild activities (2.3%–21%). Their tested solutions formed darker color producinghigherabsorbancevaluescomparedtothe blanksolutions, K. galanga, N. sativa and T. foenum-graecum seed that showed minus inhibitions, ranging from−14.2% to−0.6%. However, the negative values were justified as the inactive samples. This indicates that the activity mechanisms of these recorded components, both as antidiabetes and as obesity remedies, might be performed through other molecular targets or pathways.
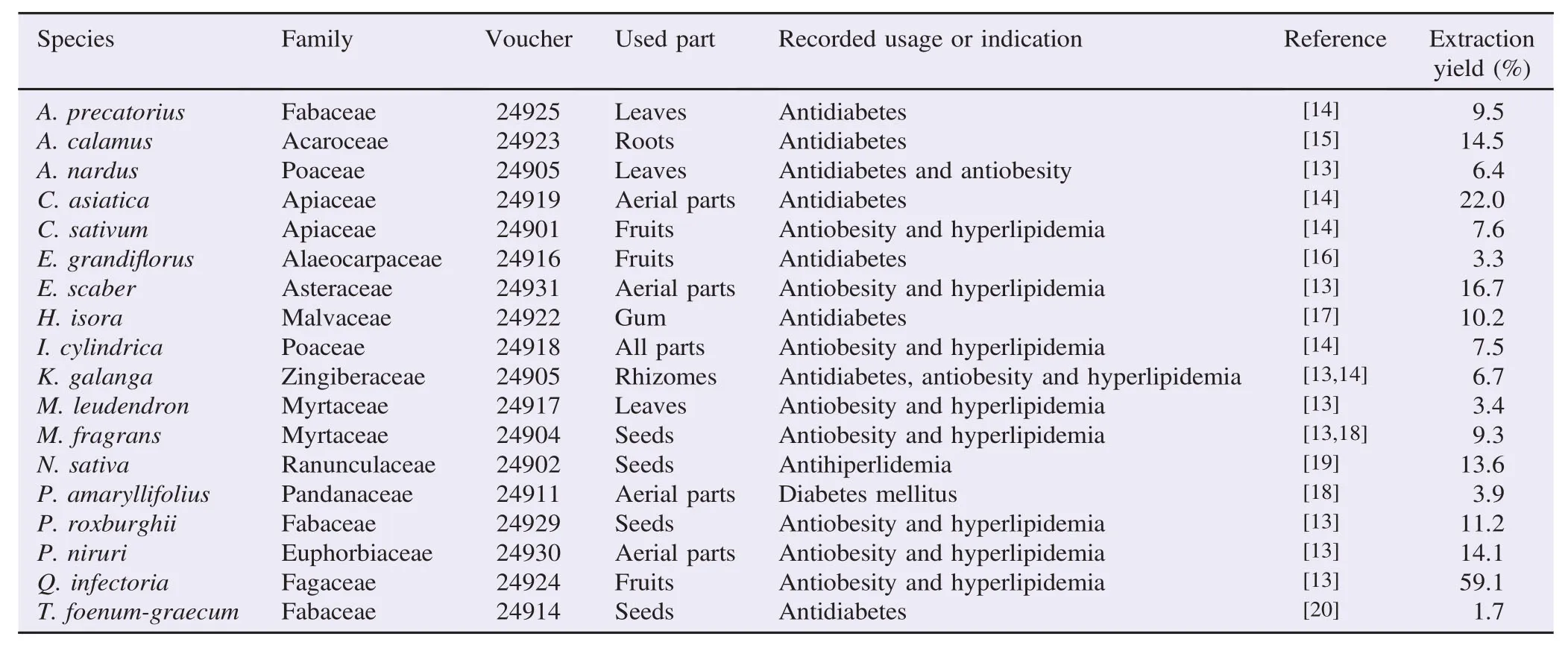
Table 1Indonesian medicinal plants, used part and usage or indication.
Further IC50examination of the samples showing more than 80% inhibitions, they demonstrated a dose dependent inhibitions with IC50values ranging from 2.05 to 13.20 μg/mL (Table 2). In particular, M. leucadendron was the most potent sample demonstrating the IC50value equal to that of the positive control RK-682 (2.05 μg/mL) and more potent compared to ursolic acid (IC50= 9.11 μg/mL). Interestingly, those potent samples were made up 44% of examined samples. Hence, their traditionally reported antidiabetes and obesity activity could be due to PTP1B inhibition.
3.2. Analyses of chemical composition of water extract
To observe the chemical constituents that could be possible to be a corresponding constituent to inhibitory activity, NMR measurement was conducted towards those potent samples. The area of diagnostics i.e. amino acids, sugars, vinyl, and aromatic chemical shift zones, both1H and13C NMR spectra that allowed us to specifically distinguish the peaks of interest. According1H NMR spectra, those extracts exhibited some dominant peaks ranging from δ 3–4.2 ppm (Figure 2).
Therefore, most of active samples showed the peaks of oxygenated methylene protons called as sugar zone. Although Blumea balsamifera and H. isora spectra showed some aliphatic signals at δ 1.2–2.6 ppm and vinyl peak signals at δ 6–7 ppm which were attributable as aliphatic and aromatic of amino acid signals, these peaks exhibited several short intensities which could be present in low quantity. For this reason, sugars must be present in much higher concentration in all active samples and contributed significantly to their activity. Meanwhile, E. scaber, A. nardus, and M. dendron extracts had some comparable intensities in both sugar and amino acid peak signals (δ 1.2–2.5 ppm) (Figure 2b, c and f). In an aqueous extract, sugars were found usually including fructose, sucrose, and glucose. Differently, amino acids frequently present are arginine, alanine, glutamate, serine, glutamine, asparagine, histidine, proline, and valine. The available organic acids included malic, ascorbic, citric, and succinic acid [24].
Considering our experiences that sugars normally have a low signal intensity in13C NMR measurements, we then choseC. burmannii and E. grandiflorus samples possessing intensive1H NMR spectra to measure. Their spectra confirmed the presence of sugar carbon signals at δ 60–90 ppm. Although13C NMR of C. burmannii showed three peaks between δ 90–105 ppm and two short peaks at δ 145 and 152 ppm, respectively, these peaks should be the part of sugar structures (Figure 3). Interestingly,1H and13C NMR spectrum of E. grandiflorus clearly revealed eleven carbon signals at δ 60.1, 62.9, 63.2, 64.2, 64.7, 70.0, 71.8, 73.2, 73.4, 74.8, 77.2, 82.2, and 104.2 ppm (Figure 3d), which were similar to those of sucrose[25]. Moreover, its1H NMR signal at δ 5.2 ppm (Figure 3c, arrow) also showed a good agreement[11,24].

Figure 1. PTP1B residual activity due to the effect of water extract of Indonesian medicinal plants at a concentration of 25 μg/mL.RK-862 and ursolic acid were used as the positive controls. Residuals higher than 100% were related to a darker tested solution resulted by three samples. Data points represent mean±SEM; n = 3. A: RK-862; B: Ursolic acid; C: A. precatorius; D: A. calamus; E: A. nardus; F: C. asiatica; G: C. sativum; H: E. grandiflorus; I: E. scaber; J: H. isora; K: L. cylindrica; L: K. galanga; M: M. leudendron; N: M. fragrans; O: N. sativa; P: P. amaryllifolius; Q: P. roxburghii; R: P. niruri; S: Q. infectoria; T: T. foenum-graecum.
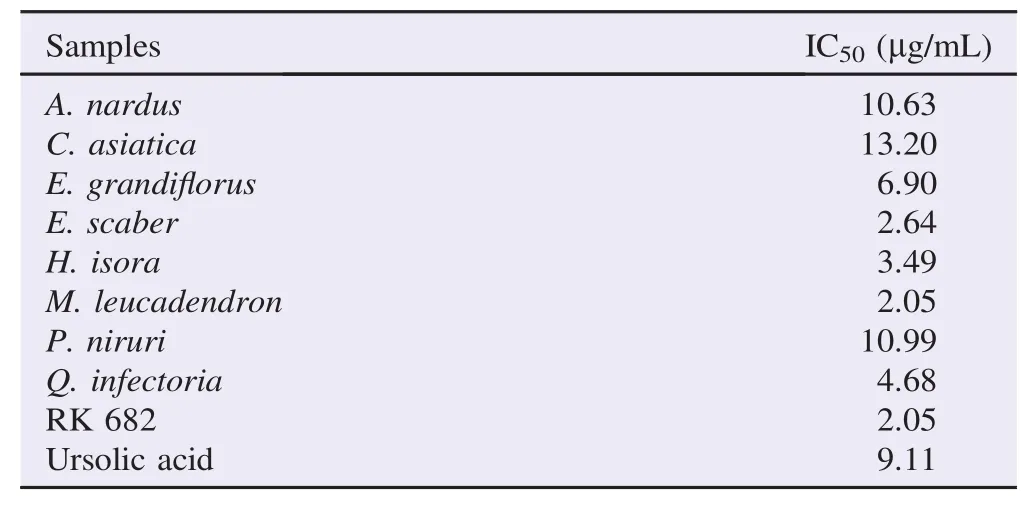
Table 2Samples with≥80% inhibition and their IC50values.
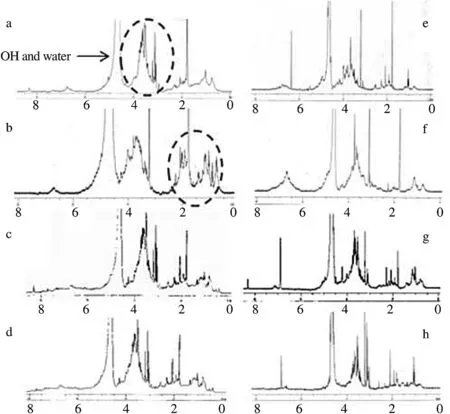
Figure 2. The representative1H NMR spectra of several water plant extracts.1H NMR spectrum of a: A. nardus; b: E. scaber; c: P. niruri; d: Syzygium aromaticum; e: Blumea balsamifera leaves; f: H. isora fruits; g: M. leucadendron; h: Syzygium polyanthum leaves. All samples showed sugar proton signals at δ 3.0–4.5 ppm. It was only E. scaber that showed a comparable intensity of amino acid signals at δ 1.5–2.5 ppm. Internal standard (3-(trimethylsilyl) propionic-2,2,3,3-d4 acid).
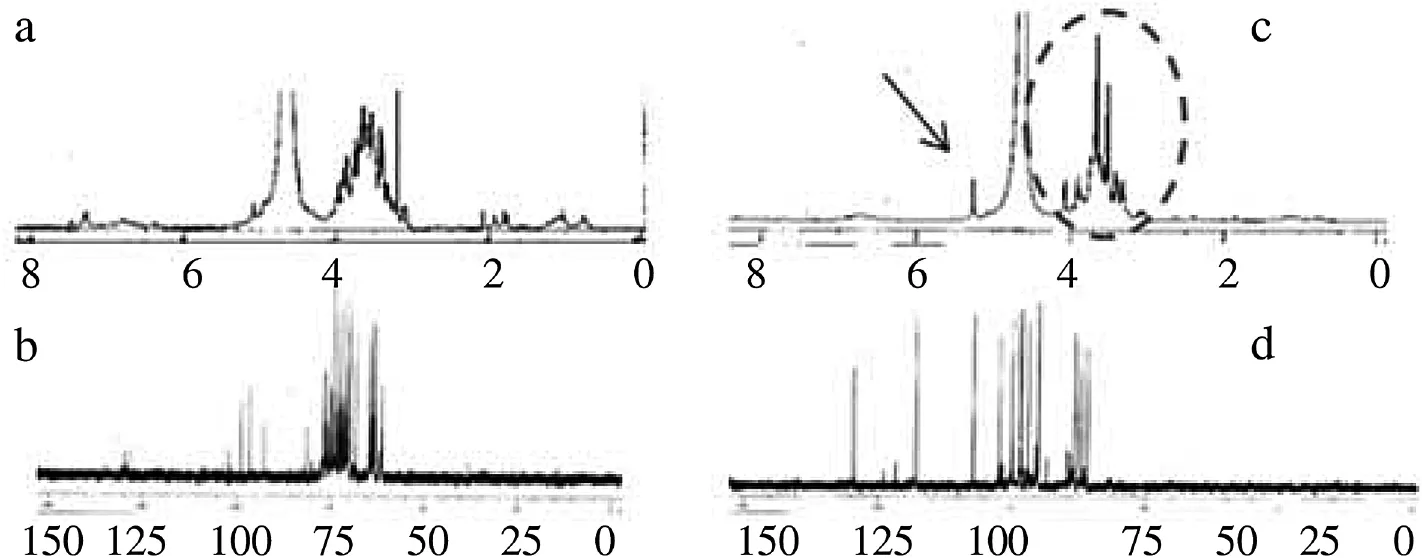
Figure 3. The representative NMR spectra of water extracts of C. burmannii and E. grandiflorus.a and b:1H and13C NMR spectra of C. burmannii leaves; c and d:1H and13C NMR spectra of E. grandiflorus fruits.
Therefore, on the basis of both NMR spectra the compound was deduced as a disaccharide which could be a succrose. The sugar remarkably showed 95% purity according to this 1D NMR analyses. Hence, this simple sugar should be the main contributor towards its extract activity. However, this case should not be taken into account for in vivo case immediately.
4. Discussion
This study was embarked from a very simple hypothesis that water extracts contain a group of polar compounds rather than secondary metabolites. Thus, based on the fact, traditional preparation have been relaying on water extraction. The water extracts of all 18 samples of Indonesian medicinal plants recorded in Jamu formulations or reported as antidiabetes or antiobesity were examined for their ability to inhibit PTP1B-mediated dephosphorylation of pNPP. Based on NMR analyses, it is convincing that instead of secondary metabolites, the activity of original Jamu medicines is due to the significant contribution of sugars or synergism or plausible effect among sugars, amino acids, and organic acids. Hence, water extracted herbal medicine has a distinguished paradigm and secondary metabolite based on Jamu medicine is another paradigm.
To date, the reports on the activities of carbohydrate class on antidiabetes related target via the inhibitory of PTP1B are still limited. It is just recently reported that saccharide from Astragalus membranaceus inhibits PTP1B and decreases PTP1B expression via relieving endoplasmic reticulum stress [26]. Saccharides from Morus alba leaves exhibited the hepatic glucose metabolism and insulin signaling by inhibiting the expression of PTP1B [27]. Acidic proteoglycan from fungi Ganoderma lucidum shows the competitively inhibiting PTP1B [28,29]. However, rather than saccharides, micromolecules have been becoming an interest in hitherto PTP1B inhibitor discoveries [30–32]. Thus, carbohydrates could also be a promising candidate to explore in future studies. Previously, we demonstrated the pairs of organic and water extracts in total of 56 extracts [12]. Of these extracts, 9 water extracts and 11 methanol extracts showed more than 70% inhibition with IC50values ranging from 2.05 to 13.4 μg/mL. From the methanol extract, we isolated the active secondary metabolite type comprising sequiterpens, triterpenes, and phloroglucinols. Hence, in our current study, the water extracts activity was contributed significantly by sugars and polar matters. Unfortunately, the use of pure water as a solvent is almost absent in the official guidances for industrial scale issued by Indonesian Drug and Food Control Agency [33]. Therefore, to some extent it should be revised based on Jamu historical preparations.
On the basis of the findings, water soluble extracts should not be ignored from utilities. It can be noted here that the paradigm of representing traditional medicine preparations with organic solvents seem to be inappropriate. For modern herbal manufacturers, reintroducing water as an extracting solvent should be considered. Although prepared in organic or water solvent, Jamu medicine must be simultaneously and scientifically confirmed regarding their pharmacological effect and chemical constituents. Finally, it is worth noting that the results of 44% those potent water extracts should also confirm the possibility of recorded Jamu components for antidiabetes and obesity through the inhibition of PTP1B activity.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors are grateful to Faculty of Pharmacy, Universitas Muhammadiyah Surakarta for financial assistantship (Ref.001/ DM-I /FF/2014) Also, we thank Indonesian Pharmacist Association Central Java Branch for donating Jamu Formulation Records II and III.
References
[1] Sujarwo W, Keim AP, Savo V, Guarrera PM, Caneva G. Ethnobotanical study of Loloh: traditional herbal drinks from Bali (Indonesia). J Ethnopharm 2015; 169: 34-48.
[2] Trihono. [Research on basic health]. Jakarta: Ministry of Health; 2013,p.47.Indonesian.[Online]Availablefrom:http://www.depkes. go.id/resources/download/general/Hasil%20Riskesdas%202013. pdf [Accessed on 15th August, 2015]
[3] Pharmacopeia of Indonesia Commission. [Pharmacopeia of Indonesia]. 4th ed. Jakarta: Pharmacopeia of Indonesia Commission; 1995, p. 9. Indonesian.
[4] Kustantinah Aspan R, Wahyu H. Monograph of Indonesia medicinal plant extracts. Vol. 1. Jakarta: Indonesia National Agency of Drugs and Food Control; 2010.
[5] Elfahmi, Woerdenbag HJ, Jamu Kayser O. Indonesian traditional herbal medicine towards rational phytopharmacological use. J Herb Med 2014; 4(2): 51-73.
[6] Gonz´alez-Rodríguez´A, Gutierrez JA, Sanz-Gonz´alez S, Ros M, Burks DJ, Valverde´AM. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 2010; 59: 588-99.
[7] Ma YM, Tao RY, Liu Q, Li J, Tian JY, Zhang XL, et al. PTP1B inhibitor improves both insulin resistance and lipid abnormalities in vivo and in vitro. Mol Cell Biochem 2011; 357: 65-72.
[8] Panzhinskiy E, Ren J, Nair S. Pharmacological inhibition of protein tyrosine phosphatase 1B: a promising strategy for the treatment of obesity and type 2 diabetes mellitus. Curr Med Chem 2013; 20: 2609-25.
[9] Lantz KA, Hart SG, Planey SL, Roitman MF, Ruiz-White IA, Wolfe HR, et al. Inhibition of PTP1B by trodusquemine (MSI-1436) causes fat-specific weight loss in diet-induced obese mice. Obes (Silver Spring) 2010; 18(8): 1516-23.
[10] Yip SC, Saha S, Chernoff J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem Sci 2010; 35(8): 442-9.
[11] Kim HK, Choi YH, Verpoorte R. NMR-based plant metabolomics: where do we stand, where do we go? Trends Biotechnol 2011; 29: 267-75.
[12] Saifudin A, Kadota S, Tezuka Y. Protein tyrosine phosphatase 1B inhibitory activity of Indonesian herbal medicines and constituents of Cinnamomum burmannii and Zingiber aromaticum. J Nat Med 2013; 67: 264-70.
[13] Gunawan H, Prabowo A, Triwara B, Rahayu NK. [Natural medicines list]. 2nd ed. Semarang: Indonesian Pharmacist Association; 2006, p. 13-26. Indonesian.
[14] Gunawan H, Prabowo A, Triwara B, Rahayu NK. [Natural medicines list]. 3rd ed. Semarang: Indonesian Pharmacist Association; 2008, p. 2-21. Indonesian.
[15] Wu HS, Zhu DF, Zhou CX, Feng CR, Lou YJ, Yang B, et al. Insulin sensitizing activity of ethyl acetate fraction of Acorus calamus L. in vitro and in vivo. J Ethnopharmacol 2009; 123: 288-92.
[16] Bualee C, Ounaroon A, Jeenapongsa R. Antidiabetic and long-term effectsofElaeocarpusgrandiflorus.NaresuanUnivJ2007;15:17-28.
[17] Kumar G, Murugesan AG. Hypolipidaemic activity of Helicteres isora L. bark extracts in streptozotocin induced diabetic rats. J Ethnopharmacol 2008; 116: 161-6.
[18] Arlina SH. [Simple and affordable in coping with various diseases]. Depok Jakarta: Agromedia Pustaka; 2003, p. 74. Indonesian.
[19] Mahendra B. [Guidelines to blend plants]. Jakarta: Penebar Swadaya; 2006, p. 76. Indonesian.
[20] Abdel-Barry JA, Abdel-Hassan IA, Al-Hakiem MH. Hypoglycemic and antihyperglycaemic effects of Trigonella foenum-graecum leaf on normal and alloxan induced diabetic rats. J Ethnopharmacol 1997; 58: 149-55.
[21] Cui L, Na M, Oh H, Bae EY, Jeong DG, Ryu SE, et al. Protein tyrosine phosphatase 1B inhibitors from Morus alba root bark. Bioorg Med Chem Lett 2006; 16: 1426-9.
[22] Carneiro VM, Trivella DB, Scorsato V, Beraldo VL, Dias MP, Sobreira TJ, et al. Is RK-682 a promiscuous enzyme inhibitor? Synthesis and in vitro evaluation of protein tyrosine phosphatase inhibition of racemic RK-682 and analogues. Eur J Med Chem 2015; 97: 42-54.
[23] Baumgartner RR, Steinmann D, Heiss EH, Atanasov AG, Ganzera M, Stuppner H, et al. Bioactivity-guided isolation of 1,2,3,4,6-Penta-O-galloyl-d-glucopyranose from Paeonia lactiflora roots as a PTP1B inhibitor. J Nat Prod 2010; 73: 1578-81.
[24] Kim HK, Choi YH, Verpoorte R. NMR-based metabolomics analysis of plants. Nat Protoc 2010; 5: 536-49.
[25] Kang J, Choi MY, Kang S, Kwon HN, Wen H, Lee CH, et al. Application of a 1H nuclear magnetic resonance (NMR) metabolomics approach combined with orthogonal projections to latent structure-discriminant analysis as an efficient tool for discriminating between Korean and Chinese herbal medicines. J Agric Food Chem 2008; 56: 11589-95.
[26] Zhao M, Zhang ZF, Ding Y, Wang JB, Li Y. Astragalus polysaccharide improves palmitate-induced insulin resistance by inhibiting PTP1B and NF-kB in C2C12 myotubes. Molecules 2012; 17: 7083-92.
[27] Ren C, Zhang Y, Cui W, Lu G, Wang Y, Gao H, et al. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int J Biol Macromol 2015; 72: 951-9.
[28] Pan D, Wang L, Hu B, Zhou P. Structural characterization and bioactivity evaluation of an acidic proteoglycan extract from Ganoderma lucidum fruiting bodies for PTP1B inhibition and antidiabetes. Biopolymers 2014; 101: 613-23.
[29] Wang N, Zhang D, Mao X, Zou F, Jin H, Ouyang J. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol Cell Endocrinol 2009; 307: 89-98.
[30] Combs AP. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J Med Chem 2010; 53: 2333-44.
[31] Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep 2012; 29: 580-606.
[32] Liu JZ, Zhang SE, Nie F, Yang Y, Tang YB, Yin W, et al. Discovery of novel PTP1B inhibitors via pharmacophore-oriented scaffold hopping from Ertiprotafib. Bioorg Med Chem Lett 2013; 23: 6217-22.
[33] Aspan R, Wahyu H. Monograph of Indonesian medicinal plant extracts. Jakarta: National Agency of Drugs and Food Control; 2014, p. 4-15.
Clinical research http://dx.doi.org/10.1016/j.apjtb.2015.10.005
*Corresponding author:Azis Saifudin, Faculty of Pharmacy, Universitas Muhammadiyah Surakarta, Pabelan, KTS Solo, Jawa Tengah 57102, Indonesia.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Healthcare waste management in selected government and private hospitals in Southeast Nigeria
- Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice
- Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
- Antifungal activity of plant extracts with potential to control plant pathogens in pineapple
- Pharmacological effects of ethanol extract of Egyptian Artemisia herba-alba in rats and mice
