Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice
Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice
Tel: +66 2 9269732
Fax: +66 2 9269711
E-mail: naowaboot@yahoo.com
Peer review under responsibility of Hainan Medical University.
Foundation Project: Supported by the research grant from the Faculty of Medicine, Thammasat University (Contract number: GEN2-05/2015).
Supaporn Wannasiri1, Pritsana Piyabhan1, Jarinyaporn Naowaboot2*1Division of Physiology, Department of Preclinical Science, Faculty of Medicine, Thammasat University (Rangsit Campus), Pathum Thani 12120, Thailand
2Division of Pharmacology, Department of Preclinical Science, Faculty of Medicine, Thammasat University (Rangsit Campus), Pathum Thani 12120, Thailand
ARTICLE INFO
Article history:
Received 20 Aug 2015
Receivedinrevisedform15 Sep2015
Accepted 8 Oct 2015
Available online 10 Nov 2015
Keywords:
Rhinacanthus nasutus
Obesity
Insulin sensitivity
Adiponectin
Peroxisome proliferators-activated receptor-α
Glucose transporter 4
ABSTRACT
Objective: To investigate the effect of Rhinacanthus nasutus (R. nasutus) leaf extract on impaired glucose and lipid metabolism in obese ICR mice.
Methods: Obesity was induced in the male ICR mice by feeding them a high-fat diet (60 kcal% fat) for 12 weeks. After the first six weeks of the diet, the obese mice were administered with the water extract of R. nasutus leaves at 250 and 500 mg/kg per day for the next six weeks. Subsequently, the blood glucose, lipid profiles, insulin, leptin, and adiponectin levels were measured. The liver and adipose tissues were excised for histopathological examination and protein expression study.
Results: After six weeks of the treatment, R. nasutus extract (at 250 and 500 mg/kg per day) was found to reduce the elevated blood glucose level, improve the insulin sensitivity, decrease the serum leptin, and increase the serum adiponectin levels. The obese mice treated with R. nasutus were found to have a reduction in the increased lipid concentrations in their serum and liver tissues. Moreover, treatment with R. nasutus reduced the fat accumulation in the liver and the large adipocyte size in the fat tissues. Interestingly, the administration with R. nasutus extract was marked by an increase in the hepatic peroxisome proliferators-activated receptor alpha, fat cell adiponectin, and glucose transporter 4 proteins.
Conclusions: To the best of our knowledge, the present study is the first report on the impact of R. nasutus extract in improving the impaired glucose and lipid metabolism in high-fat diet-induced obesity in mice via stimulating the insulin sensitivity in the liver and adipose tissues.
1. Introduction
Abnormal lipid metabolism in obesity can impair insulin signaling by inhibiting the release of glucose from the liver and its uptake by the fat and muscle cells [1]. An overloaded long-term high-fat diet (HFD) leads to obesity, which can induce insulin resistance in many tissues such as the liver, skeletal muscle, and adipose tissues. In the insulin-resistant state, insulin is unable to inhibit lipolysis, which results in increased circulating free fatty acid (FFA) [2]. The elevated FFA levels increase the chronic hyperglycemia and hypertriglyceridemia. Hepatic insulin resistance can increase the hepatic gluconeogenesis and lipogenesis [3]. FFA accumulation in the liver results in hepatic steatosis and contributes towards dysfunctional insulin signaling [4]. Moreover, the adipose tissues in the obese state are largely expanded and function abnormally in the regulation of cytokine release as well as fatty acid metabolism and its storage [4].
Insulinisthemostimportanthormonefortheregulationofblood glucose level. Glucose transport, the rate-limiting step in carbohydrate metabolism, is facilitated by glucose transporters (GLUT) across the cell membranes. GLUT4, a major GLUT, stimulates glucose uptake into fat and muscle cells. Its function is crucial for the condition of insulin sensitivity[5]. It has been reported that the overexpression of GLUT4 gene reduces the hyperglycemic and hyperinsulinemic conditions in HFD-fed transgenic mice [6]. Adiponectin is an adipokine secreted by the adipose tissues thatregulates glucose and lipid metabolism [7]. Peroxisome proliferators-activated receptor alpha (PPARα) plays an important role in the regulation of lipid metabolism in the liver. PPARα enhancestheuptake,utilization,andcatabolismofthefattyacidsby up-regulating the genes, which are involved in the transport, binding, and β-oxidation of fatty acids[8]. It has been shown that PPARα agonist improves hepatic and muscle insulin resistance, decreases hepatic and intramuscular fat content, decreases plasma FFA, and enhances adiponectin expression in rodents[9–13].
Rhinacanthus nasutus (R. nasutus) is found naturally in India, South China, and Southeast Asia including Thailand. R. nasutus has been reported to have several bioactivities such as anti-allergic[14], neuroprotective[15], antidiabetic[16], antitumor [17], and neuroprotective activities [18]. However, the effects of the water extract of R. nasutus leaves on obesity have not been clearly demonstrated yet. Therefore, given the increasing incidence of obesity, the aim of this study was to investigate the effect of the extract of R. nasutus on impaired glucose and lipid metabolism in HFD-fed mice.
2. Materials and methods
2.1. Plant extraction
Leaves of R. nasutus were collected from Buriram, Thailand, between Julyand September2014.Avoucherherbariumspecimen (SKP 001 18 14 01) was given by the Faculty of Pharmaceutical Sciences,Princeof Songkla University,Thailand.Thedriedleaves wereextractedwithwaterat100°Cfor30min.Theextractwasthen concentrated and freeze-dried. After this procedure, the yield was 25.11% of the initial dry weight of the leaves. The obtained R. nasutus extract was kept at−20°C untilit was further used. The amount ofthe total polyphenol and flavonoid were calculatedtobe (91.10±0.97) mg gallic acid equivalents per gram of extract and (85.65±0.40) mg catechin/g extract, respectively. The prior was determinedbyFolin-Ciocalteumethod[19],whilethelaterwasfrom the method derived by Sumczynski et al.[20].
2.2. Animals, diets, and obesity induction
All animal experiment protocols were approved by the Animal Careand Use Committeeof Thammasat University,Pathum Thani, Thailand (Record No. AE 006/2014). Thirty-two male ICR mice weighing 2025 g were obtained from the National Laboratory Animal Center of Mahidol University, Nakhon Pathom, Thailand. Theyweremaintainedinanair-conditionedroom[(25±2)°C]ata 12 h light/12 h dark cycle and fed with normal diet and water ad libitum for a week. After random selection, the mice were fed with normal diet (Research Diets, New Brunswick, NJ, USA) containing 10% of energy as fat (cholesterol in lard was 0.72 mg/g), 70% as carbohydrate, and 20% as protein (total energy was 3.85 kcal/g) or HFD (Research Diets, New Brunswick, NJ, USA) containing 60% as fat (cholesterol in lard was 0.72 mg/g), 20% as carbohydrate, and 20% as protein (total energy was 5.24 kcal/g) for 12 weeks. After the first six weeks of HFD, the obesity condition was observed.
2.3. Experimental study
The obese animals were weighed and randomly divided into three groups with eight mice per group and were orally treated as follows: obese control mice (OB) treated with 5% gum arabic, obese mice treated with R. nasutus 250 mg/kg per day, and obese mice treated with R. nasutus 500 mg/kg per day. In the normal control group (NC), the mice were also treated with 5% gum arabic. All mice were administered for six weeks. Gum arabic at 5% concentration was used for dissolving R. nasutus extract. The doses of the extract were selected based on the preliminary test. We also studied an acute toxicity test and found that the R. nasutus water extract did not show any toxicity in mice at the doses of 250, 500, 1000, and 2000 mg/kg for a week (data have not been shown). Changes in the body weight, food consumption, and caloric intake were monitored weekly. After six weeks of treatment, mice were fasted for 6 h and anesthetized with isoflurane. Blood samples were collected from the hearts of all mice to determine their blood glucose, lipid profile, insulin, leptin, and adiponectin levels. After the blood collection, the liver and epididymal fat tissues were removed from the mice and subjected to histological examinations. The rest of these tissues were stored at−80°C until analysis.
2.4. Intraperitoneal glucose tolerance test (IPGTT)
After five weeks of treatment, an IPGTT was performed on the mice to evaluate the effect of each treatment on their glucose tolerance. After 6 h of fasting, the mice were injected with glucose (2 g/kg), followed by the collection of blood samples from the tail vein at 0, 20, 60, and 120 min.
2.5. Measurement of serum insulin, leptin and
adiponectin levels
After six weeks of treatment, the serum insulin, leptin, and adiponectin concentrations in mice at fasting condition were measured by using ELISA kit (EMD Millipore, Billerica, MA, USA). The index of the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as an indicator of insulin sensitivity according to the following formula: [insulin (μIU)×glucose (mmol/L)]/22.5.
2.6. Measurement of serum and liver lipid profiles
After six weeks of treatment, serum total cholesterol (TC), triglyceride (TG), and non-esterified fatty acid (NEFA) concentrations were determined by using commercial kits (Wako, Osaka, Japan).
For determination of liver TG and NEFA accumulations, 100 mg of the liver was used to extract lipids according to the previous study [21]. TG and NEFA concentrations were measured by using commercial kits (Wako, Osaka, Japan).
2.7. Western immunoblotting
Liver and epididymal fat tissues were homogenized and extracted with TPER®and Halt®protease inhibitor (Thermo Scientific, Rockford, IL, USA). For Western blot analysis, 40 μg of liver PPARα and 20 μg of fat cell adiponectin and GLUT4 proteins were separated by 12% sodium dodecyl sulfatepolyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5% skimmed milk in Tris-buffered saline containing 0.01% Tween 20 for 1 h. The blocked membrane was incubatedovernight with anti-PPARα, anti-adiponectin, anti-GLUT4, and anti-actin primary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C. After incubation with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature, the immunoreactive proteins were developed and the intensities of each band were quantified by densitometry with an ImageQuant™400 imager (GE Healthcare Life Sciences, Piscataway, NJ, USA). Actin was used as a loading control to normalize the target proteins.
2.8. Histopathology
Liver and epididymal fat tissues were fixed in 10% formalin and embedded in paraffin. Three-micron sections were cut and stained with hematoxylin and eosin for examination of the liver and adipose tissue histology (CX31; Olympus, Tokyo, Japan). The fat cell size was calculated by using an Image J Software program (National Institute of Health, Bethesda, MD, USA).
2.9. Statistical analyses
Data were expressed as the mean±SEM. The groups of data were compared by One-way ANOVA followed by the Tukey's post hoc test (SigmaStat Software, CA, USA). Statistical analyses with the P value less than 0.05 were considered significant.
3. Results
3.1. Effect of R. nasutus on metabolic abnormalities in HFD-induced obese mice
After six weeks of induced obesity, the body weight was found to be significantly higher in the OB group compared to the NC group (Figure 1A). However, the body weight of OB mice treated with either 250 or 500 mg/kg R. nasutus did not show any significant decrease in comparison to the OB group. No significant differencewasobservedinfoodintakeamongthegroups(Figure1B). However, all of the OB groups showeda significant increase in the energy intake compared to the NC group (Figure 1C).
The OBgroupsignificantlyincreasedtheepididymalfatweight compared to the NC group (Figure 2A). Interestingly, the obese micetreatedwithboth250and500mg/kg R.nasutusdemonstrated the reduction in the body fat. Moreover, their enlarged fat cell size significantly decreased compared to the OB group (Figure 2B,C).
After six weeks of R. nasutus (250 and 500 mg/kg) treatment, the fasting blood glucose was significantly reduced compared to the OB group (Figure 3A). The administration of R. nasutus (250 and 500 mg/kg) showed a significant reduction in the elevated insulin level too (Figure 3B). The reduced fasting blood glucose and insulin levels in the obese mice treated with 250 and 500 mg/kg R. nasutus were found to be effective in decreasing the index of HOMA-IR (Figure 3C). The serum leptin level wassignificantly increased in OB group compared to the NC group, but it reduced significantly after being treated with 250 and 500 mg/kg R. nasutus (Figure 3D). After six weeks of treatment with R. nasutus, the serum adiponectin levels were significantly higher than that of the obese control mice group (Figure 3E).

Figure 1. Effect of R. nasutus on body weight (A), food intake (B), and energy intake (C) in HFD-induced obese mice.Values are represented as mean±SEM; (n = 8).#: P<0.05 when compared with the NC group;*: P<0.05 when compared with the OB group.
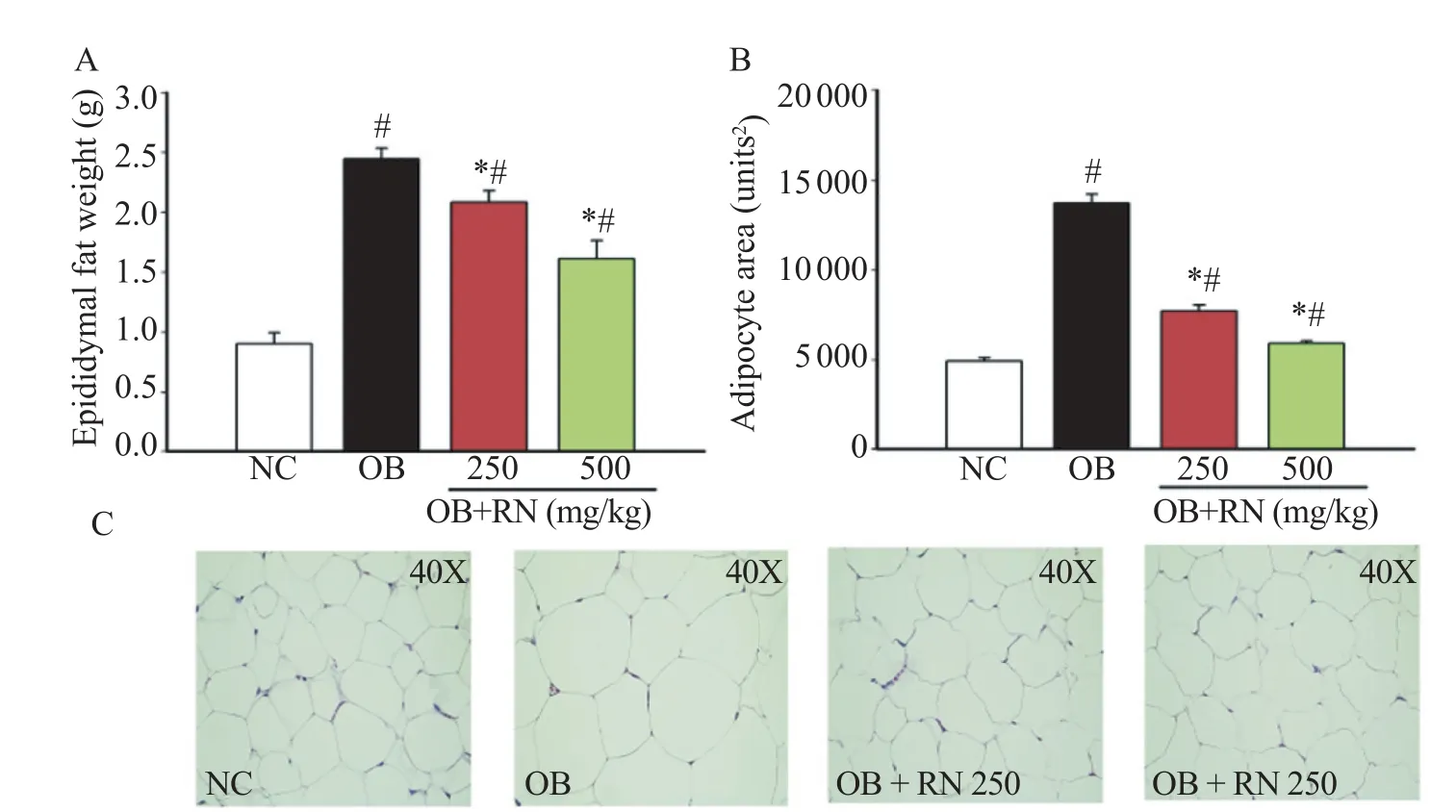
Figure 2. Effect of R. nasutus on epididymal fat weight (A), adipocyte area (B), and histology of epididymal fat tissue (C) in HFD-induced obese mice. Epididymal fat tissue was stained with hematoxylin and eosin (40×magnification). Using an Image J Software program, the average adipocyte area was estimated. The R. nasutus administration for six weeks significantly induced small fat cells.Values are represented as mean±SEM; (n = 8).#: P<0.05 when compared with the NC group;*: P<0.05 when compared with the OB group.
After glucose injection, the blood glucose level of the obese controlgroupwassignificantlyincreasedcomparedtotheNCgroup (Figure 3F). However, the obese mice treated with R. nasutus (250 and 500 mg/kg) showed a significant reduction in the high blood glucose levels at 20, 60, and 120 min compared to the OB group.
After six weeks of treatment, the R. nasutus (250 and 500 mg/ kg) slightly decreased the serum TC, which was not significant compared to the OB group (Figure 4A). In comparison to the OB group, the groups treated with R. nasutus showed a significant reduction in the serum TG and NEFA levels (Figure 4B,C). The weight of the liver of the OB group increased compared to the NC group, but the obese mice treated with R. nasutus recorded a decrease in the weight of their livers (Figure 4D). Moreover, the storage of hepatic TG and NEFA were significantly reduced by R. nasutus treatment (Figure 4E,F). The accumulation of lipid droplets in the liver tissue was related to the results of hepatic TG and NEFA content. The OB group clearly showed increased hepatic fat accumulation, but its treatment with R. nasutus markedly reduced the fat accumulation (Figure 4G).
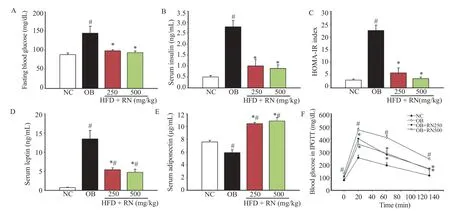
Figure 3. Effect of R. nasutus on fasting blood glucose (A), serum insulin (B), HOMA-IR index (C), serum leptin (D), serum adiponectin (E), and blood glucose levels in IPGTT (F) in HFD-induced obese mice.Values are represented as mean±SEM; (n = 8).#: P<0.05 when compared with the NC group;*: P<0.05 when compared with the OB group.

Figure 4. Effect of R. nasutus on serum total cholesterol (A), serum triglyceride (B), serum non-esterified fatty acid (C), liver weight (D), liver triglyceride (E), liver non-esterified fatty acid (F), and histology of liver (hematoxylin and eosin-staining, 40×) (G) in HFD-induced obese mice.Liver histological examination confirmed that the RN administration for six weeks decreased lipid accumulation in the liver. Hepatocytes of obese mice were filled with macrovesicular fat deposits while microvesicular fat deposits to a lesser extent were found in obese mice treated with RN; Values are represented as mean±SEM; (n = 8).#: P<0.05 when compared with the NC group;*: P<0.05 when compared with the OB group.
3.2. Effect of R. nasutus on PPARα, adiponectin, and GLUT4 protein expressions
The OB group showed a significant reduction in hepatic PPARα, fat cell adiponectin, and fat cell GLUT4 proteins expression as compared with the NC group (Figure 5A,B and C, respectively). Interestingly, R. nasutus strongly reversed the decreased protein expressions of PPARα, adiponectin, and GLUT4.
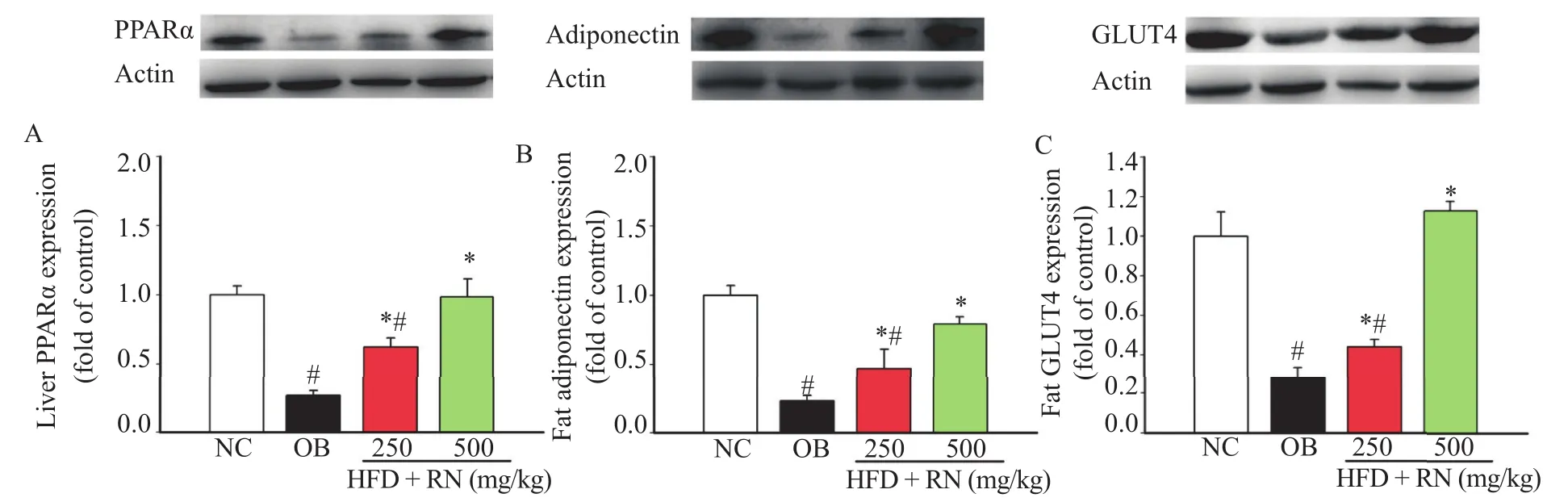
Figure 5. Effect of R. nasutus treatment on liver PPARα(A), fat adiponectin (B), and fat GLUT4 (C) protein expressions in HFD-induced obese mice. The RN administration for six weeks significantly increased the expressions of PPARα, adiponectin, and GLUT4 proteins.Values are represented as mean±SEM; (n = 8).#: P<0.05 when compared with the NC group;*: P<0.05 when compared with the OB group.
4. Discussion
To the best of our knowledge, the present study is the first to demonstrate the effect of R. nasutus on impaired glucose and lipid metabolism in the HFD-induced obese mice. Our findings stated that HFD can produce a hyperglycemic condition with decreased glucose tolerance and insulin sensitivity, and increased insulin and leptin levels in obese mice. This HFD-induced obesity model also showed an increase in the serum TC, TG, and NEFA levels, which were similar to those in the case of human obesity. Furthermore, the HFD-fed mice showed a large amount of lipid accumulation and increased TG and NEFA levels in the liver tissue.
The weight of the body and visceral adipose tissue of the HFD-fed mice (60% kcal fat) was more than that of the mice fed with a low-fat diet [22,23]. From our study, we found that the HFD-fed mice also showed a significant increase in the epididymal fat weight. However, the R. nasutus treatment slightly reduced the body weight, epididymal fat weight, and the amount of food intake of the obese mice. The energy intake was, yet, significantly higher in HFD mice groups.
The feeding of HFD increased the cholesterol levels via an increase in the cholesterol absorption by the small intestine[24]. The hypertriglyceridemia observed in the HFD model may be due to the increased absorption and formation of TG, and decreased TG uptake by the fat tissues [25]. Many studies have reported that the elevated fat storage in the liver tissue and the abnormal circulating FFA are also associated with the increased insulin resistance [26–28]. Noticeably, all doses of R. nasutus treatment strongly decreased the hepatic serum TG and NEFA levels by decreasing the hepatic fat accumulation in the histological examination.
PPARα is the master regulator of lipid metabolism [29]. PPARα agonists stimulate lipid oxidation, decrease the levels of circulating TG, increase high-density lipoprotein cholesterol, and exhibit anti-atherosclerotic activity [30,31]. In our studies, we found that R. nasutus can reduce the high levels of serum lipid profiles and hepatic fat accumulation. Therefore, the effect of R. nasutus on the expression of PPARα protein is an interesting aspect to investigate. After six weeks of R. nasutus treatment, the protein expression of PPARα significantly improved compared to the obese control mice. This may lead to the possibility of an improvement in the impaired lipid metabolism by inhibiting the serum lipid profiles, liver fat accumulation, and stimulating the PPARα expression through R. nasutus treatment.
Either deficiency of insulin or insulin-resistant condition can induce the dysfunction of GLUT4 resulting in its retention inside the cell. This dysfunction causes a reduction in the glucose uptake into the muscle and adipose tissues leading to the increase in the blood glucose levels. These reduction and retention may be the reason for causing the obese state [6]. In our study, the HFD-fed mice showed elevated blood glucose, increased serum insulin levels, and increased HOMA-IR values. Interestingly, the OB mice treated with R. nasutus can improve the insulin sensitivity by reducing the levels of high blood glucose, insulin, and HOMA-IR. Restoration of GLUT4 levels would be helpful in controlling the hyperglycemic and hyperinsulinemic conditions via an enhancing glucose uptake by the fat tissues. The present study also showed how the small adipocyte in the R. nasutus-treated groups could help in increasing the GLUT4 protein expression. These results indicated that R. nasutus may be stimulating insulin sensitivity in adipose tissues.
Leptin is an adipokine related to the impaired insulin sensitivity condition. It has been reported that leptin is more likely to be increased in the obesity state[32,33]. In our study, the OB mice showed an increase in the serum leptin levels, but the levels were reduced after six weeks of R. nasutus treatment. Adiponectin has an effect on glucose homeostasis. The circulating level of adiponectin correlates with insulin sensitivity both in the humans and rodents [34–36], and it is found to be reduced in humans with obesity and type 2 diabetes [37]. The induction of adipose tissue adiponectin expression and consequently, the increase in circulating adiponectin levels could represent a novel potential enhancement of whole-body insulin sensitivity [38]. After the obese mice had been treated with R. nasutus for six weeks, we found that the levels of serum adiponectin and proteinexpression levels increased significantly. Thus, the treatment with R. nasutus may cause an increase in the peripheral insulin sensitivity in the obesity-induced insulin resistant condition.
In conclusion, R. nasutus administration has an impressive effect on the glucose and lipid metabolism in HFD-induced obese mice. The effects are as follows: (a) reduction of serum TG and NEFA levels and lipid accumulation in the liver, (b) reduction of blood glucose and improvement of insulin sensitivity, and (c) increase of small adipocyte numbers in fat tissue. Furthermore, R. nasutus may mediate these effects via its positive effect in stimulating hepatic PPARα, fat adiponectin, and fat GLUT4 protein expressions. Thus, to the best of our knowledge, our findings are the first to support the usefulness of R. nasutus in regulating the abnormalities of glucose and fat metabolism in HFD-induced obesity condition.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This research was supported by the research grant from the Faculty of Medicine, Thammasat University (Contract number: GEN2-05/2015).
References
[1] Snel M, Jonker JT, Schoones J, Lamb H, de Roos A, Pijl H, et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol 2012; http:// dx.doi.org/10.1155/2012/983814.
[2] Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011; 60: 2441-9.
[3] Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014; 510: 84-91.
[4] McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013; 4: 52.
[5] Govers R. Cellular regulation of glucose uptake by glucose transporter GLUT4. Adv Clin Chem 2014; 66: 173-240.
[6] Atkinson BJ, Griesel BA, King CD, Josey MA, Olson AL. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat diet-fed transgenic mice. Diabetes 2013; 62: 2249-58.
[7] Lee B, Shao J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord 2014; 15: 149-56.
[8] Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol 2015; 62: 720-33.
[9] Ye JM, Iglesias MA, Watson DG, Ellis B, Wood L, Jensen PB, et al. PPARalpha/gamma ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am J Physiol Endocrinol Metab 2003; 284: E531-40.
[10] Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 2001; 50: 411-7.
[11] Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, et al. Peroxisome proliferator-activated receptor-alpha agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes 2003; 52: 1770-8.
[12] Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, et al. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem 2000; 275: 16638-42.
[13] Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun 2005; 336: 747-53.
[14] Tewtrakul S, Tansakul P, Panichayupakaranant P. Anti-allergic principles of Rhinacanthus nasutus leaves. Phytomedicine 2009; 16: 929-34.
[15] Brimson JM, Brimson SJ, Brimson CA, Rakkhitawatthana V, Tencomnao T. Rhinacanthus nasutus extracts prevent glutamate and amyloid-beta neurotoxicity in HT-22 mouse hippocampal cells: possible active compounds include lupeol, stigmasterol and beta-sitosterol. Int J Mol Sci 2012; 13: 5074-97.
[16] Visweswara Rao P, Madhavi K, Dhananjaya Naidu M, Gan SH. Rhinacanthus nasutus ameliorates cytosolic and mitochondrial enzyme levels in streptozotocin-induced diabetic rats. Evid Based Complement Altern Med 2013; http://dx.doi.org/10.1155/2013/ 486047.
[17] Siripong P, Yahuafai J, Shimizu K, Ichikawa K, Yonezawa S, Asai T, et al. Antitumor activity of liposomal naphthoquinone esters isolated from Thai medicinal plant: Rhinacanthus nasutus KURZ. Biol Pharm Bull 2006; 29: 2279-83.
[18] Brimson JM, Tencomnao T. Rhinacanthus nasutus protects cultured neuronal cells against hypoxia induced cell death. Molecules 2011; 16: 6322-38.
[19] Gonzalez de Mejia E, Song YS, Ramirez-Mares MV, Kobayashi H. Effect of yerba mate (Ilex paraguariensis) tea on topoisomerase inhibition and oral carcinoma cell proliferation. J Agric Food Chem 2005; 53: 1966-73.
[20] Sumczynski D, Bubelova Z, Sneyd J, Erb-Weber S, Mlcek J. Total phenolics,flavonoids, antioxidant activity, crude fibre and digestibility in non-traditional wheat flakes and muesli. Food Chem 2015; 174: 319-25.
[21] Naowaboot J, Somparn N, Saentaweesuk S, Pannangpetch P. Umbelliferone improves an impaired glucose and lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats. Phytother Res 2015; 29: 1388-95.
[22] Lim E, Lim JY, Shin JH, Seok PR, Jung S, Yoo SH, et al. DXylose suppresses adipogenesis and regulates lipid metabolism genes in high-fat diet-induced obese mice. Nutr Res 2015; 35: 626-36.
[23] Rong X, Li Y, Ebihara K, Zhao M, Naowaboot J, Kusakabe T, et al. Angiotensin II type 1 receptor-independent beneficial effects of telmisartan on dietary-induced obesity, insulin resistance and fatty liver in mice. Diabetologia 2010; 53: 1727-31.
[24] Chandak PG, Obrowsky S, Radovic B, Doddapattar P, Aflaki E, Kratzer A, et al. Lack of acyl-CoA: diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice. Biochim Biophys Acta 2011; 1811: 1011-20.
[25] Khalifeh-Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med 2014; 20: 175-83.
[26] Bermudez JA, Velasquez CM. [Profile of free fatty acids (FFA) in serum of young Colombians with obesity and metabolic syndrome]. Arch Latinoam Nutr 2014; 64: 248-57. Spanish.
[27] Yu X, Ye L, Zhang H, Zhao J, Wang G, Guo C, et al. Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J Ginseng Res 2015; 39: 199-205.
[28] Neuman MG, Cohen LB, Nanau RM. Biomarkers in nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2014; 28: 607-18. [29] Shimizu M, Tanaka T, Moriwaki H. Obesity and hepatocellular carcinoma: targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin Immunopathol 2013; 35: 191-202.
[30] Gai YT, Shu Q, Chen CX, Lai YL, Li WJ, Peng L, et al. [Antiatherosclerosis role of N-oleoylethanolamine in CB2]. Yao Xue Xue Bao 2014; 49: 316-21. Chinese.
[31] Li L, Li L, Chen L, Lin X, Xu Y, Ren J, et al. Effect of oleoylethanolamide on diet-induced nonalcoholic fatty liver in rats. J Pharmacol Sci 2015; 127: 244-50.
[32] Nascimento AF, Luvizotto RA, Leopoldo AS, Lima-Leopoldo AP, Seiva FR, Justulin LA Jr, et al. Long-term high-fat diet-induced obesity decreases the cardiac leptin receptor without apparent lipotoxicity. Life Sci 2011; 88: 1031-8.
[33] Carvalho KM, Marinho Filho JD, de Melo TS, Araujo AJ, Quetz Jda S, da Cunha Mdo P, et al. The resin from Protium heptaphyllum prevents high-fat diet-induced obesity in mice: scientific evidence and potential mechanisms. Evid Based Complement Altern Med 2015; http://dx.doi.org/10.1155/2015/106157.
[34] Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79-83.
[35] Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005; 26: 439-51.
[36] Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep 2003; 3: 207-13.
[37] Kim AY, Park YJ, Pan X, Shin KC, Kwak SH, Bassas AF, et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat Commun 2015; 6: 7585.
[38] Tozzo E, Bhat G, Cheon K, Camacho RC. Pioglitazone increases whole body insulin sensitivity in obese, insulin-resistant rhesus monkeys. PloS One 2015; 10: e0126642.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.08.004
*Corresponding author:Jarinyaporn Naowaboot, Division of Pharmacology, Department of Preclinical Science, Faculty of Medicine, Thammasat University (Rangsit Campus), Pathum Thani 12120, Thailand.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Healthcare waste management in selected government and private hospitals in Southeast Nigeria
- Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
- Antifungal activity of plant extracts with potential to control plant pathogens in pineapple
- Potent water extracts of Indonesian medicinal plants against PTP1B
- Pharmacological effects of ethanol extract of Egyptian Artemisia herba-alba in rats and mice
