Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
Tel: +86 13906174047
E-mail: ysong@jiangnan.edu.cn
Peer review under responsibility of Hainan Medical University.
Foundation Project: Supported by National Natural Science Foundation of China (31271812), the National High Technology Research and Development Program of China (863 Program 2012AA022105C).
Gbago Onivogui1, Rebaone Letsididi2, Mohamed Diaby2, Liping Wang2, Yuanda Song1,2*1Center of Excellence for Functional Food and Health, Jiangnan University, Wuxi, China
2State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, China
ARTICLE INFO
Article history:
Received 11 Aug 2015
Received in revised form 25 Aug 2015
Accepted 5 Sep 2015
Available online 10 Nov 2015
Keywords:
Anisophyllea laurina R. Br. ex
Sabine
Pulp
Seed
Antimicrobial activity
Antioxidant activity
ABSTRACT
Objective: To evaluate the influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits.
Methods: The antibacterial activities of pulp and seed extracts were tested by using disk diffusion method against eight bacterial strains and three fungal strains. Total phenolic, flavonoid, monomeric anthocyanin and tannin contents, and antioxidant activities were determined by spectrometric methods.
Results: The antioxidant analysis of pulp extract revealed the strong radical scavenging capacity and total phenolic content (4329.66 mg of gallic acid/100 g), while seed extract showed the high antioxidant activity and total tannin content (5326.78 mg catechin equivalent/100 g). Antibacterial and antifungal activities of methanol and ethanol extracts exhibited potent growth inhibitory activity against Aeromonas hydrophila, Bacillus subtilis, Escherichia coli O157:H7, Pseudomonas aeruginosa, Salmonella Typhimurium and Staphylococcus aureus ATCC 6538 with minimum inhibitory concentration values ranged from 125 to 250 μg/mL. However, seed extract had the strongest potential activity against Aspergillus niger and Candida albicans with minimum inhibitory concentration value of 500 μg/mL compared to pulp extract.
Conclusions: Ourresultsthereforedemonstratedthatethanolandmethanolextractionswere more efficient in extracting antioxidants and bioactive compound in pulp and seed. These results support that these plant extracts can be used for the treatment of bacterial infections.
1. Introduction
The human diet often comprises foods and beverages with significant amounts of phenolic compounds such as fruits, vegetables, wines and teas. A considerable weight of evidence has been gathered suggesting that consumption of fruit and vegetables is beneficial for human health and may help in the prevention of chronic diseases, because they contain phenolic compounds [1]. Due to their antibacterial, antifungal and antiviral activity, phenolic compounds and antioxidant biomolecules were the subject of anti-infective research for many years[2].
The food antimicrobials are usually classified into traditional or natural and synthetic substances depending on their origin. Antimicrobials are called traditional substances when they have been used for many years and many countries approve them for inclusion in foods. Although, many synthetic antimicrobials are found naturally (benzoic acid in cranberries, sorbic acid in rowanberries, citric acid in lemons, malic acid in apples, tartaric acid in grapes, etc.), the perception of natural has become important for many consumers [3].
Anisophylleaceae comprise of 29–34 species in four genera: Anisophyllea with 2 species in South America, 5–9 in mainland Africa, 1 in Madagascar and 15–19 in Malaysia. It is the common mangrove and consequently accounts for a considerable growth area[4,5]. A decoction of the leaves is used as a mouth rinse for toothache and the ground leaves are said to have medicinal properties to treat diabetes and emetics [6]. The leaves of Anisophyllea laurina R. Br. ex Sabine (A. laurina) plant were identified and are well-known as traditional medicine for malariain Guinea[7].Varioussolventextractsfromleavesandstembarkof A. laurina were previously tested in vitro antimicrobial activity. Ethanol and methanol extracts of the leaves and stem bark have shown the potential antibacterial and antifungal activities[8]. The study conducted by Kargbo et al. showed that the ethanol crude extract from leaves of A. laurina exerted an inhibitory effect on α-glycosidase and α-amylase[9]. A. laurina fruits have a pleasant taste of sweet cherries and have great importance from nutritional and economic points of view (Figure 1). They are consumed in different ways, either eaten fresh or boiled in jam. Information on phenolic compounds and antioxidant activities from pulp and seed of A. laurina fruit will contribute to a more comprehensive assessment of their nutritional value. Previous studies that focused on the chemical composition and nutritional properties of A.laurinafruithaverevealedthatpulpandseedhaveahighcontent of essential nutrients and organic acidswhich if wellexploited and promoted can address many nutritional related disorders and also beusefulinfoodindustryforproductionofavarietyofvalueadded products [10]. To our knowledge, no previous study had directly examined the contributions of antimicrobial and antioxidant activities of A. laurina fruits. Thus, the aim of this present study was to evaluate in vitro antibacterial, antifungal and antioxidant activities of various solvent extracts of the pulp and seed of A. laurina fruits.

Figure 1. Monkey apple fruit (a), pulp (b), seed (c) and kernel (d) of A. laurina.
2. Materials and methods
2.1. Collection and preparation of plant extracts
Fresh mature whole fruits of A. laurina were collected in Coyah of Kindia region in September 2014 and identified by Valorization Center on Medicinal Plants, Dubr´eka, Guinea. A voucher specimen of the plant was deposited with the number 5HK4 at the herbarium of the center. About 10 g of each powder materials were extracted by sonication over an ice bed with methanol/water 80:20 (v/v), ethanol/water 80:20 (v/v) and ethyl acetate/water 1:5 (w/v) for 15 min. The clear filtrates were dried under vacuum using a rotary evaporator and gave the extract yields. The samples were stored at−20°C.
2.2. Quantification of phenolic compound
The total phenolic content (TPC) was determined using the Folin–Ciocalteu reagent as described by Gouveia and Castilho [11]. TPC was expressed as mg of gallic acid equivalents (GAE) per 100 g of dry weight (DW) through a calibration curve (0–400 μg/mL range). Total flavonoid content (TFC) was measured as described by Gouveia and Castilho [11]. TFC was expressed as mg of quercetin equivalent (QE) per 100 g of DW through a calibration curve of quercetin (0–400 μg/mL). The total tannin content (TTC) was determined using the vanillin-methanol solution as described by Sun et al. [12]. TTC was expressed as mg (+)-catechin equivalents (CE) per 100 g of DW through a calibration curve (0–400 μg/mL). The total monomeric anthocyanin content (TMAC) of the extracts was determined using the pH-differential method previously described [13]. TMAC was expressed as mg of cyanidin-3-O-glucoside (C3G) per 100 g of DW.
2.3. Determination of antioxidant activities
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay followed a reported method by Gouveia and Castilho[11]. The DPPH radical scavenging effect of the sample was expressed based on the Trolox calibration curve, as μmol Trolox equivalent (TE) per 100 g of dried fruit weight. Ferricreducing antioxidant power (FRAP) assay was conducted according to Lu et al.[14]. A standard curve was made with Trolox and the results were expressed as μmol TE per 1 g DW of the fruit powders.
The 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity assay was performed according to the procedures of Gouveia and Castilho[11]. Results were expressed as μmol TE per 100 g of extract.
2.4. In vitro antimicrobial assay
Antimicrobial activity of extracts was evaluated according to the method reported by Onivogui et al. [8]. The extracts were tested for activity against eight strains of bacteria: Staphylococcus aureus (S. aureus) ATCC 6538, S. aureus ATCC 29213, Bacillus subtilis ATCC 6059 (B. subtilis), Escherichia coli (E. coli) ATCC 25922, E. coli O157:H7, Salmonella Typhimurium ATCC 14028 (S. Typhimurium), Aeromonas hydrophila ATCC 7966 (A. hydrophila) and Pseudomonas aeruginosa (Schroeter) Migula ATCC 27853 (P. aeruginosa) and three fungal strains: Candida albicans CMCC 98001 (C. albicans), Aspergillus niger MCC 98003 (A. niger) and Aspergillus flavus AS3.3554 (A.flavus). All these bacteria and fungi were collected from Beijing Institute of Biotechnology.
2.5. Statistical analysis
Results obtained were reported as mean±SD of triplicate measurements. Significance differences for multiple comparisons were determined by One-way ANOVA followed by Duncan test with P = 0.05 using SPSS (version 19).
3. Results
3.1. Percent yield, total phenolic,flavonoid, monomeric anthocyanin and tannin contents
Total phenolic,flavonoid, monomeric anthocyanin and tannin contents were determined for methanol, ethanol, ethyl acetate and water extracts of pulp and seed of A. laurina fruit, separately and were presented in Table 1. Their values showed great variations in various solvents. The yield extracts for the pulp were as follows: 47.62% for ethanol, 32.67% for methanol, 27.66% for ethyl acetate and 11.09% for water while the crude extract for seeds were 48.43% for ethanol, 34.45% for methanol, 17.49% for ethyl acetate and 14.41% for water. It was further noted that the extract yields in descending order followed this trend: ethanol>methanol>ethyl acetate>water in both pulp and seed. The TPC in extracts, expressed as GAE per 100 g of dry extract weight were ranged from 1858.53 to 4329.66 mg GAE/100 g for pulp and 1997.35 to 2679.84 mg GAE/100 g for seed, TFC ranged from 103.87 to 549.40 mg QE/100 g for pulp and 129.44 to 297.47 mg QE/100 g for seed, TMAC ranged from 32.27 to 63.27 mg C3G/100 g for pulp and 12.45 to 40.59 mg C3G/100 g for seed and TTC ranged from 157.53 to 817.42 mg CE/100 g for pulp extract and 2437.02 to 5326.78 mg CE/100 g for seed. The TPC, TFC and TMAC amounts in pulp extract were higher than in seed, while the TTC in seed extracts (5326.78 mg CE/100 g) was higher than in pulp extract (817.42 mg CE/100 g). It was further found out that ethanol and methanol were the best efficient solvents for extraction of phenolic compounds in pulp and seed.
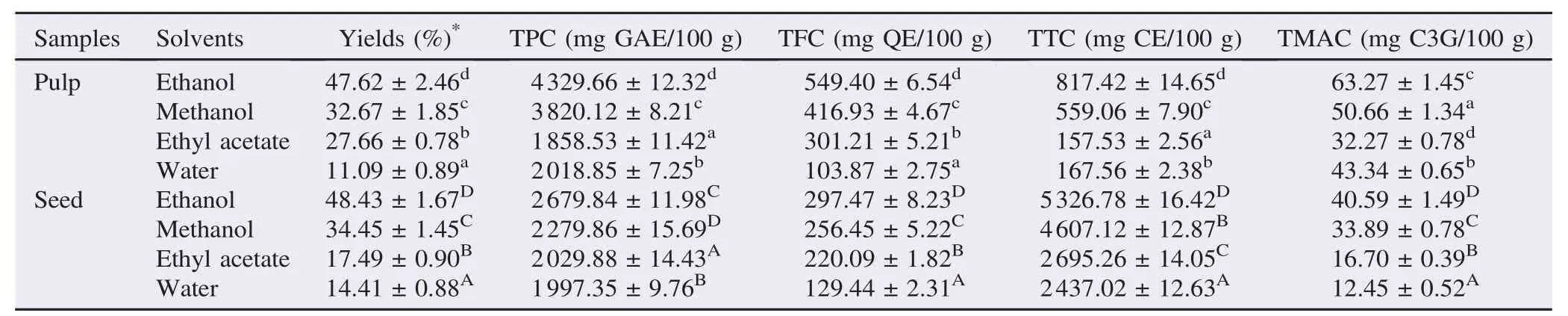
Table 1Total phenolic,flavonoid, anthocyanin and tannin contents of various solvent extracts from pulp and seed of A. laurina (DW extract).
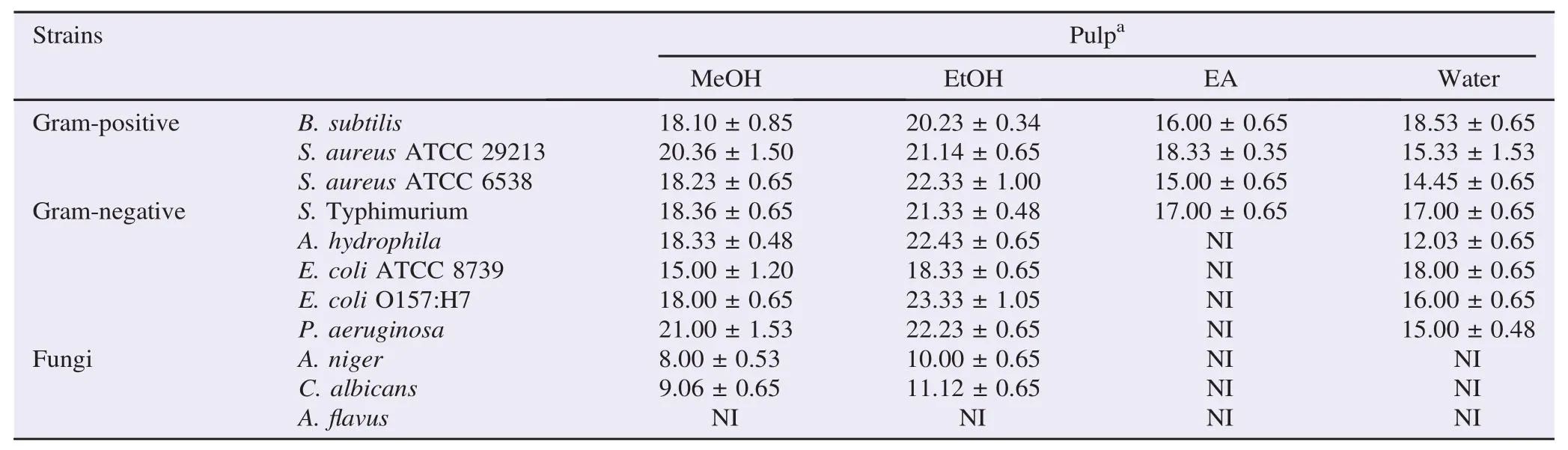
Table 2Antibacterial and antifungal activities of various solvent extracts of the leaves and stem bark by agar-well diffusion method.
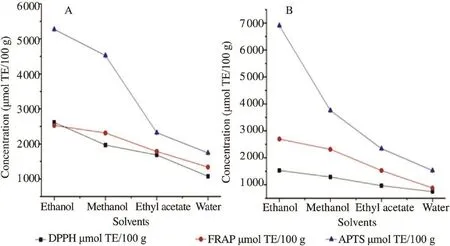
Figure 2. Antioxidant capacity of various solvent extracts from pulp and seed of A. laurina fruits (DW extract).A: Pulp; B: Seed.
3.2. Antioxidant activities
Results for the antioxidant activities of the pulp and seed as determined by ABTS, DPPH and FRAP assay using the different extractions were shown in Figure 2. The ethanol extract of pulp showed the highest DPPH scavenging activity values within the range from 1073.83 to 2617.89 μmol TE/100 g while the lowest DPPH values (745.83 to 1528.57 μmol TE/100 g) were obtained from extract of seed (Figure 2A). However, the highest levels activities evaluated by the ABTS and FRAP assay were noted in ethanol extract of seed (6906.34 μmol TE/100 g and 2696.474 μmol TE/100 g, respectively) (Figure 2B). Figure 2 showed that ethanol extraction was more efficient in extracting antioxidants in pulp (Figure 2A) and seed (Figure 2B) compared to methanol, ethyl acetate and water.
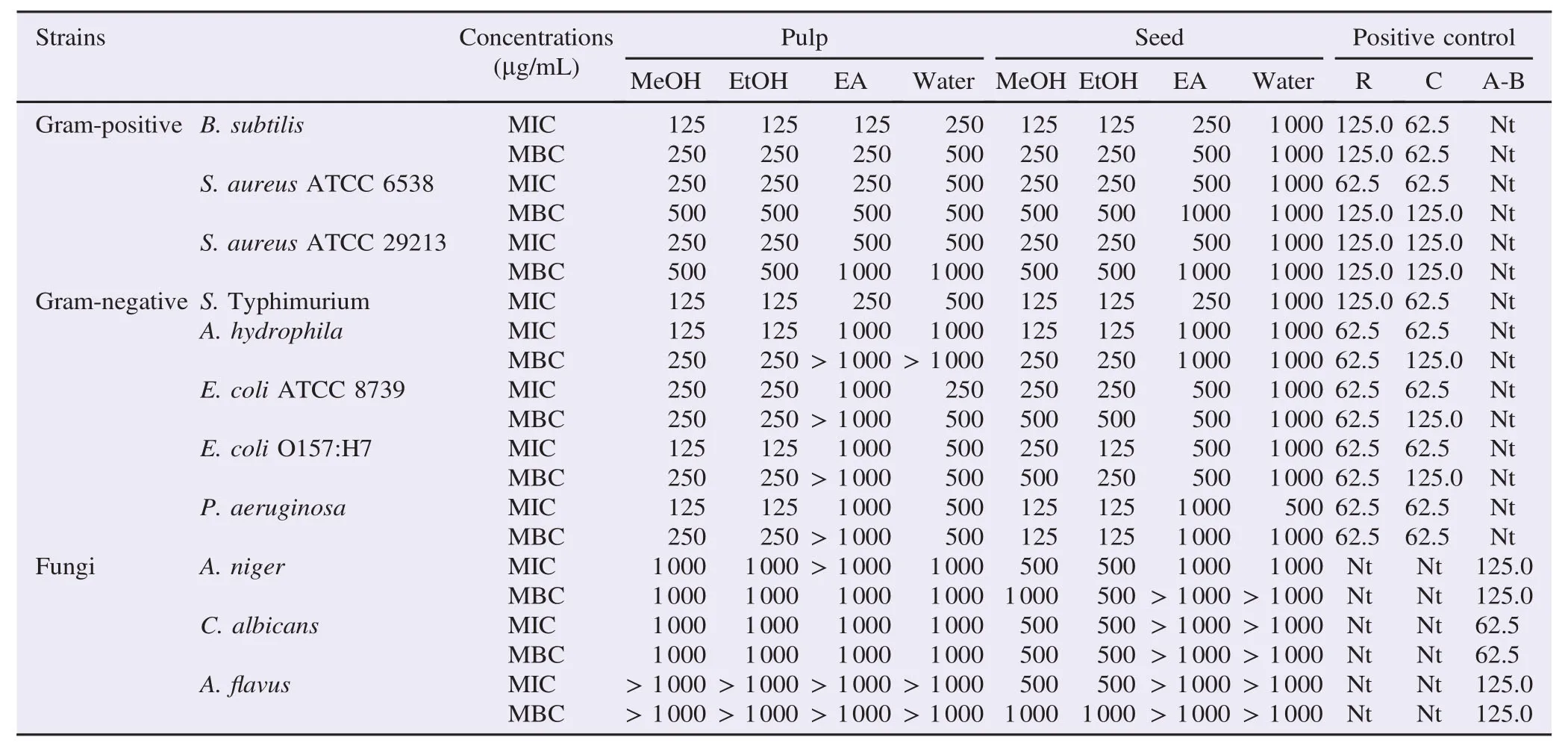
Table 3MIC and MBC/MFC values.μg/mL.
3.3. In vitro antimicrobial assay
The crude extracts of pulp and seed exhibited antibacterial activity against bacterial strains and the fungus (Table 2). According to the results obtained from the disc diffusion assay given in Table 2. Ethanol extract of pulp showed the highest activity against E. coli O157:H7 (23.33 mm) followed by A. hydrophila (22.43 mm), S. aureus ATCC 6538 (22.33 mm) and P. aeruginosa (22.23 mm). Ethyl acetate showed the moderate antibacterial activity against Gram-positive bacteria and did not show any inhibitory effect on all Gram-negative bacteria except S. Typhimurium (17.00 mm).
Compared to the pulp, ethanol and methanol extracts of seed showed the best inhibition against all bacteria. Antibacterial activity of ethanol extract varied greatly among the different pathogenic bacteria and the highest activity was observed against P. aeruginosa (25.10 mm) followed by S. aureus ATCC 6538 (23.00 mm), B. subtilis (21.00 mm), E. coli O157:H7 (21.00 mm) and S. Typhimurium (20.00 mm).

SeedaPositive controls MeOH EtOH EA Water R C A-B 19.00±1.00 21.00±1.50 14.00±0.58 NI 27.33±0.65 24.00±0.65 Nt 15.02±0.33 18.34±0.48 13.00±0.33 NI 25.00±0.65 22.00±0.48 Nt 16.07±0.36 23.00±1.53 12.00±0.65 NI 24.00±1.00 24.00±1.00 Nt 19.33±0.65 20.00±0.65 16.33±0.65 NI 26.53±1.00 20.03±0.48 Nt 18.33±0.65 15.00±0.58 11.00±0.65 NI 24.33±0.58 24.00±0.65 Nt 16.08±0.65 19.00±0.33 14.33±0.65 NI 22.00±1.00 23.00±0.87 Nt 15.00±0.65 21.00±1.00 15.00±0.33 NI 23.00±0.48 23.00±1.00 Nt 23.00±1.53 25.10±1.00 11.00±0.58 14.00±0.65 27.00±0.48 24.00±0.48 Nt 9.00±0.65 12.03±0.65 NI NI Nt Nt 18.00±0.65 10.00±0.65 14.06±0.65 NI NI Nt Nt 23.00±0.65 NI NI NI NI Nt Nt 19.00±0.65
The antifungal activities of the pulp and seed extracts were tested against three fungal species as C. albicans, A. niger and A.flavus (Table 2). Ethanol and methanol extracts of pulp showed a low inhibition against A. niger (10.00 and 8.00 mm respectively) and C. albicans (11.12 and 9.06 mm whereas there was no activity against A.flavus). However, water and ethyl acetate extracts of pulp did not show any activity against A. niger and C. albicans.
Contrary to the pulp extracts, ethanol extract of seed showed the moderate inhibition activity against A. niger (12.03 mm) and C. albicans (14.06 mm). Similarity to the pulp extract, water and ethyl acetate extracts of seed did not show any antibacterial activity against A. niger and C. albicans. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) or minimum fungicidal concentration (MFC) values of pulp and seed extracts were determined by using two-fold broth micro-dilution method. As showed in Table 3, the methanol and ethanol extracts of the pulp showed antibacterial activity against all bacteria with MIC values of 125–250 μg/mL and MBCvalues of 250–500 μg/mL and water had moderate antibacterial activity against all bacteria except A. hydrophila with MIC value 1000 μg/mL while ethyl acetate extract had the lowest antimicrobial activity with MIC values of>1000 μg/mL against A. hydrophila, E. coli O157:H7, E. coli ATCC 8739 and P. aeruginosa. Further methanol and ethanol extracts of pulp showed low antifungal activity against C. albicans and A. niger, with MFC values of 1000 μg/mL while MFC values of methanol and ethanol extracts of pulp were 1000 μg/mL for C. Albicans and A. niger, and A.flavus (MIC>1000 μg/mL).
Compared to the pulp, MIC values of methanol and ethanol extracts of seed ranged from 125 to 250 μg/mL and MBC values 500 μg/mL. Among the bacteria used, the most sensitive bacteria were all Gram-negatives bacteria with MIC value ranged from 125 to 250 μg/mL, whereas ethyl acetate extracts of seed were moderately active against all bacteria with MIC values ranged from 250 to 500 μg/mL, and MBC values ranged from 500 to 1000 μg/mL. However, all bacteria were almost resistant to the water extracts of seed with the MIC of>1000 μg/mL except P. aeruginosa with MIC value of 500 μg/mL and MBC values of 1000 μg/mL. Methanol and ethanol extracts of seed were moderately sensitive against C. albicans MIC values of 500 μg/ mL and MFC values of 500 μg/mL, A. niger and A.flavus with MIC values 500 μg/mL and MFC value ranged from 500 to 1000 μg/mL. However, all fungus were almost resistant to the ethyl acetate and water extracts of seed with the MIC of >1000 μg/mL.
4. Discussion
The results of this study confirmed that both ethanol and methanol are very effective to extract phenolics due to their high polarity and good solubility for phenolic compounds [15]. Comparing these results with literature, the values of the TPC obtained in this study were found to be higher than those obtained in other fruit species such as sapodilla (13.5 mg of GAE/100 g), jackfruit (29.0 mg of GAE/100 g) and pineapple (38.1 mg of GAE/100 g) as reported by Almeida et al. [16]. TMAC value in pulp was higher than pineapple (11.62 mg/ 100 g), cashew apple (7.32 mg/100 g), guava (7.62 mg/ 100 g), papaya (1.87 mg/100 g) and tamarind (2.92 mg/100 g) as reported by Ribeiro da Silva et al.[17].
Comparing with some other tropical fruits, amount of FRAP of A. laurina fruit was higher than those fruits studied by Contreras-Calder´on et al. except banana passionfruit (175 μmol TE/g) and Brazilian guava (39.9 μmol TE/g) [18]. These results are in agreement with that of a previous study reported by Korekar et al. who found that FRAP was higher in seed of sea buckthorn (Hippophae rhamnoides L.) than in pulp [19]. On the other hand, the pulp and seed extracts were found to be a good source of antioxidants by ABTS than tamarind (Tamarindus indica) (8.32 μmol TE/g), pineapple (3.78 μmol TE/g), murici (Byrsonima crassifolia) (15.73 μmol TE/g) and mangaba (10.84 μmol TE/g) as reported by Almeida et al.[16]. In this present study, DPPH antioxidant capacity of seed and pulp showed better activity than some exotic fruit, such as tamarind (Tamarindus indica) (2.04 μmol TE/g), murici (Byrsonima crassifolia) (6.46 μmol TE/g), mangaba (5.27 μmol TE/g) pulps as reported by Almeida et al. [16]. However, higher ABTS, DPPH and FRAP values were observed after extraction with ethanol and methanol as compared to extraction by ethyl acetate and water. Furthermore, correlation tests (Pearson correlation) study between total tannin, phenolic content and antioxidant activities (DPPH, ABTS, FRAP) in the pulp and seed extracts revealed a positive correlation (r = 0.999, P<0.01), which mean that TTC increased as the concentration of total phenolic and antioxidant activities increased. Significant correlations between total phenolic and antioxidant activities have also been reported by Contreras-Calder´on et al. [18].
According to the antibacterial activities of methanol, ethanol, ethyl acetate and water crude extracts of pulp, we confirmed previous studies which reported that ethanol and methanol were among the best solvents used for extraction of antimicrobial substances compared to ethyl acetate and water [20–22]. We demonstrated that the pulp and seed extracts of A. laurina fruits possess antibacterial activities. Similar studies elsewhere recorded antibacterial activity of the fruits extracts against activities against Gram-positive and Gram-negative bacteria [23,24].
Nevertheless, ethyl acetate extract of seed showed moderate inhibitory effect against all bacteria studied. Water extract of seed did not show any antibacterial activity against all Grampositive and Gram-negative except P. aeruginosa. Similar results have been reported where aqueous extracts had low or no antimicrobial activity [25]. A. hydrophila, which is already known to be multi-drugs resistant, was inhibited by pulp and seed extracts. These results are important due to the fact that A. hydrophila can produce several types of enterotoxins that cause dysenteric gastroenteritis [8,26].
In the study, the extracts from pulp and seed of A. laurina fruits showed that ethanol and methanol were a better extraction solvents of TPC, TFC, TMAC and TTC compared to ethyl acetate and water. However, the TTC in seed extracts was higher than pulp extracts. They were also found to be the most effective against both Gram-positive and Gram-negative bacteria strains. However, the fungal strains showed low sensitivity to pulp extract compared to seed extract. Additionally, our results revealed a significant and strong correlation between phenolic compounds and antioxidant activities. This study further requires the isolation and identification of bioactive compounds in the pulp and seed used.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (31271812), the National High Technology Research and Development Program of China (863 Program 2012AA022105C) for providing financial support for the study and Jeannine Sogony Koivogui from Guinea for facilitating transportation of raw materials to China.
References
[1] Rodríguez-Carpena JG, Morcuende D, Est´evez M. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: effect on lipid composition, oxidative stability and quality traits. Meat Sci 2012; 90: 106-15.
[2] Plazoni'c A, Bucar F, Males Z, Mornar A, Nigovi'c B, Kujundzi'c N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009; 14(7): 2466-90.
[3] Negi PS. Plant extracts for the control of bacterial growth: efficacy, stability and safety issues for food application. Int J Food Microbiol 2012; 156: 7-17.
[4] Zhang LB, Simmons MP, Renner SS. A phylogeny of Anisophylleaceae based on six nuclear and plastid loci: ancient disjunctions and recent dispersal between South America, Africa, and Asia. Mol Phylogenet Evol 2007; 44: 1057-67.
[5] Juncosa AM, Tomlinson PB. A historical and taxonomic synopsis of Rhizophoraceae and Anisophylleaceae. Ann Mo Bot Gard 1988; 75(4): 1278-95.
[6] Neuwinger HD. African traditional medicine: a dictionary of plant use and applications with supplement: search system for diseases. Stuttgart: Medpharm Scientific; 2000, p. 589.
[7] Traore MS, Bald´e MA, Diallo MS, Bald´e ES, Dian´e S, Camara A, et al. Ethnobotanical survey on medicinal plants used by Guinean traditional healers in the treatment of malaria. J Ethnopharmacol 2013; 150: 1145-53.
[8] Onivogui G, Diaby M, Chen X, Zhang H, Kargbo MR, Song Y. Antibacterial and antifungal activities of various solvent extracts from the leaves and stem bark of Anisophyllea laurina R. Br ex Sabine used as traditional medicine in Guinea. J Ethnopharmacol 2015; 168: 287-90.
[9] Kargbo MR, Onivogui G, Song Y. In vitro anti-diabetic activity and phenolic compound profile of ethanol extracts of Anisophyllea laurina R. Br. ex Sabine leaves and stem bark. Eur Acad Res 2015; 2(12): 16089-106.
[10] Onivogui G, Zhang H, Mlyuka E, Diaby M, Song Y. Chemical composition, nutritional properties and antioxidant activity of monkey apple (Anisophyllea laurina R. Br. ex Sabine). J Food Nutr Res 2014; 2(6): 281-7.
[11] Gouveia S, Castilho PC. Antioxidant potential of Artemisia argentea L'H´er alcoholic extract and its relation with the phenolic composition. Food Res Int 2011; 44(6): 1620-31.
[12] Sun B, Ricardo-da-Silva JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem 1998; 46(10): 4267-74.
[13] Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int 2005; 88(5): 1269-78.
[14] Lu M, Yuan B, Zeng M, Chen J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res Int 2011; 44(2): 530-6.
[15] Fern´andez-Agull´o A, Pereira E, Freire MS, Valentão P, Andrade PB, Gonz´alez-´Alvarez J, et al. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod 2013; 42: 126-32.
[16] Almeida MMB, de Sousa PHM, Arriaga AMC, do Prado GM, de Carvalho Magalhães CE, Maia GA, et al. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res Int 2011; 44(7): 2155-9.
[17] Ribeiro da Silva LM, Teixeira de Figueiredo EA, Silva Ricardo NM, Pinto Vieira IG, Wilane de Figueiredo R, Brasil IM, et al. Quantification of bioactive compounds in pulps and byproducts of tropical fruits from Brazil. Food Chem 2014; 143: 398-404.
[18] Contreras-Calder´on J, Calder´on-Jaimes L, Guerra-Hern´andez E, García-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res Int 2011; 44(7): 2047-53.
[19] Korekar G, Stobdan T, Singh H, Chaurasia OP, Singh SB. Phenolic content and antioxidant capacity of various solvent extracts from seabuckthorn (Hippophae rhamnoides L.) fruit pulp, seeds, leaves and stem bark. Acta Aliment 2011; 40(4): 449-58.
[20] Tekwu EM, Pieme AC, Beng VP. Investigations of antimicrobial activity of some Cameroonian medicinal plant extracts against bacteria and yeast with gastrointestinal relevance. J Ethnopharmacol 2012; 142(1): 265-73.
[21] Hasson SS, Al-Balushi MS, Sallam TA, Idris MA, Habbal O, Al-Jabri AA. In vitro antibacterial activity of three medicinal plants-Boswellia (Luban) species. Asian Pac J Trop Biomed 2011; 1(Suppl 2): S178-82.
[22] Naz R, Bano A. Antimicrobial potential of Ricinus communis leaf extracts in different solvents against pathogenic bacterial and fungal strains. Asian Pac J Trop Biomed 2012; 2(12): 944-7.
[23] Saravanan S, Parimelazhagan T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci Hum Wellness 2014; 3(2): 56-64.
[24] Khan AV, Ahmed QU, Mir MR, Shukla I, Khan AA. Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains. Asian Pac J Trop Biomed 2011; 1(6): 452-5.
[25] De Las Llagas MC, Santiago L, Ramos JD. Antibacterial activity of crude ethanolic extract and solvent fractions of Ficus pseudopalma Blanco leaves. Asian Pac J Trop Dis 2014; 4(5): 367-71.
[26] Malekinejad H, Tukmechi A, Ebrahimi H, Bazargani-Gilani B. One step forward to improve the latest method of antibacterial susceptibility testing of vitro-cultured bacteria: an implication for antibacterial efficacy of enrofloxacine on Aeromonas Hydrophila. World J Microbiol Biotechnol 2011; 27(1): 147-51.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.09.026
*Corresponding author:Yuanda Song, Center of Excellence for Functional Food and Health, School of Food Science and Technology, Jiangnan University, Wuxi, No. 1800 Lihu Road, 214122, China.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
- Comparative studies of elemental composition in leaves and flowers of Catharanthus roseus growing in Bangladesh
- Formulation and evaluation of semisolid jelly produced by Musa acuminata Colla (AAA Group) peels
- Prevalence of multi-drug resistant uropathogenic Escherichia coli in Potohar region of Pakistan
- Antibiotic resistance profile and RAPD analysis of Campylobacter jejuni isolated from vegetables farms and retail markets
