Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
E-mail: moussaoui.fadila@gmail.com
Peer review under responsibility of Hainan Medical University.
Fadila Moussaoui*, Tajelmolk Alaoui
Laboratory of Environment and Health, University Moulay Ismail, Faculty of Sciences, BP11201, Meknes, Morocco
ARTICLE INFO
Article history:
Received 9 Mar 2015
Received in revised form 17 Apr, 2nd revised form 26 Aug 2015
Accepted 19 Sep 2015
Available online 14 Nov 2015
Keywords:
Essential oils
Antibacterial
Synergies
Additive
Antagonistic
ABSTRACT
Objective: To demonstrate the in vitro antibacterial properties of five essential oils against ten bacterial strains and study the synergistic effect of the combination of essential oils with standard antibiotics.
Methods: Origanum compactum, Chrysanthemum coronarium, Thymus willdenowii Boiss, Melissa officinalis and Origanum majorana L. were used alone and combined used with standard antibiotics to evaluate their antimicrobial activities. The disk diffusion method was employed.
Results: The results showed that the combined application of the essential oils of the plants with antibiotics led to a synergistic effect in some cases, but antagonistic effect was also observed in some bacteria.
Conclusions: This study shows that the combination of essential oils of the five plants with antibiotics may be useful in the fight against emerging microbial drug resistance.
1. Introduction
Antibiotic resistance is a phenomenon as old as the advent of antibiotics. Antibiotics are from natural substances produced by fungi but also by certain bacteria to“defend”against other bacteria. The bacteria are not suicidal; the first who learned to synthesize antibiotics developed at the same time the means to protect themselves[1]. The development and spread of resistance to currently available antibiotics is a global concern[2]. With the increase in bacterial resistance to antibiotics, antimicrobials plant products have gained attention in the scientific research. The use of natural antimicrobial compounds is important not only in food preservation, but also in the control of human diseases and plant microbial origin[3]. The use of natural products with therapeutic properties, whether mineral, vegetable and animal, for a long time were the main sources of important therapeutic agents as well as important raw materials for the manufacture of traditional and modern medicines [4]. Medicinal plants are considered an important source of new chemical substances with potential therapeutic effects [5]. They contain a wide range of substances that can be used to treat chronic diseases, and infectious diseases. Essential oils are a very interesting group of secondary metabolites that are potentially useful sources of antimicrobial compounds. Many studies have been published on the antimicrobial activity of essential oils [6–9].
According to Enrico et al. [10], the essential oils, unlike antibiotics, are composed of many molecules so that bacteria cannot resist in mutant. Preventively and curatively, they are especially known for their potent antibacterial, antiviral, anti inflammatory, anti fungal, anti parasitic, antipyretic, expectorant, and mucolytic effects. The combination of essential oils with antibiotics therapeutic approach may lead to new ways to treat infectious diseases. Many researchers have studied experimentally the synergistic effect resulting from the combination of antibiotics with different plant extracts [11–14]. Indeed this combination therefore allowed reducing bacterial resistance to drugs [15]. This work was carried out in order to demonstrate the in vitro antibacterial properties of five essential oils against ten bacterial strains by disc diffusion method and study the synergistic effect of the combination of essential oils with standard antibiotics.
2. Materials and methods
2.1. Plant materials
Samples of Origanum compactum (O. compactum), Chrysanthemum coronarium (C. coronarium) and Thymuswilldenowii Boiss (T. willdenowii) were harvested in Kh´enifra while Melissa officinalis (M. officinalis) and Origanum majorana L. (O. majorana) were harvested in the Marrakech Region. The sample collection was conducted in the months of May to June, 2014. The samples were dried in the shade for 10 days before the steam distillation.
2.2. Hydrodistillation
The extraction of the essential oils was carried out by hydrodistillation in a Clevenger-type apparatus [16]. The essential oils were stored at 4°C in the dark and in the presence of anhydrous sodium sulfate.
2.3. Microorganisms
The antibacterial activity was evaluated using Gram positive bacteria: Staphylococcus aureus (S. aureus), and Gram negative bacteria: Escherichia coli (E. coli), E. coli (ATCC 25921), Klebsiella pneumoniae (K. pneumoniae), Proteus mirabilis (P. mirabilis), Pseudomonas aeruginosa (P. aeruginosa), P. aeruginosa (ATCC 27853), Pseudomonas putida (P. putida), Salmonella enteritidis (S. enteritidis) and Enterobacter aerogenes (E. aerogenes). All bacterial strains were provided from the microbiology laboratory of the hospital Mehemmed VI. Bacterial strains were maintained by subculture on nutrient agar favorable to their growth for 24 h in the dark at 37°C.
2.4. Antibiotics
The antibiotics–standard gentamicin (10 μg), tobramycin (30 μg), imipenem (10 μg) and ticarcillin (75 μg) were used.
2.5. Antimicrobial activity
The antimicrobial activity of the extracts was determined by the disk diffusion method which is based on the spread of antimicrobial compound in solid medium [17]. The Mueller–Hinton agar was poured in sterile petri dishes (90 mm diameter). The paper discs (6 mm diameter) that were impregnated with 2 μL of each pure essential oil and antibiotic and tested standard discs were placed on the inoculated agar surface. Petri dishes were allowed to stand for 30 min at room temperature before incubation at 37°C for 24 h. The effect of essential oils was reflected by the appearance around disc with a transparent circular zone corresponding to the absence of growth. The diameter of inhibition zone was measured in mm. The larger the diameter of the area the more susceptible the strain [18]. To evaluate the synergistic effect of the combination of the essential oils and antibiotics which are in the form of ready to use discs, 2 μL of each essential oil was saturated to the antibiotic disc to determine the zones of inhibition [19]. The obtained results were compared with those of the antibiotics tested on the same strains alone and by the same method.
2.6. Statistical analysis
All experiments were repeated three times. Results were presented as mean±SEM.
3. Results
3.1. T. willdenowii Boiss
T. willdenowii Boiss essential oil showed significant antibacterial characters against the tested microorganisms with exception of P. putida, P. aeruginosa and P. aeruginosa ATCC 27853 (Table 1).
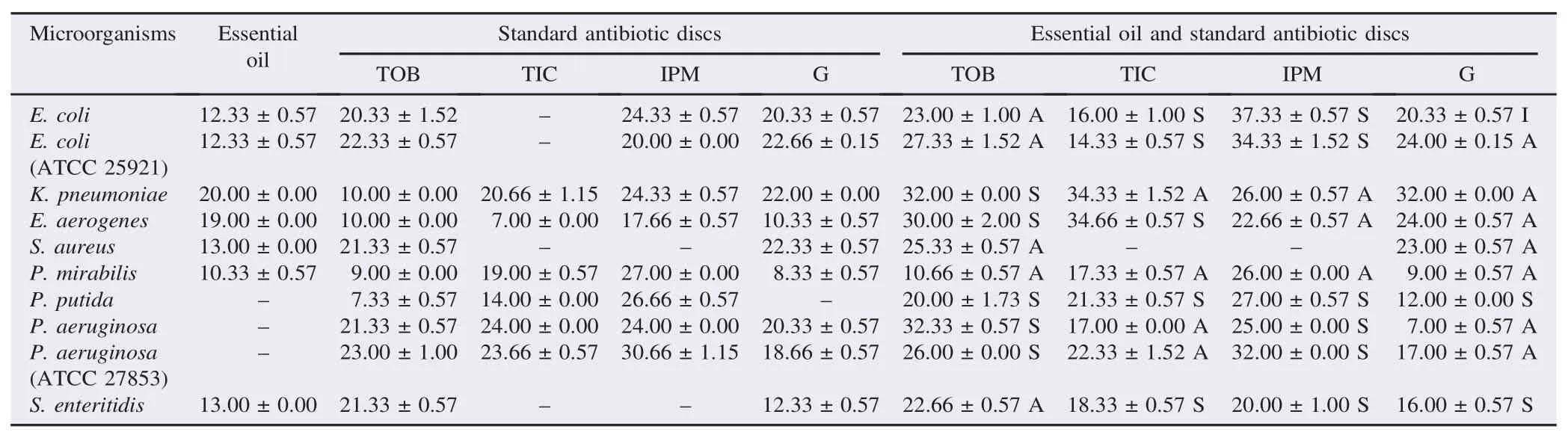
Table 1The antimicrobial activities (zones of inhibition) of essential oil of T. willdenowii and its synergistic effect with antibiotics. mm.
According to the obtained results, the combination of essential oil of T. willdenowii Boiss with tobramycin antibiotics showed an antagonistic effect against six tested bacteria (Table 1). A synergistic effect was observed in K. pneumoniae, P. aeruginosa, P. aeruginosa ATCC 27853, P. putida and E. aerogenes. The application of ticarcillin with the essential oil of T. willdenowii Boiss led to a synergistic effect on E. coli (ATCC 25921), E. coli, P. putida, S. enteritidis, E. aerogenes and P. aeruginosa. An antagonistic effect was observed in other bacteria. Combination of the essential oil of T. willdenowii Boiss with imipenem had an antagonistic effect against K. pneumoniae, E. aerogenes, P. mirabilis, and a synergistic effect against E. coli, E. coli (ATCC 25921), P. putida, P. aeruginosa, S. enteritidis and P. aeruginosa ATCC 27853. A synergistic effect was observed in P. putida, S. enteritidis, an undifferentiated effect in E. coli and an antagonistic effect were observed on the other tested bacteria when there was a combination of the essential oil of T. willdenowii Boiss and gentamisine.
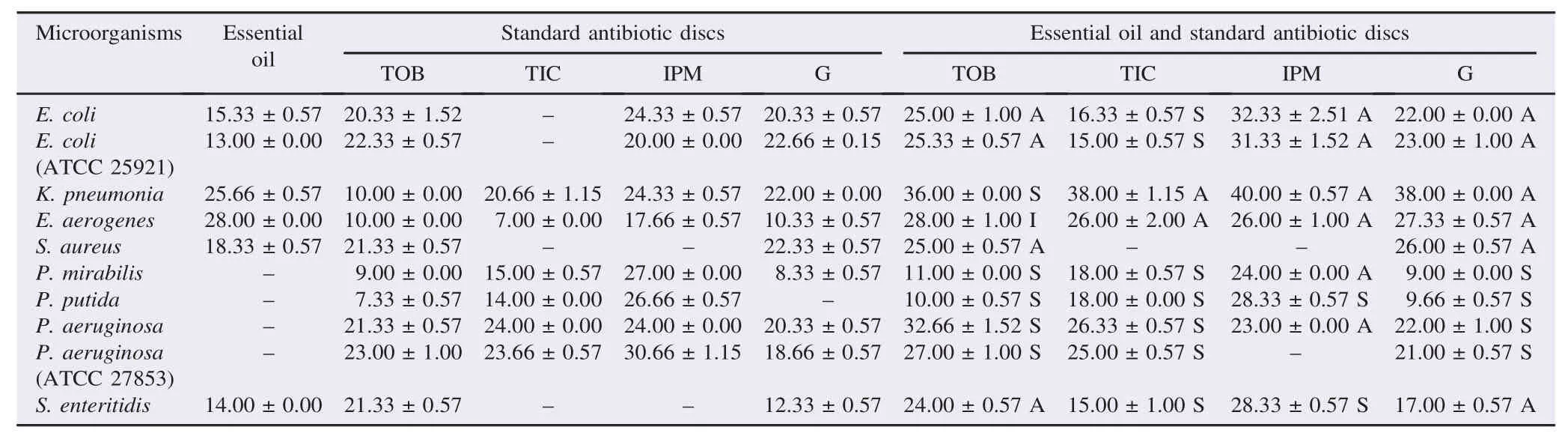
Table 2The antimicrobial activities (zones of inhibition) of essential oil of O. compactum L. and its synergistic effect with antibiotics. mm.
3.2. O. compactum L.
The essential oil of O. compactum L. showed a significant inhibitory effect against the studied microorganisms except for P. aeruginosa, P. aeruginosa (ATCC 27853), P. mirabilis and P. putida (Table 2).
A synergistic effect was observed in K. pneumoniae, P. putida, P. aeruginosa (ATCC 27853), P. aeruginosa, P. mirabilis, when there was a combination of O. compactum L. with tobramycin, while an antagonistic effect was seen on other bacteria. Ticarcillin with O. compactum L. had a synergistic effect in most bacteria, antagonistic effect was observed in K. pneumoniae and E. aerogenes. Combination of the essential oil of O. compactum L. with imipenem antibiotic disc led to a synergistic effect against P. putida and S. enteritidis and an antagonist effect in the rest of bacteria. A synergistic effect was also observed in P. putida, P. mirabilis, P. aeruginosa (ATCC 27853) and P. aeruginosa when the combination of essential oils of O. compactum L. with gentamicin was applied. The antagonistic effect has been observed in other bacteria.
3.3. O. majorana L.
Essential oils of O. majorana L. showed an average activity against E. coli, E. coli (ATCC 25921), K. pneumoniae, E. aerogenes, S. auereus and S. enteritidis with zones of inhibitions between (8.00±1.00) mm and (10.00±1.00) mm, while no effect was detected in P. putida, P. mirabilis, P. aeruginosa (ATCC 27853) and P. aeruginosa (Table 3).

Table 3The antimicrobial activities (zones of inhibition) of essential oil of O. majorana L. and its synergistic effect with antibiotics. mm.
From Table 3, it can be seen that the combination of the essential oil of O. majorana L with antibiotic discs tobramycin led to a synergistic effect against K. pneumoniae and P. putida. An antagonistic effect was observed in the other tested bacteria. An additive effect was observed in E. aerogenes, a synergistic effect in E. coli ATCC 25921, S. enteritidis and E. coli, and an antagonistic effect was seen in the other bacteria when the combination of essential oils of O. majorana and ticarcillin was applied. The combination of imipenem with the essential oil of O. majorana L. had a synergistic effect in E. coli (ATCC 25921), P. aeruginosa (ATCC 27853), P. aeruginosa, S. enteritidis and P. putida, while the antagonist effect was in other bacteria tested.
The combination of essential oil with gentamicin led to a synergistic effect against K. pneumoniae, an additive effect against E. aerogenes and an antagonistic effect against the rest of the bacteria. No inhibitory effect has been recorded on P. putida, P. mirabilis, P. aeruginosa.
3.4. M. officinalis L.
Essential oils of M. officinalis L. exhibited low activity against most of the bacteria with a zone of inhibition of(8.00±1.00) mm to (11.00±1.00) mm. And M. officinalis L. showed no activity against P. putida, P. aeruginosa (ATCC 27853), S. enteritidis and P. aeruginosa (Table 4).

Table 4The antimicrobial activities (zones of inhibition) of essential oil of M. officinalis L. and its synergistic effect with antibiotics. mm.
Applying ticarcillin with the essential oil of M. officinalis L. had a synergistic effect on E. coli, P. putida and K. pneumoniae (Table 4), an antagonist effect was observed in E. coli (ATCC 25921), P. mirabilis and P. aeruginosa. No inhibitory effect was found for the rest of the bacteria. The synergistic effect was observed in gentamicin and imipenime against P. mirabilis and S. enteritidis, respectively. An additive effect was observed in P. putida and P. aeruginosa, while a synergistic effect was observed in K. pneumoniae and P. mirabilis. Also, indifferent effect on S. aureus and antagonist effect in other bacteria were observed when there was a combination between essential oil of M. officinalis L. and tobramycin.
3.5. C. coronarium L.
Essential oil of C. coronarium L. had no antibacterial activity against the tested bacteria except for K. pneumoniae having a small zone of inhibition of (9.66±1.15) mm (Table 5).
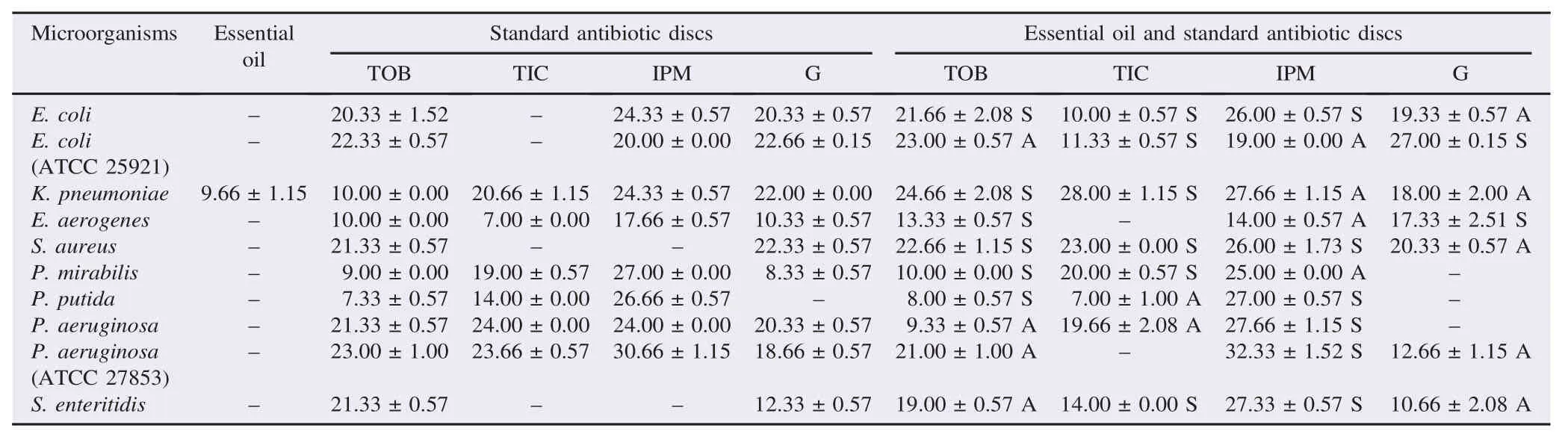
Table 5The antimicrobial activities (zones of inhibition) of essential oil of C. coronarium L. and its synergistic effect on antibiotics. mm.
The essential oil of C. coronarium L. exerted a synergistic effect against K. pneumoniae, P. mirabilis, P. putida, S. aureus, E. aerogenes and E. coli, and an antagonistic effect against E. coli (ATCC 25921), P. aeruginosa (ATCC 27853), P. aeruginosa and S. enteritidis when it was combined with tobramycin. The combination of ticarcillin with the essential oil of C. coronarium L. had a synergistic effect in E. coli, E. coli (ATCC 25921), K. pneumoniae, P. mirabilis and S. auereus, and an antagonistic effect in other bacteria. The application of imipenem with the essential oil of C. coronarium L. led to an antagonistic effect on E. coli (ATCC 25921), E. aerogenes, K. pneumoniae and P. mirabilis and a synergistic effect was observed in the other tested bacteria. A synergistic effect was observed in E. coli (ATCC 25921) and E. aerogenes, while the antagonistic effect was seen in the rest of the bacteria when there was a combination of the essential oil of C. coronarium L. with gentamicin.
4. Discussion
From this study, we can see that all antibiotics showed antibacterial activity against different bacterial strains, but at different levels. All the tested bacteria were more or less sensitive to five essential oils with the exception of P. aeruginosa. According to the literature P. aeruginosa is usually very sensitive to essential oils[20]. Khadir also showed that P. aeruginosa resisted the action of the essential oil of Thymus lanceolatus[21]. The essential oil of T. willdenowii Boiss showed significant antibacterial characters on the microorganisms tested with the exception of P. putida and P. aeruginosa. According to the literature, the essential oils of several species of thyme areknown for their antibacterial activities[22,23]. The application of T. willdenowii Boiss with ticarcillin, imipenem, gentamicin and tobramycin increased the antimicrobial activity of all tested antibiotics. However, an antagonistic effect was seen in some bacteria. Toroglu reported that the combination of essential oil of Thymus eigii and standard antibiotics in vitro led to an antagonistic effect on the tested bacteria [19].
The essential oil of O. compactum L. used in this study has antimicrobial activity against the tested strains with different diameters of inhibition zones from one strain to another with the exception of P. aeruginosa, P. aeruginosa (ATCC 27853), P. mirabilis and P. putida. Bouhdid et al. [24] also showed antimicrobial activity of essential oil of O. compactum L. against all tested bacteria except for P. aeruginosa which showed resistance. Our results were similar to the study. The application of O. compactum L. with ticarcillin, imipenem, gentamicin and tobramycin had a synergistic effect on all tested antibiotics against P. putida. While the antagonistic effect was also observed in some bacteria. Our result showed that the combined application of O. majorana L. with tested antibiotics led to the decrease in antimicrobial activity.
The essential oil M. officinalis L. showed relatively little effect against six bacterial strains with a zone of inhibition between (8.00±1.00) mm and (11.33±0.57) mm, but had no effect on P. putida, P. aeruginosa (ATCC 27853); P. aeruginosa and S. enteritidis. M. officinalis L. has been used internally and externally since ancient times for its sedative, digestive, appetizing, analgesic, antibacterial, antiviral and antioxidant activities[25]. The application of M. officinalis L. with ticarcillin, imipenem, gentamicin and tobramycin driven decreased antimicrobial activity against the bacteria, but a synergistic effect was also detected against some bacteria.
Essential oils of C. coronarium L. showed no antibacterial effect against all strains tested except K. pneumoniaee with very low sensitivity of (9.66±1.15) mm.
According to Felice et al., the essential oils of C. coronarium L. had no activity against E. coli, S. aureus and P. aeruginosa [26], which is consistent with the results of our study. The application of C. coronarium L. with ticarcillin, imipenem, gentamicin and tobramycin resulted in the decrease of the antimicrobial activity against the bacteria tested, but a synergistic effect was also detected against some bacteria.
This study indicates that the combination of essential oils of the five medicinal plants and the standard antibiotics has significant potential for the development of new antimicrobial treatment and reduction of drug resistance, which will permit to find the treatment of several diseases caused by microorganisms. From the results obtained, the essential oils acts in synergy with the tested standard antibiotics. This synergy could lead to new options for the treatment of infectious diseases and emerging drug resistance. There is a need for more studies on the molecular basis of the synergistic interaction to understand the synergistic mechanism that is fundamental for the development of pharmacological agents to treat bacterial infections using medicinal plants. Therefore, research should be focused in that direction to identify medicinal plants that have a synergistic behavior.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Lozniewski A, Rabaud C, Nancy. [Bacterial antibiotic resistance]. Lyon: CCLIN Sud-Est; 2010. French. [Online] Available from: http://nosobase.chu-lyon.fr/recommandations/cclin_arlin/ cclinSudEst/2010_ResistanceAntibiotiques_CClinSE.pdf [Accessed on 25th January, 2015]
[2] Chanda S, Rakholiya K. Combination therapy: synergism between natural plant extracts and antibiotics against infectious diseases. In: M´endez-Vilas A, editor. Science against microbial pathogens: communicating current research and technological advances. Badajoz: Formatex Research Center; 2011, p. 520-9.
[3] Choi HS. Antioxidative activity. In: Sawamura M, editor. Citrus essential oils:flavor and fragrance. New Jersey: John Wiley & Sons, Inc; 2010, p. 231-43.
[4] Ishrat JB, Laizuman N, Farhana AR, Obaydul H. Antibacterial, cytotoxic an antioxidant activity of chloroform, n-hexane and ethyl acetate extract of plant Amaranthus spinosus. Int J Pharm Tech Res 2011; 3(3): 1675-80.
[5] Blumenthal M. Herbal medicines: expanded commission E monographs. Boston: Integrative Medicine Communications; 2000, p. 419-23.
[6] Mihajilov-Krtev T, Radnovic D, Kitic D, Stojanovic-Radic Z, Ziatkovic B. Antimicrobial activity of Satureja hortensis essential oil against pathogenic microbial strains. Arch Biol Sci Belgr 2010; 62(1): 159-66.
[7] Sokovi'c M, Glamoˇclija J, Marin PD, Brki'c D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010; 15: 7532-46.
[8] Coisin M, Burzo I, Stefan M, Elida Rosenhech, Zamfirache MM. Chemical composition and antibacterial activity of essential oils of three Salvia species, widespread in Eastern Romania. Sci Ann Alexandru Ioan Cuza Univ Iasi New Ser Sect 2 Veg Biol 2012; 58: 51-8.
[9] Sepahvand R, Delfan B, Ghanbarzadeh S, Rashidipour M, Veiskarami GH, Ghasemian-Yadegari J. Chemical composition, antioxidant activity and antibacterial effect of essential oil of the aerial parts of Salvia sclareoides. Asian Pac J Trop Med 2014; 7(Suppl 1): S491-6.
[10] Enrico V, Andrea P, Francesca B, Min ZJ. [Experimental study of reinforcement of immunity by syrup of father Michel (POE 20)]. Italy: Institute of Naturopathic Sciences (ISN); 2004. French. [Online] Available from: http://www.crao.ch/data/fichiers/Article% 20SPM%206%20pages.pdf [Accessed on 12th January, 2015]
[11] Ghaleb A, Mhanna M. Synergistic effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimens. Middle East J Sci Res 2008; 3(3): 134-9.
[12] Aiyegoro OA, Afolayan AJ, Okoh AI. Synergistic interaction of Helichrysum pedunculatum leaf extracts with antibiotics against wound infection associated bacteria. Biol Res 2009; 42: 327-38.
[13] Stefanovic O, Comic L. Synergistic antibacterial interaction between Melissa officinalis extracts and antibiotics. J Appl Pharm Sci 2012; 2: 1-5.
[14] Olajuyigbe OO, Afolayan AJ. Synergistic interactions of methanolic extract of Acacia mearnsii De Wild. with antibiotics against bacteria of clinical relevance. Int J Mol Sci 2012; 13: 8915-32.
[15] Abascal K, Yarnell E. Herbs and drug resistance: part 2—clinical implications of research on microbial resistance to antibiotics. Altern Complement Ther 2002; 8: 284-90.
[16] Clevenger JF. Apparatus for the determination of volatile oil. J Am Pharm Assoc 1928; 17(4): 345-9.
[17] Lesueur D, de Rocca Serra D, Bighelli A, Hoi TM, Ban NK, Thai TH, et al. Chemical composition and antibacterial activity of essential oil of Michelia faveolata Meryll ex Dandy from Vietnam. Flavour Fragr J 2007; 22: 317-21.
[18] Choi YM, Noh DO, Cho SY, Suh HJ, Kim KM, Kim JM. Antioxidant and antimicrobial activities of propolis from several regions of Korea. LWT Food Sci Technol 2006; 39: 756-61.
[19] Toroglu S. In vitro antimicrobial activity and antagonistic effect of essential oils from plant species. J Environ Biol 2007; 28: 551-9.
[20] De Martino L, De Feo V, Nazzaro F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009; 14: 4213-30.
[21] Khadir A, Bendahou M, Benbelaid F, Abdoune MA, Abdelouahid DE.[Antimicrobialpowerof Thymuslanceolatus Desf., harvested in Algeria]. Phytoth´erapie 2013; 11(6): 353-8. French.
[22] Amarti F, Satrani B, Aafi A, Ghanmi M, Farah A, Aberchane M, et al. [Chemical composition and antimicrobial activity of the essential oils of moroccan Thymus capitatus and Thymus bleicherianus]. Phytoth´erapie 2008; 6: 342-7. French.
[23] Cheurfa M, Allem R, Sebaihia M, Belhireche S. [Effect of essential oil of Thymus vulgaris on bacterial pathogens responsible for gastroenteritis]. Phytoth´erapie 2013; 11: 154-60. French.
[24] Bouhdid S, Abrini J, Zhiri A, Espuny MJ, Manresa A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J Appl Microbiol 2009; 106(5): 1558-68.
[25] Carron CA, Vouillamoz J, Baroffio C. [Melissa officinalis: agrotextile cover and dry matter yield, essential oil and rosmarinic acid]. Rev suisse Vitic Arboric Hortic 2013; 45(5): 276-82. French.
[26] Felice S, Rigano D, De Fusco R, Bruno M. Composition of the essential oil from flowerheads of Chrysanthemum coronarium L. (Asteraceae) growing wild in Southern Italy. Flavour Fragr J 2004; 19: 149-52.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.09.021
*Corresponding author:Fadila Moussaoui, Laboratory of Environment and Health, University Moulay Ismail, Faculty of Sciences, BP11201, Meknes, Morocco.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.09.024
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Isolation of aerobic bacteria from ticks infested sheep in Iraq
- Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice
- Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
- Antifungal activity of plant extracts with potential to control plant pathogens in pineapple
- Potent water extracts of Indonesian medicinal plants against PTP1B
