The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
E-mail: pratiwi@fa.itb.ac.id
Peer review under responsibility of Hainan Medical University.
Pratiwi Wikaningtyas*, Elin Yulinah Sukandar
Pharmacology and Clinical Pharmacy Research Group, School of Pharmacy, Bandung Institute of Technology,
Ganesa Street 10, Bandung 40132, Indonesia
ARTICLE INFO
Article history:
Received 29 Apr 2015
Received in revised form 24 Jun, 2nd revised form 3 Jul, 3rd revised form 4 Jul 2015
Accepted 20 Aug 2015
Available online 10 Nov 2015
Keywords:
Medicinal plants
Antibacterial activity
Minimum inhibitory concentration Resistance bacteria
Secondary metabolites
ABSTRACT
Objective: To evaluate the antibacterial activity of eight plants against methicillinresistant Staphylococcus aureus (MRSA), extended spectrum beta-lactamase and carbapenemase-resistant Enterobacteriaceae, which are the most prevalent causes of infections in inpatients.
Methods: The antibacterial activity was calculated based on the minimum inhibitory concentration using Mueller–Hinton broth in a microdilution method.
Results: The best antibacterial activity, calculated as minimum inhibitory concentration values, against MRSA was shown by the Kaempferia pandurata (Roxb) (K. pandurata) extract (256 μg/mL) and the Senna alata (S. alata) extract (512 μg/mL). Phytochemical screening of dried S. alata leaf and its extract showed the presence of flavonoids, alkaloids, saponins, quinones, tannins and sterols, while dried K. pandurata and its extract only showed the presence of flavonoids and sterols/triterpenoids.
Conclusions: K. pandurata and S. alata have the potential to be developed as antibacterial agents, especially against MRSA strain, but further in vivo research and discovery of the mode of its action are still needed to shed light on the effects.
1. Introduction
Antibiotic resistance has become a serious and widespread problem in developing countries, both in hospitals and the community, causing high mortality each year [1]. Inappropriate usage of antibiotics is the most influential factor of antibiotic resistance and the global emergence of multi-drug resistant bacteria is increasingly limiting the effectiveness of current drugs and significantly causing treatment failure [2]. Antibiotic resistance results in reduced efficacy of antibacterial drugs, making the treatment of patients difficult, costly, or even impossible. The impact on particularly vulnerable patients is most obvious, resulting in prolonged illness and increased mortality [3]. New therapy classes of antibiotics have become a popular choice to reduce antibiotic resistance. However, antibiotic resistance is difficult to reduce. One strategy to avoid this is by using alternative therapeutic agents from plants that are effective against antibiotic resistant bacteria, safe and have low cost. Consequently, one of the objectives of our research group is to investigate the potential antibacterial properties of traditional plants. In the present study, we used eight plants that have the potential to be used as antibacterial agents against non-resistant bacteria [4–11], to conduct antibacterial activity assays against resistant bacteria isolated from inpatients, such as methicillin-resistant Staphylococcus aureus (S. aureus) (MRSA), extended spectrum beta lactamase (ESBL)-producing bacteria and carbapenemase-resistant Enterobacteriaceae (CRE). The eight plants used in this study were Curcuma xanthorrhiza (C. xanthorrhiza), Ocimum sanctum (O. sanctum), Senna alata (S. alata), Kaempferia pandurata (Roxb) (K. pandurata), Zingiber officinale (Z. officinale), Moringa oleifera (M. oleifera), Tamarindus indica (T. indica) and Pangium edule (P. edule).
2. Materials and methods
2.1. Plants
Fresh plant parts were collected from the Manoko field in Bandung at winter season. The collected plants were identified and classified according to the Herbarium Bandungense at theSchool of Life Sciences and Technology research centre. They were then dried under sunlight for two days. The dried plants were then milled into fine powder using a milling machine.
2.2. Materials
The used materials were ninety-six microwell plates, autoclave, shaker, laminar air flow, Eppendorf, micropipette, separation funnel, glass set, chromatography set and MRSA collected from hospital, ethanol, n-hexane, ethyl acetate, water, dimethyl sulfoxide, Mueller–Hinton broth and Mueller–Hinton agar, curved.
2.3. Preparations of extracts
Dried C. xanthorrhiza, O. sanctum, S. alata, K. pandurata, Z. officinale and M. oleifera were extracted by the reflux method using ethanol 96% while T. indica and P. edule were extracted by maceration using distilled water (aqua dest). The solvent in the extracts was completely removed by using a rotary evaporator to obtain a semi-solid mass and the yield was calculated based on the weight of the dried plants. A portion of the resulting crude extract was fractioned by a separation funnel using n-hexane, ethyl acetate, and water as solvents. Eluates were collected in 1 L Erlenmeyer flasks and each fraction was subjected to evaporation under reduced pressure in a rotary evaporator. Fractions were stored at 4°C until assayed.
2.4. Bacterial preparation
The MRSA, ESBL-producing bacteria and CRE were taken from isolated specimens which exhibited resistance to some antibiotics in hospitalized patients. They were taken based on ethical clearance approval from the ethical committee in the hospital. The bacteria were cultured over night (18–24 h) at 37°C on nutrient broth for the preparation of cell suspensions. The bacteria cell suspensions were homogenized and adjusted to 0.5 McFarland standards (5×105CFU/mL) using spectrophotometry.
2.5. Antimicrobial susceptibility assays
The minimum inhibitory concentrations (MICs) of plant extracts were initially determined using Mueller–Hinton broth microdilution[12]. MIC determination was performed by a serial dilution technique using 96-well microtiter plates. Plant extract (100 μL) was placed into the well/plate. Then, 100 μL bacterial cell suspensions were placed in each well/plate. Microplates were incubated for 24 h at 37°C. The lowest concentrations without visible growth completely inhibited the bacteria (MICs). Dimethyl sulfoxide was used as a control and Mueller–Hinton broth as negative control. Tetracycline and vancomycin were used as positive controls for MRSA, while cefotaxime and meropenem were used as positive controls for ESBL–producing bacteria and CRE. The assay was repeated twice with three replicates per assay.
2.6. Phytochemical screening
The selected plants which showed the MIC were screened for the presence of different classes of secondary metabolites, including alkaloids,flavonoids, alcaloid, saponins, tannins, quinones and sterols/triterpenes using previously described methods[13].
3. Results
3.1. Extracts yield
The ethanolic extracts of eight plants and the water extracts of two plants were calculated for the yield. T. indica showed the highest yield in water solvent, which showed that its constituents were relatively polar (Table 1).
3.2. The antibacterial activity
The antibacterial activity of the eight extracts were assayed in vitro by the agar microdilution method against three resistant bacteria. The antibacterial activity against each bacterium was observed to be varied. Table 2 shows that among the eight plants, K. pandurata exhibited the smallest value of MIC against MRSA (256 μg/mL) (Figure 1), while S. alata and C. xanthorrhiza also had the same activity against MRSA (Figures 2 and 3). This showed that K. pandurata, S. alata and C. xanthorrhiza had the potential to be developed as antibacterial agents for MRSA strains.
3.3. Phytochemical analysis
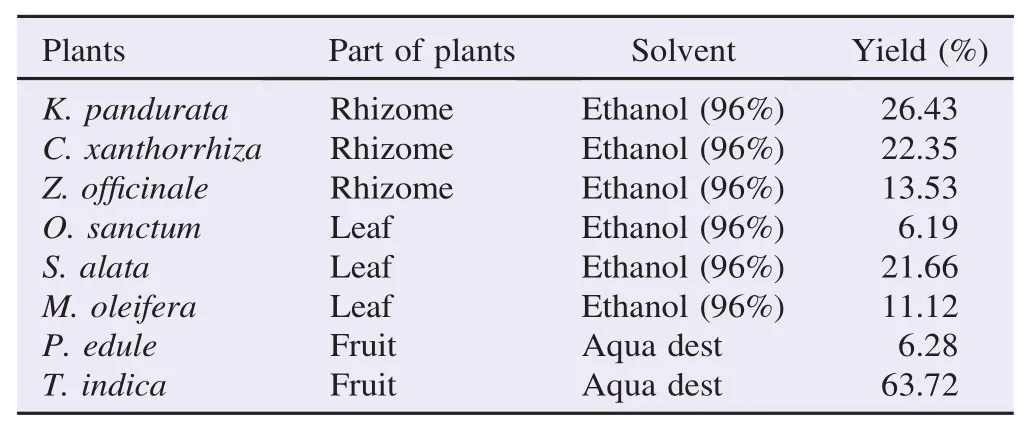
Table 1The extract yield.

Table 2The antibacterial activity of eight plants towards MRSA, ESBL and CRE.
The screening of the phytochemical composition was conducted only for the two dried plants, S. alata and K. pandurata, as well as their extracts, that showed the lowest MIC, because they have the potential to be developed as antibacterial agents. The secondary metabolites are shown in Table 3. All testedsecondary metabolites were present in the S. alata extract. In K. pandurata, only flavonoids and steroid/triterpenoids were present.
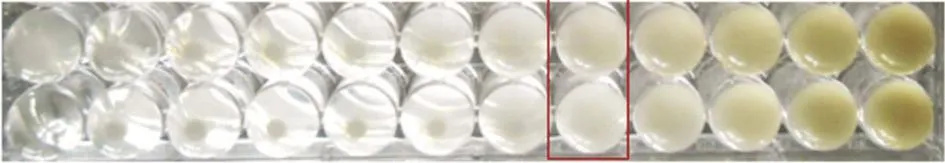
Figure 1. MIC value of K. pandurata against MRSA at 256 μg/mL.

Figure 2. MIC value of S. alata against MRSA at 512 μg/mL.

Figure 3. MIC value of C. xanthorrhiza against MRSA at 512 μg/mL.
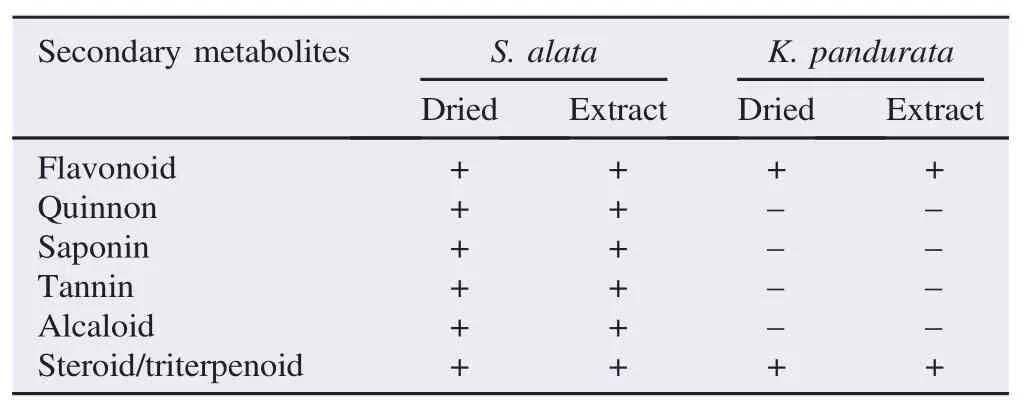
Table 3Results of phytochemical analysis of dried S. alata and its extracts.
4. Discussion
The research for new antibacterial agents has become a very important endeavor, especially in recent times, considering the escalating levels of antibiotic resistance among pathogenic bacteria. One of the efforts in this research is focused on the use of medicinal plants, which are widely available resources, less if no side effects, less expensive and have shown antimicrobial properties [14]. Also, the therapeutic properties of medicinal plants are well recognized at a global level, especially for antibiotic development. Thus, the research of alternative and effective medicines from plants against such resistant bacteria has become an important concern all over the world [15]. Antibiotic misuse has been considered as a major cause of antibiotic resistant bacteria. As a result, bacteria become resistant to antibiotics, which are in turn less effective after extended periods of use. Pharmaceutical companies whose efforts are focused on the production and manufacture of antibiotics strive to manufacture new generations of antibiotics which are capable of treating such antibiotic-resistant bacterial strains [16].
The evolution of antibiotic-resistant bacteria has left researchers scrambling to develop new and stronger antibiotics. MRSA, ESBL-producing bacteria and CRE are commonly occurring drug resistant bacteria in hospitals. MRSA is a major pathogen causing nosocomial and community acquired infection throughout the world. It is also one of the leading causes of skin, soft tissue, bone, joint, abscess and normal heart valve infections and its infections in humans have been associated with excess morbidity, mortality and increased length of hospitalization [14]. ESBL is an enzyme that mediates resistance to third generation cephalosporins (e.g., ceftazidime, cefotaxime and ceftriaxone) and monobactams (aztreonam), but do not affect cephamycins (cefoxitin and cefotetan) or carbapenems (meropenem and imipenem). Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca are the most common ESBL-producing pathogens[17].
The microdilution method was used in the present study because it is a quantitative reference method routinely used in clinical laboratories. In this method, susceptibility panels in 96-well microtiter plates contained various concentrations of antimicrobial agents. Then, standardized numbers of bacteria were inoculated into the wells of the microtiter plates and incubated overnight at 35°C. The MIC value was observed as the lowest concentration where no viability was observed in the wells after incubation. Compared with agar-based methods, broth microdilution can decrease much labor and time[18].
Each of the tested extracts displayed antibacterial activity against MRSA, ESBL-producing bacteria and CRE. Furthermore, all extracts showed highly varying MIC values against those resistant bacteria, but the lowest MIC value belonged to S. alata leaf extract (512 μg/mL) and K. pandurata (256 μg/mL) against MRSA. A previous study has shown that S. alata is susceptible to Streptococcus pyogenes and S. aureus, and the ethanolic extract of K. pandurata had antibacterial activity against MRSA, methicillin-susceptible S. aureus and Salmonella typhi at varying values of MIC[7]. MRSA has the ability to grow in the presence of beta-lactams and its derivatives, including cephalosporin and penicillin. This resistance is intrinsic and can be transferred to susceptible strains through horizontal transfer of the mecA gene[16]. All plant extracts showed no antibacterial activity with their high MIC values against ESBL-producing bacteria and CRE.
The results obtained from the present study provide evidence that ethanolic extracts of S. alata and K. pandurata exhibit antibacterial activities against isolated MRSA strain, which suggests that they may be clinically useful. Further research in respect of these findings are needed and are promising.
Preliminary phytochemical analyses revealed that K. pandurata only consisted of flavonoids and sterols/triterpenoids while S. alata contained flavonoids, alkaloids, saponins, tannins, quinones, and sterols/triterpenoids. These bioactive compounds have been reported to be used by plants for protection against bacterial and are responsible for antimicrobal
activity [7,19,20].
K. pandurata and S. alata were shown to be potentially developed as antibacterial agents, especially for MRSA strain. Further in vivo research and discovery of mode action are needed to shed light on their antibacterial effects.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We would like to acknowledge Directorate General for Higher Education of Republic of Indonesia for providing funding for this research.
References
[1] Gyles C. The growing problem of antimicrobial resistance. Can Vet J 2011; 52(8): 817-20.
[2] Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement Altern Med 2013; 13: 164.
[3] World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014. [Online] Available from: http://apps.who.int/iris/bitstream/10665/ 112647/1/WHO_HSE_PED_AIP_2014.2_eng.pdf [Accessed on 1st April, 2015]
[4] Diastuti H, Syah YM, Juliawaty LD, Singgih M. Antibacterial Curcuma xanthorrhiza extract and fractions. J Math Fundam Sci 2014; 46(3): 224-34.
[5] Mathew S. An evaluation of the antimicrobial activity of various concentrations of Ocimum sanctum against various species of bacteria: an in vitro study. Int J Adv Appl Sci 2014; 3(1): 33-6.
[6] Ogunjobi AA, Abiala MA. Antimicrobial activity of Senna alata and Phyllanthus amarus. Glob J Pharmacol 2013; 7(2): 198-202.
[7] Sukandar EY, Sunderam N, Fidrianny I. Activity of Kaempferia pandurata (Roxb.) rhizome ethanol extract against MRSA, MRCNS, MSSA, Bacillus subtilis and Salmonella typhi. Pak J Biol Sci 2014; 17(1): 49-55.
[8] Hasan HA, Raauf AMR, Razik BMA, Hassan BAR. Chemical composition and antimicrobial activity of the crude extracts isolated from Zingiber officinale by different solvents. Pharm Anal Acta 2012; 3: 9.
[9] Oluduro AO. Evaluation of antimicrobial properties and nutritional potentials of Moringa oleifera Lam. leaf in South-Western Nigeria. Malays J Microbiol 2012; 8(2): 59-67.
[10] Gupta C, Prakash D, Gupta S. Studies on the antimicrobial activity of tamarind (Tamarindus indica) and its potential as food biopreservative. Int Food Res J 2014; 21(6): 2437-41.
[11] Mora E, Emrizal Mulyantika E. [Isolation of compound from ethyl acetate of kepayang leaves (Pangium edule Reinw) and its antibacterial activity test]. Farmasains 2014; 2(3): 1-6. Indonesian.
[12] Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility test for bacterial that grow aerobically. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
[13] Wadood A, Ghufran M, Jamal SB, Naeem M, Khan A, Ghaffar R, et al. Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochem Anal Biochem 2013; 2: 144.
[14] Rubens DM, Constantin OO, Moevi AA, Nathalie GK, Daouda T, David NJ, et al. Anti Staphylococcus aureus activity of the aqueous extract and hexanic fraction of Thonningia sanguinea (Cote ivoire). Int J Pharmacogn Phytochem Res 2015; 7(2): 301-6.
[15] Adnan M, Bibi R, Mussarat S, Tariq A, Shinwari ZK. Ethnomedicinal and phytochemical review of Pakistani medicinal plants used as antibacterial agents against Escherichia coli. Ann Clin Microbiol Antimicrob 2014; 13: 40.
[16] Abouzeed YM, Elfahem A, Zgheel F, Ahmed MO. Antibacterial in-vitro activities of selected medicinal plants against methicillin resistant Staphylococcus aureus from Libyan environment. J Environ Anal Toxicol 2013; 3: 194.
[17] Madhavan HN, Murali S. Mechanisms of development of antibiotic resistance in bacteria among clinical specimens. J Clin Biomed Sci 2011; 1(2): 42-8.
[18] Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. 10th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
[19] Najafi S. Phytochemical screening and antibacterial activity of leaf extract of Ziziphus mauritiana Lam. Int Res J Appl Basic Sci 2013; 4(11): 3274-6.
[20] Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013; 2013: 162750.
Original article http://dx.doi.org/10.1016/j.apjtb.2015.09.023
*Corresponding author:Pratiwi Wikaningtyas, Pharmacology and Clinical Pharmacy Research Group, School of Pharmacy, Bandung Institute of Technology, Ganesa Street 10, Bandung 40132, Indonesia.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants
- Isolation of aerobic bacteria from ticks infested sheep in Iraq
- Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice
- Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
- Antifungal activity of plant extracts with potential to control plant pathogens in pineapple
- Potent water extracts of Indonesian medicinal plants against PTP1B
