Antifungal activity of plant extracts with potential to control plant pathogens in pineapple
Antifungal activity of plant extracts with potential to control plant pathogens in pineapple
Tel: +55 27 33357251
E-mail: debora.dummer.meira@gmail.com
Peer review under responsibility of Hainan Medical University.
Foundation Support: Supported by the Espírito Santo Research Foundation and National Council for Scientific and Technological Development for research grants (Proc.: 46704469/2009 and 307752/2012-7).
Maria Diana Cerqueira Sales1,2, Helber Barcellos Costa1,2, Patrícia Machado Bueno Fernandes1,
Jos´e Aires Ventura1,3, Debora Dummer Meira4*1Center for Biotechnology, Federal University of Espírito Santo-UFES, Campus Maruípe, Vit´oria, Espírito Santo, Brazil
2Laboratory of Research, Development and Innovation in Natural Products and Biotechnology-College of Santa Casa de
Miseric´ordia de Vit´oria, Vit´oria, Espírito Santo, Brazil
3Capixaba Institute of Research, Technical Assistance and Rural Extension-Incaper, Vit´oria, Espírito Santo, Brazil
4Department of Biological Sciences, Federal University of Espírito Santo-UFES, Campus Maruípe, Vit´oria, Espírito Santo, Brazil
ARTICLE INFO
Article history:
Received 21 Aug 2015
Received in revised form 2 Sep 2015
Accepted 27 Sep 2015
Available online 14 Nov 2015
Keywords:
Ananas comosus var. comosus
Bioassay
Fungi
Natural plant products
Mother tincture
ABSTRACT
Objective: To evaluate the in vitro antifungal activity of extracts, resins, oils and mother tinctures from plants against the filamentous fungi Fusarium guttiforme (F. guttiforme) and Chalara paradoxa, and to evaluate the control of the pineapple fusariosis in situ using mother tinctures.
Methods: The screening of the antifungal potential of 131 extract forms from 63 plant species was performed in vitro by using plate-hole method. To control pineapple fusariosis in situ, preventive and post-infection treatments were performed on detached pineapple leaves of cv. P´erola (susceptible).
Results: The quantitative study indicated that among the 49 mother tincture samples analyzed, 46% were effective against F. guttiforme and 29%for the Chalara paradoxa. The natural plant extracts, mother tincture of Glycyrrhiza glabra (MTGG1), mother tincture of Myroxylon balsamum (MTBT2), mother tincture of Aloe vera (MTAV3), mother tincture of Allium sativum (MTAS4), resin of Protium heptaphyllum (RESAM5) and crude extracts of Rhizophora mangle (CEMV6), exhibited an antifungal activity against F. guttiforme. In the preventive treatment against pineapple fusariosis, MTAV3, MTAS4 and MTGG1 were statisticallysimilartothetreatmentwithtebuconazolfungicide.Thecurativetreatmentswith MTAV3, MTAS4, MTGG1 and MTBT2 presented similar activity to fungicide (P<0.05). Conclusions: The findings of the present study concluded that mother tinctures can effectively control phytopathogens. The mother tincture extract of Myroxylon balsamum showed antifungal activity and was used here for the first time for inhibition of phytopathogenic fungi. This study paves the way for the development of bioactive natural products with phytosanitary applications, with the added benefits of an environmentally safe and economically viable product.
1. Introduction
Food security exists when all people, at all times, have physical, social and economic access to sufficient, safe and nutritious food [1]. Currently, the consequences derived from application of fungicides in traditional agricultural production systems for control of crop diseases have impacted negatively this activity [2].
Brazil is a major pineapple producer, but this crop has serious phytosanitary diseases. Moreover, the yield of pineapple, Ananas comosus (L. Merril) var. comosus (Coopens & Leal) is still considered low particularly due to fusariosis, a disease caused by the fungus, Fusarium guttiforme Nirenberg & O'Donnell (F. guttiforme) (synonym: Fusarium subglutinans f. sp. ananas Ventura, Zambolim & Gilb). Losses can reach up to 100% of the fruit production under certainconditions that engender plant susceptibility. Other diseases such as butt rot in suckers and black rot of post-harvest fruit, caused by the fungus Chalara paradoxa (C. paradoxa) (synonym: Thielaviopsis paradoxa), are also responsible for losses in propagative material, naturally consumed fruit and fruit destined for the processing industry [3]. Therefore, new substances and methods for the control of post-harvest diseases are needed [4].
Chemical fungicides has often been used to control these diseases, but this conduct is associated to negative environmental impacts, potential human exposure to pesticides, and deposition of residues on the fruits. However, the effectiveness of synthetic fungicides has been reduced by the frequent development of resistance by the pathogens. Hence there is a great demand for safer, alternative and effective chemotherapeutic agents [5,6].
Currently, the search for natural products with novel uses, particularly related to pest management is very active. Aromatic and medicinal plants have attracted interests in the field of plant disease control, particularly plant extracts with antimicrobial properties and contain a spectrum of secondary metabolites such as alkaloids, quinones,flavonoids, glycosides, saponins, tannins and terpenoids. The concentration of these bioactive compounds in each plant species depends on the environmental conditions and pathosystem [6–8].
Studies have shown that the extraction method of medicinal plants have profound effect on the isolation of antimicrobial chemical principles [9]. Bioactive natural products can be extracted from species with significant antifungal activity, such as Aloe vera (L.) Burm F. (A. vera) (aloe) [10–12], Glycyrrhiza glabra L. (G. glabra) (licorice) [13–15], and Allium sativum L. (A. sativum) (garlic) [16], as well as from Myroxylon balsamum L. (M. balsamum) (balsam of Tolu), by different extraction methods. To date, the balsam of Tolu was used in medicine as an antiseptic, antiparasitic, and cicatrizant agent [17], but has not been related as effective against phytopathogenic fungi.
In this work, the present study was intended to explore the in vitro antifungal potential of extracts, resins, oils and mother tinctures against the filamentous fungi F. guttiforme and C. paradoxa, and to evaluate in situ, the pineapple fusariosis control by mother tinctures, on detached pineapple leaves of cv. P´erola, a variety of the Ananas comosus (L. Merril) susceptible to diseases caused by these plant pathogens.
2. Materials and methods
2.1. Plant extracts
Plant extract samples, such as mother tincture (MT), resin (RES), oil (OIL), and crude extracts (CE) and their fractions were obtained from plant species. The extract forms, MTs, represented 92% of the total researched plant extracts and 67% of the species selected in the screening performed in the diffusion assay in vitro. These MTs and the Copaifera reticulata (Copaíba) plant oil were prepared following standardized techniques in industrialized laboratories (Almeida Prado Homeopathic Laboratory, São Paulo, Brazil).
The latices from Jatropha curcas (Pinhão manso) and Synadenium sp. were obtained from plants cultivated at the Viana experimental farm (Capixaba Institute of Research, Technical Assistance and Rural Extension–Incaper). The resins from the Protium heptaphyllum (P. heptaphyllum) and samples of the mangrove species: Avicennia sp., Laguncularia racemosa, and Rhizophora mangle (R. mangle), were collected in 2008 at the Ruschi Marine Biology Station in Santa Cruz, Aracruz, Espírito Santo, Brazil. The extracts (barks and leaves of the mangrove species) were prepared using maceration at the Pharmacognosy Laboratory of the Brazilian College (Univix), Vit´oria, Espírito Santo, Brazil. The solvent used was 96% ethanol for 7 days. After filtration, extracts were evaporated in a rotational evaporator at a temperature below 50°C and at 90 r/min to constant weight. Dry extracts were then dissolved in 10% dimethyl sulfoxide (DMSO).
The other extract forms such as crude extracts and fractions were prepared by the Technology Laboratory of Natural Products (LPPN) of the Fluminense Federal University (UFF), Brazil.
2.2. Fungi
Reference fungi, F. guttiforme (E-203) and C. paradoxa (E-411), were obtained from the fungi collection of the Incaper Phytopathology Laboratory and cultivated in potato dextrose agar (PDA; Acumedia Laboratories, Michigan, USA) medium and incubated at 25°C. The fungi strains were maintained by using Castellani's method[18].
2.3. In vitro screening using plate-hole diffusion method
The antifungal activity of 131 samples of extracts was assayed through screening of the F. guttiforme and C. paradoxa fungi, by the diffusion technique on PDA growth medium[16,19]. The fungal suspension was standardized to 106conidia/mL in sterile saline solution (0.85%) and 100 μL of each fungal suspension was spread onto the surface of the Petri dishes. After 10 min of rest, 5-mm-diameter holes were punched and filled with 100 μL of the previously prepared extract samples. As control samples for each experiment, DMSO, hydroethanolic solution was used at 70% and the fungicide tebuconazole (TEB) (Folicur®20EC) was used at 0.1%. Subsequently, the plates were incubated at (28±2)°C. Each extract form was evaluated with 3 repetitions, and the assessment was conducted after 72 h by measuring the diameter of the inhibition of the fungi mycelial growth (clear zone of inhibition formed around were considered indicative of antifungal activity).
2.4. Control of fusariosis (in situ)
Assays were performed on detached leaves from the pineapple cv. P´erola, a susceptible variety, which was previously disinfected using sodium hypochlorite (1%) followed by sterile distilled water. Each leaf was inoculated by making a 5 mm diameter wound, in which 100 μL of F. guttiforme inoculum (previously adjusted to approximately 106conidia/mL) was applied. Treatments consisted of MT of G. glabra (MTGG1), MT of M. balsamum (MTBT2), MT of A. vera (MTAV3), MT of A. sativum (MTAS4), resin of P. heptaphyllum (RES-AM5), and crude extracts of R. mangle (CEMV6), and the controls consisted of sterile distilled water (GC1), hydroethanolic solution (GC3) at 70% and fungicide TEB (Folicur®) at 0.1%.

The efficacy of fusariosis control in the detached leaves was determined by calculating the control index (CI), using the following formula (1): where, CI is control index; LC is lesion in sample with sterile distilled water (negative control); LT is lesion in sample with the treatment (plant extracts or TEB).
2.4.1. Preventive control
The preventive control was performed before inoculation with the pathogen and pineapple leaves received 100 μL of each treatment and controls. The inoculated leaves were realized at 0 h (T0), 8 h (T8), and 24 h (T24), after each treatment, at the same deposition point. The leaves were maintained in a wet chamber formed by Gerbox™boxes (11 cm×11 cm×3.5 cm) at room temperature (25±2)°C, and the diameter (mm) of the leaf lesion was measured at 48 h.
2.4.2. Curative control
The initial procedure was inoculation of the leaves at 0 h (T0), 8 h (T8), and 24 h (T24). The treatment with the extracts was conducted in post-infected pineapple cv. P´erola leaf tissues and were applied at the same inoculation point.
2.5. Data analysis
All experiments were conducted in a completely randomized design with three repetitions, for each treatment. The statistical analysis of the results was conducted by analysis of variance (ANOVA) statistics program 3.0 OSU Press, Ohio.
3. Results
3.1. In vitro screening of extracts presenting antifungal activity
Of the tested samples obtained from plant species, 3 species showed antifungal activity only against C. paradoxa, 16 species showed antifungal activity only against F. guttiforme, 24 species showed antifungal activity against both fungi and 14 species did not exhibit antifungal activity against the tested fungi (Table 1).
All the samples tested gave different results than that of the reference TEB, especially for the C. paradoxa fungus. The MTGG1, MTAS4 and CEMV6 species showed efficiency with an inhibition hale greater than 20 mm, especially for the F. guttiforme fungus (Table 2). The TEB showed strong antifungal inhibition at a dose of 0.1% (Table 1). MTGG1, MTBT2, MTAV3, and MTAS4, RES-AM5 and CEMV6 showed antifungal activity against F. guttiforme and C. paradoxa, in relation to the mycelial growth at concentrations of 89.5 μg/mL, 83.7 μg/ mL, 90.1 μg/mL, 90.5 μg/mL, 88.1 μg/mL and 86.5 μg/mL, respectively. MTGG1 and MTAS4 and CEMV6 were statistically different from the tested samples with regard to the growth inhibition against the F. guttiforme (P<0.05). The MTGG1 sample also showed antifungal activity against C. paradoxa, whereas the CEMV6 sample showed the lowest inhibition against this fungus. The effects of MTBT2 and RES-AM5 were statistically different from the control samples, and the MTBT2 sample showed the lowest inhibition against the F. guttiforme (Table 2).

Table 1Antifungal inhibitory activity of the plant extracts using hole-plate diffusion method.
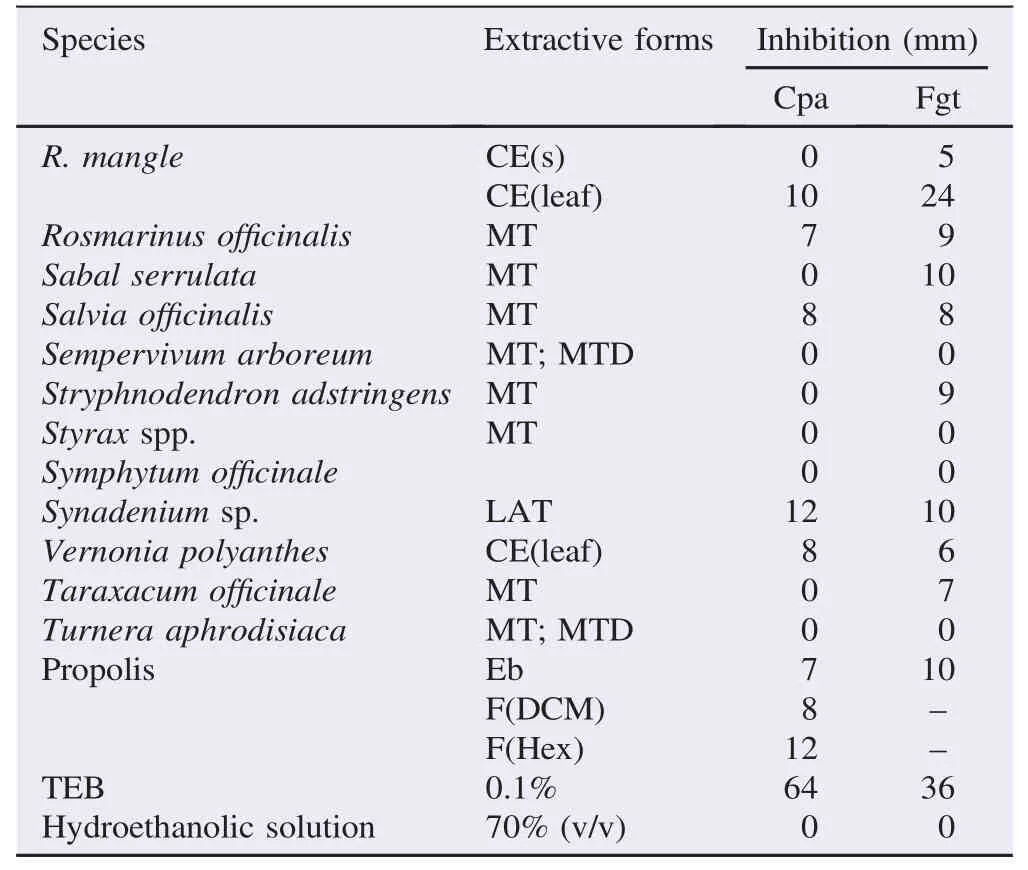
Table 1 (continued)
3.2. In situ control of fusariosis
3.2.1. Preventive control
The results were statistically significant in different times (T0, T8and T24) after the preventive treatment with extracts (MT, RES, and EB), when compared with the negative controls, sterile distilled water (GC1) and hydroethanolic solution at 70% (v/v) (GC3), and the positive control, TEB at 0.1% (Table 3).

Table 2Inhibitory activity of extract forms by agar dilution method, against two phytopathogenic fungi, C. paradoxa and F. guttiforme.
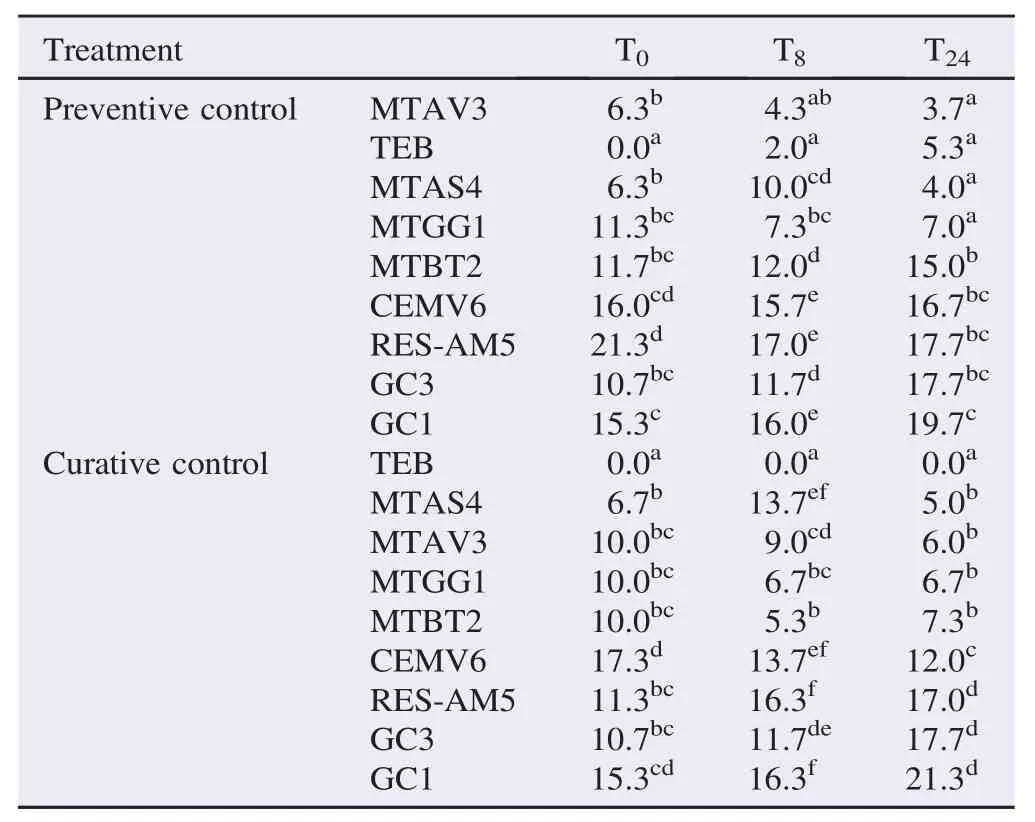
Table 3Lesion size in pineapple leaves of cv. P´erola, inoculated with F. guttiforme, preventively and curatively treated with potential fungicide, in different times (0, 8 and 24 h), before and after the inoculation. mm.
Preventive treatments (24 h prior to inoculation) with MTAV3, MTAS4 and MTGG1 were efficient, and the results did not differ from that of TEB. The diameter of the lesions showed that, the treatments with MTBT2, RES-AM5 and CEMV6 were inferior to the accepted standard control, TEB. The crude extract of the leaf of R. mangle did not show fungi control and was not statistically different (P>0.05) from the control samples of GC3 or GC1. In the GC3 treatment, a lower mean of the leaf lesion size was verified, especially in the mean values obtained with the initial inoculations (T0and T8). However, when assessed 24 h after inoculation, it did not yield different results from RES-AM5 in the samples used as negative controls (GC3 and GC1), which did not exhibit activity in the control of Fusarium (Table 3).
3.2.2. Curative control
The mean values of the different treatments, for all the periods after inoculation, showed significant differences (Table 3). Treatments MTAS4, MTAV3, MTGG1 and MTBT2 showed antifungal activities with values that were drastically different from negative control groups (P<0.05), GC1 and GC3. The treatments, at time T24, with MTAS4 and MTAV3, have lower values than those obtained for the times T0and T8. The treatment with MTAS4 was effective at the concentration of 100 μL/ mL, at time T24, and suggested an action of this tincture in the control of the infection in pineapple leaves, as its activity at earlier times (T0and T8) was not different from the GC1 control.
Treatment with CEMV6 showed increase in efficiency in the treatments with 8 and 24 h after inoculation. The RES-AM5 did not control the fungus F. guttiforme and was statistically equal to the samples, GC3 and GC1 (Table 3). The results of the control observed with treatment GC3 at times 0 and 8 h after inoculation may be attributed to the presence of alcoholic solutions at 70%,which have a sanitizing action, but were not different from the treatment RES-AM5.
3.2.3. CI of fusariosis
The treatments, with MTAV3, MTAS4, and MTGG1 showed efficacy for the CI of fusariosis with reduction of the lesion at 81%, 80%, 64%, respectively, and TEB showed CI of 73%, when compared to sample GC1 (sterile water). Efficacy of the control of fusariosis, when treatments were added 24 h postinoculation with the fungus for tinctures of A. sativum, A. vera, G. glabra, and M. balsamum was evident with a reduction of the size of fungal lesions to 76%, 72%, 69%, and 65%, respectively, compared to the control, GC1. The TEB, as expected, was effective in the control of the disease, presenting complete (100%) inhibition of the leaf lesions.
4. Discussion
Plant extract forms that include ethanol extracts and its fractions, resins and essential oils have been reported to have antifungal activity and show a potential for the control of phytopathogenic fungi[7,20,21]. These forms involve simple extraction methods with low production costs [22], and have potential for technological development easily implemented in agribusiness industries.
The in vitro trials showed that the selected species, A. sativum, A. vera, G. glabra, M. balsamum, R. mangle and P. heptaphyllum, displayed consistent antifungal activity against F. guttiforme and C. paradoxa. The results showed that the different extract forms varied in their effectiveness in inhibiting fungi growth. The ethanol extracts and mother tinctures, showed high diffusion in the growth media owing to their hydrophilic character[19], making active chemical groups bioavailable to the assayed fungi, which represents an important characteristic in the evaluation of new compounds.
The diversity in the biocomposition of chemical components of plant extracts, i.e., the secondary metabolites of plants, even those obtained from the same species, may result in different responses, especially with regard to the potential for microorganism inhibition. Other associated factors include solubility, pH, volatility, diffusion characteristics in growth medium, and the type of microorganism under evaluation [8,19]. The Copaíba oil (Copaifera reticulata) and resinous samples have compromised solubility owing to their high lipophilicity, indicating that the absolute values in the inhibition zone are relatively significant. The specificity of stem dicholoromethane fraction of the species Erythroxylum ovalifolium and Kielmeyera membranacea, and leaf dichloromethane fraction of the specie Passiflora mucronata on the fungi tested may be attributed to the extraction methods and chemical reagents used.
The stability of fungicide activity in the MTAS4 sample was observed in the experiment on the PDA growth medium, represented by the inhibition of mycelial growth even after 5 days of culture. MT of A. sativum (hydroalcoholic extraction) contains secondary metabolites derived from amino acids produced by hydrolysis, in which allicin (a low-molecular-weight compound) has powerful antimicrobial activity. Most of therapeutic effects are ascribed to specific oil and water-soluble organosulphur compounds [14,15].
MTAV3 showed an efficient antifungal activity that is normally attributed to the presence of anthraquinones such as aloin, barbaloin, and isobarbaloin [10,11]. This fact was confirmed by Rawat and Shivani [12], who demonstrated that the hydroethanolic 70% extract, from A. vera, showed an antifungal activity against mycelial growth of Botrytis gladiolorum, Fusarium oxysporum f. sp. gladioli, Heterosporium pruneti, and Penicillium gladioli. MTGG1 (G. glabra), obtained from the root, may have high antifungal activity owing to the presence of saponins (glycyrrhizin or glycyrrhizic acid),flavonoids, coumarins, and essential oils[13].
The literature describes the use of MTBT2 (M. balsamum) in medicine as an antiseptic, cicatrizant, and antiparasitic agent[17], but no data have been published regarding the pharmacological activity of M. balsamum as a fungicide or fungistat toward the fungi tested in our study. However, it deserves special attention, as the resin isolated from this plant contains (~70%–80%) cinnamic and benzoic esters of the resinous alcohol known as tolu-resinotannol, which is essentially composed of benzyl benzoate and benzyl cinnamate [17]. Recently, the presence of other esters, such as eugenol, vanillin, ferilic acid, 1,2-diphenylethane, mono- and sesquiterpenes (oxidized or not), as well as triterpenes, has been described in the plant, which largely explains its potential as a fungicide against plant pathogens[17,20].
The sample RES-AM5 (P. heptaphyllum), showed considerably greater results in relation to the few reports in the literature, showing volatile resinous oils and gums, which are formed by steroids and triterpenes[23], with the relevance of the latter for antifungal activity. The antifungal activity shown by CEMV6 (R. mangle) is supported by the presence of active secondary metabolites such as tannins; these metabolites are naturally produced in traditional herbal medicines rich in polyphenols (vegetable tannins).
The inoculation method using F. guttiforme in detached leaves from the pineapple cv. P´erola (susceptible) was found to be efficient for assessing the antifungal activity of bioactive extract forms of the selected species A. sativum (MTAS4), A. vera (MTAV3), G. glabra (MTGG1), M. balsamum (MTBT2), P. heptaphyllum (RES-AM5) and R. mangle (CEMV6). The antifungal activity exhibited by the MT of A. sativum might be due to the bioactive metabolite, allicin, which is reported to have a powerful antimicrobial activity [14,15]. It may also be derived from the expression of substances with polar characteristics [19], such as saponins and bioflavonoids, promoters of anti-free-radical action synergetic to the biological activity of allicin, which is powered by the maceration of ethanolic extraction of the plant.
Recent studies have shown that the antimicrobial activity of medicinal plants might be due to the presence and synergistic activity of diverse bioactive metabolites [9].
The antifungal effect of MTAV3 (A. vera), when applied 24 h after fungus inoculation, i.e., when the plant tissue was already infected, may also be attributed to the direct action of the active substances present in the MT on the host. This would interfere with pathogenesis by changing the relationship between pathogen and host. The efficacy in control of the lesion with MTAV3 and MTAS4 may be related to the chemical and prophylactic properties of the species involved, especially the activity of biologically active products with potential to induce resistance in the leaf tissues of cv. P´erola.
The results obtained with the treatment of MTs of species G. glabra (MTGG1) and M. balsamum (MTBT2) applied 24 hafter inoculation are likely associated with the action of bioactive compounds present in these species of the Fabaceae[24].
The MT extract of M. balsamum showed antifungal activity and was used here for the first time for inhibition of phytopathogenic fungi. The activity of M. balsamum, related to the substance benzyl benzoate, is particularly important in this regard. Benzoate (butylparaben), an ester obtained from the esterification of p-hydroxybenzoic acid, is considered one of the top phenolic antimicrobial preservatives. The chemical composition of the paraben groups, methyl and propylparaben, confers antifungal activity to this compound, and it is widely used in the pharmaceutical industry. The antifungal activity shown by treatment with MTBT2 is possibly linked to the residual effect of resinous substances present in the tincture resin. An important factor to be considered is that high dose substances in commercial synthetic forms are considered toxic, and the active chemical groups diluted in the MT may possibly be used with a degree of safety as a phytosanitary, chemical, and prophylactic product in agriculture.
In the preventive control of fusariosis, the tinctures, MTAV3, MTAS4, and MTGG1 showed significant antifungal activity in the control of F. guttiforme in the foliar tissues of the pineapple plants and did not significantly differ from the standard treatment using the fungicide TEB (P>0.05).
The findings of the present study envisaged that MTs can effectively control phytopathogens, and this study paves the way for development of bioactive natural products with phytosanitary applications, with the added benefits of an environmentally safe and economically viable product.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We are thankful to the researchers from Technology Laboratory with Natural Products, Federal Fluminense University, Rio de Janeiro-RJ, Brazil and from Almeida Prado Homeopathic Laboratory, São Paulo-SP, Brazil, for their support and for providing valuable information during this work. We thank the Espírito Santo Research Foundation and National Council for Scientific and Technological Development for research grants (Proc.: 46704469/2009 and 307752/2012-7) to support this study.
References
[1] Food and Agriculture Organization of the Unitd Nations. Food security statistics. Rome: Food and Agriculture Organization of the Unitd Nations; 2015. [Online] Available from: http://www.fao.org/ economic/ess/ess-fs/en [Accessed on 8th July, 2015]
[2] Castillo F, Hernandez D, Gallegos G, Rodriguez R, Aguilar CN. Antifungal properties of bioactive compounds from plants. In: Dhanasekaran D, Thajuddin N, Panneerselvam A, editors. Fungicides for plant and animal diseases. Rijeka: InTech; 2012, p. 82-106.
[3] Korres AM, Buss DS, Ventura JA, Fernandes PM. Candida krusei and Kloeckera apis inhibit the causal agent of pineapple fusariosis, Fusarium guttiforme. Fungal Biol 2011; 115(12): 1251-8.
[4] Martinez JA. Natural fungicides obtained from plants. In: Dhanasekaran D, Thajuddin N, Panneerselvam A, editors. Fungicides for plant and animal diseases. Rijeka: InTech; 2011, p. 3-28.
[5] Liu C, Zhao C, Pan HH, Kang J, Yu XT, Wang HQ, et al. Chemical constituents from Inonotus obliquus and their biological activities. J Nat Prod 2014; 77(1): 35-41.
[6] Balakumar S, Rajan S, Thirunalasundari T, Jeeva S. Antifungal activity of Aegle marmelos (L.) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pac J Trop Biomed 2011; 1(4): 309-12.
[7] Gahukar RT. Evaluation of plant-derived products against pests and diseases of medicinal plants: a review. Crop Prot 2012; 42: 202-9.
[8] Gillitzer P, Martin AC, Kantar M, Kauppi K, Dahlberg S, Lis D, et al. Optimization of screening of native and naturalized plants from Minnesota for antimicrobial activity. J Med Plants Res 2012; 6(6): 938-49.
[9] Manilal A, Idhayadhulla A. Potential in vitro antimicrobial efficacy of Holigarna arnottiana (Hook F). Asian Pac J Trop Biomed 2014; 4(1): 25-9.
[10] de Rodríguez JD, Hernandez-Castillo D, Rodríguez-García R, Angulo-Sanchez JL. Antifungal activity in vitro of Aloe vera pulp and liquid fraction against plant pathogenic fungi. Ind Crops Prod 2005; 21(1): 81-7.
[11] Patel DK, Patel K, Tahilyani V. Barbaloin: a concise report of its pharmacological and analytical aspects. Asian Pac J Trop Biomed 2012; 2(10): 835-8.
[12] Rawat P, Shivani Anand J. Immunomodulatory properties of some herbal plants against Candida albicans: a review. Biotech Int 2012; 5(2): 52-68.
[13] Roshan A, Verma NK, Kumar CS, Chandra V, Singh DP, Panday MK. Phytochemical constituent, pharmacological activities and medicinal uses through the millenia of Glycyrrhiza glabra Linn: a review. Int Res J Pharm 2012; 3(8): 45-55.
[14] Bhagwat MK, Datar AG. Antibacterial activity of herbal extracts against five plant pathogenic bacteria. Arch Phytopathol Plant Prot 2014; 47(7): 892-9.
[15] Alok S, Jain SK, Verma A, Kumar M, Mahor A, Sabharwal M. Herbal antioxidant in clinical practice: a review. Asian Pac J Trop Biomed 2014; 4(1): 78-84.
[16] Thangavelu R, Devi PG, Gopi M, Mustaffa MM. Management of Eumusae leaf spot disease of banana caused by Mycosphaerella eumusae with Zimmu (Allium sativum×Allium cepa) leaf extract. Crop Prot 2013; 46(1): 100-5.
[17] Cust´odio DL, Veiga-Junior VF. True and common balsams. Rev Braz J Pharmacogn 2012; 22(6): 1372-83.
[18] Figueiredo MB. [Study on the application of the method of Castellani for the conservation of fungi pathogens in plants]. O Biol´ogico 1967; 33(1): 9-13. Portuguese.
[19] Talibi I, Askarne L, Boubaker H, Boudyach EH, Msanda F, Saadi B, et al. Antifungal activity of some Moroccan plants against Geotrichum candidum, causal agent of postharvest citrus sour rot. Crop Prot 2012; 35(1): 41-6.
[20] Garcia R, Alves ESS, Santos MP, Aquile GMFV, Fernandes AAR, dos Santos RB, et al. Antimicrobial activity and potential use of monoterpenes as tropical fruits preservatives. Braz J Microbiol 2008; 39(1): 163-8.
[21] Kuster RM, Arnold N, Wessjohann L. Anti-fungal flavonoids from Tibouchina grandifolia. Biochem Syst Ecol 2009; 37(1): 63-5.
[22] Rocha L. [Preparation of Tinctures for homeopathic use]. In: Sharapin N, Rocha LM, Carvalho ES, L´ucio EMRA, dos Santos EVM, de Almeida JML, editors. [Technology fundamentals of herbal products]. Santa F´e de Bogot´a: CAB-CYTED; 2000, p. 63-72. Spanish.
[23] Maia RM, Barbosa PR, Cruz FG, Roque e Miguel Fascio NF. [Triterpenes from the resin of Protium heptaphyllum March (Burseraceae): characterization in binary mixtures]. Quím Nova 2000; 23(5): 623-6. Portuguese.
[24] Fenner R, Betti AH, Mentz LA, Rates SMK. [Plants with potential antifungal activity employed in Brazilian folk medicine]. Rev Bras Ciˆencias Farm 2006; 42(3): 369-94. Portuguese.
*Corresponding author:Debora Dummer Meira, PhD, Department of Biological Sciences, Federal University of Espírito Santo-UFES, Campus Maruípe, Vit´oria, Espírito Santo, Brazil.
 Asian Pacific Journal of Tropical Biomedicine2016年1期
Asian Pacific Journal of Tropical Biomedicine2016年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The antibacterial activity of selected plants towards resistant bacteria isolated from clinical specimens
- Healthcare waste management in selected government and private hospitals in Southeast Nigeria
- Rhinacanthus nasutus leaf improves metabolic abnormalities in high-fat diet-induced obese mice
- Influence of extraction solvents on antioxidant and antimicrobial activities of the pulp and seed of Anisophyllea laurina R. Br. ex Sabine fruits
- Potent water extracts of Indonesian medicinal plants against PTP1B
- Pharmacological effects of ethanol extract of Egyptian Artemisia herba-alba in rats and mice
