超声心动图分层应变技术评价肥厚型梗阻性心肌病改良扩大Morrow术后左心室游离壁逆重构及预测影响因素
王婧金,肖明虎,孙欣,张茗卉,张金萍,陈海波,朱昌盛,王水云,王浩
超声心动图分层应变技术评价肥厚型梗阻性心肌病改良扩大Morrow术后左心室游离壁逆重构及预测影响因素
王婧金,肖明虎,孙欣,张茗卉,张金萍,陈海波,朱昌盛,王水云*,王浩
摘要
关键词心肌病,肥厚型; 心脏外科手术;超声心动描记术; 心室功能障碍,左
资助项目:首都卫生发展科研专项基金(2011-4003-05);北京协和医学院研究生创新基金(2013-1002-55)
作者单位:100037 北京市,北京协和医学院 中国医学科学院 国家心血管病中心 阜外医院 超声影像中心(王婧金、肖明虎、孙欣、张茗卉、张金萍、王浩);成人外科中心(陈海波、朱昌盛、王水云)
*共同通讯作者:王水云 Email: wsymd@sina.com
Predictor and Risk Factor Evaluation of Left Ventricular Free Wall Reverse Remodeling in Patients With Obstructive Hypertrophic Cardiomyopathy After Modified Morrow Procedure by Three-layer Speckle Tracking of Echocardiography
WANG Jing-jin, XIAO Ming-hu, SUN Xin, ZHANG Ming-hui, ZHANG Jin-ping, CHEN Hai-bo, ZHU Chang-sheng, WANG Shui-yun, WANG Hao.
Department of Echocardiography, Cardiovascular Institute and Fu Wai Hospital, CAMS and PUMC, Beijing (100037), China
Co-corresponding Authors: WANG Hao, Email: fwwanghao@gmail.com and WANG Shui-yun, Email: wsymd@sina.com
Abstract
Objectives: To evaluate the predictor and risk factor of left ventricular (LV) free wall reverse remodeling in patients with obstructive hypertrophic cardiomyopathy (HCM) after modified Morrow procedure by three-layer speckle tracking of echocardiography.
Methods: Our investigation included 2 groups: HCM group, n=60 patients who had successful modified Morrow procedure in our hospital from 2014-06 to 2014-12, there were 41 (68.3%) male with the average age of (39.1 ± 15.2) years. Control group, n=40 healthy subjects. Three-layer speckle tracking echocardiography was conducted to analyze pre- and post-operative LV free wall three-layer myocardium (endocardial, mid, and epicardial layers) changes at longitudinal strain (LS) and circumferential strain (CS). Clinical and echocardiography information were collected at pre- and (6-24) months post-operation. The impact factors for LV free wall reverse remodeling was identified by liner regression analysis and the segment’s thickness ≥ 15mm was defined as the hypertrophic LV free segment.
Results: In HCM group, compared with pre-operative condition, the post-operative thickness of LV free wall including anterior, anterolateral and inferolateral were reduced; while both post-operative LS and CS elevated (-13.8 ± 4.8) % vs (-17.0 ± 5.2) % and (-23.7 ± 3.8) % vs (-25.4 ± 3.7) %, P<0.05. LV mass index by echocardiography was larger than LV mass index by surgical resection (13.5 ± 30.9) g/m2vs (3.4 ± 2.0) g/m2, P<0.05. Liner regression analysis indicated that the number of preoperative hypertrophic segments (r=-0.680, P<0.001) and age (r=0.638, P<0.001) were the independent impact factors for post-operative LS; △left ventricular outflow tract (LVOT) gradient (r=0.386, P=0.005) was the independent impact factor for post-operative CS.
Conclusion:①After modified Morrow procedure, LVOT obstruction disappeared which leaded LV free wall reverse remodeling in HCM patients, ②three-layer myocardium of LV free wall all had reverse remodeling, ③better improved LVOT gradient were with less number of hypertrophic segments; the elder patients usually had the better post-operative reverse remodeling.
Key words Cardiomyopathy, hypertrophic; Cardiac surgical procedure; Ventricular dysfunction, left; Ultrasonography
(Chinese Circulation Journal, 2016,31:60.)
左心室流出道(LVOT)梗阻是肥厚型心肌病相关心力衰竭和死亡的独立预测因子[1]。2011年美国心脏病学院基金会/美国心脏协会(ACCF/AHA)指南中[2],改良扩大Morrow术是肥厚型梗阻性心肌病(HOCM)理想的治疗方法,解除LVOT梗阻可以提高患者生活质量和延长寿命。有研究表明,改良扩大Morrow术后左心室整体功能改善,包括左心室舒张末期容积、压力和心肌功能[3, 4]。但局部心肌功能的改善及其影响因素,尚少见系统研究。超声心动图分层应变技术是一项新技术,可分析左心室心内膜下心肌、中层心肌和心外膜下心肌的应变,不同于既往的斑点追踪技术(分析全层心肌,且存在心肌包裹不全的缺点)。目前,尚少见应用该技术分析HOCM左心室游离壁的研究。本研究应用超声心动图分层应变技术,分析HOCM改良扩大Morrow术后左心室游离壁逆重构即心内膜下心肌、中层心肌和心外膜下心肌的纵向应变和环形应变,并预测发生左心室逆重构的影响因素。
1 资料与方法
临床资料:本研究入选我院2014-06到2014-12期间成功接受改良扩大Morrow术式[5]的HOCM患者60例(HOCM组),男性 41例(68.3%);平均年龄(39.1 ± 15.2)岁,其中年龄<21岁的患者11例(18.3%),21 ~40岁之间的21例 (35.0%),>40岁的28例(46.7%)。 入选标准:(1)HOCM的诊断主要依据2011年ACCF/AHA指南[2];(2)内科药物治疗后症状仍未缓解,或不能解释的晕厥;(3)静息状态下或激发试验后,超声心动图测量LVOT压差≥50 mmHg(1 mmHg=0.133 kPa);(4)前间隔切除部位厚度≥16 mm。排除标准:(1)合并心脏瓣膜疾病;(2)合并右心室流出道梗阻;(3)超声心动图图像质量差。60例患者中,入院症状为胸痛胸闷者47例(78.3%),呼吸困难者25例(41.7%),心悸者13例(21.7%),晕厥及晕厥前反应者15例(25.0%);HOCM家族史6例(10.0%);8例(13.3%)合并冠心病或肌桥的患者同时行冠状动脉旁路移植术,1例(1.7%)合并心房颤动的患者同时行迷宫射频消融术。术后随访6~24个月。对照组:同期选取入选40例门诊且超声心动图图像质量良好的正常人。
外科手术治疗:通过直视下手术切除前间隔组织,减小室间隔厚度,达到LVOT压差小于30 mmHg的目的。手术切除距离因患者情况不同而个体化,最远可达心尖水平。
超声心动图检测:60例患者均采集超声心动图图像,采用GE E9超声诊断仪(挪威霍尔滕GE医疗公司)。△值为术前值-术后值。LVOT压差通过简化伯努利方程计算。心腔大小的测量和左心室舒张功能分级均遵照美国超声心动图协会指南和推荐[6,7]。二尖瓣反流分度分为:0为无; 1+为少量,2+为中量, 3+为中大量; 4+为大量[8]。根据AHA推荐,本研究采用17节段模型[9]。本研究的定义:(1)左心室游离壁包括前壁基底段、中间段和心尖段、侧壁基底段、中间段和心尖段、后壁基底段和中间段、下壁基底段、中间段和心尖段,共11节段(除外心尖帽)。(2)增厚左心室游离壁节段:上述的11节段中,任一节段的室壁厚度≥15 mm者即为增厚左心室游离壁节段。
心肌应变检测:采用分层应变技术,脱机分析术前和术后左心室游离壁11节段的应变(ECHOPAC软件BT 113版)。可获得左心室游离壁各节段的平均应变以及三层心肌应变(包括心内膜下心肌、中层心肌和心外膜下心肌)。本研究分析两个方向的应变,即(1) 纵向应变:测量的切面包括心尖四腔心切面、心尖三腔心切面和心尖两腔心切面;(2)环形应变:测量的切面包括,左心室基底段短轴、左心室中间段短轴和左心室心尖段短轴。二维超声心动图图像帧频范围为55~90帧/s。 选取两名有经验的超声心动图医师,采用单纯随机抽样法选取20例观察病例,评价同一观察者和不同观察者之间测量的变异性。
统计学方法:采用SPSS 20.0软件进行分析,计量资料以均数±标准差表示,术前HOCM和正常对照组计量资料比较采用独立样本t检验,术前和术后HOCM组计量资料比较采用配对样本t检验。计数资料以率和构成比表示,采用卡方检验。两组以上计量资料比较采用方差分析或Welch分析。同一观察者和不同观察者的变异性采用组内相关系数(ICC)方法评价。用线性回归法识别逆重构的预测因素,计算相关系数,回归系数和常数。P<0.05表示差异有统计学意义。
2 结果
两组患者一般资料比较:HOCM组与正常对照组间,年龄[(38.3±17.8)岁 vs (39.1± 15.2 )岁]、男性比例 (65% vs 68.3%)和体表面积[(1.6±0.2) m2vs (1.7±0.2) m2]差异均无统计学意义(P>0.05)。
两组超声心动图检测指标的比较(表1): 与正常对照组比较,HOCM组患者术前及术后最厚室壁厚度、LVOT压差、左心房前后径、左心室射血分数、二尖瓣血流舒张早期E峰速度 /二尖瓣环室间隔侧组织多普勒舒张早期E’峰速度 [二尖瓣 E/E’(室间隔侧)],左心室质量指数、室壁厚度均增加;左心室舒张末期内径、左心室舒张末期容积、左心室收缩末期容积和每搏输出量、游离壁纵向应变和游离壁环形应变(心内膜下心肌除外,术后的平均值除外)均降低,差异均有统计学意义(P<0.05)。
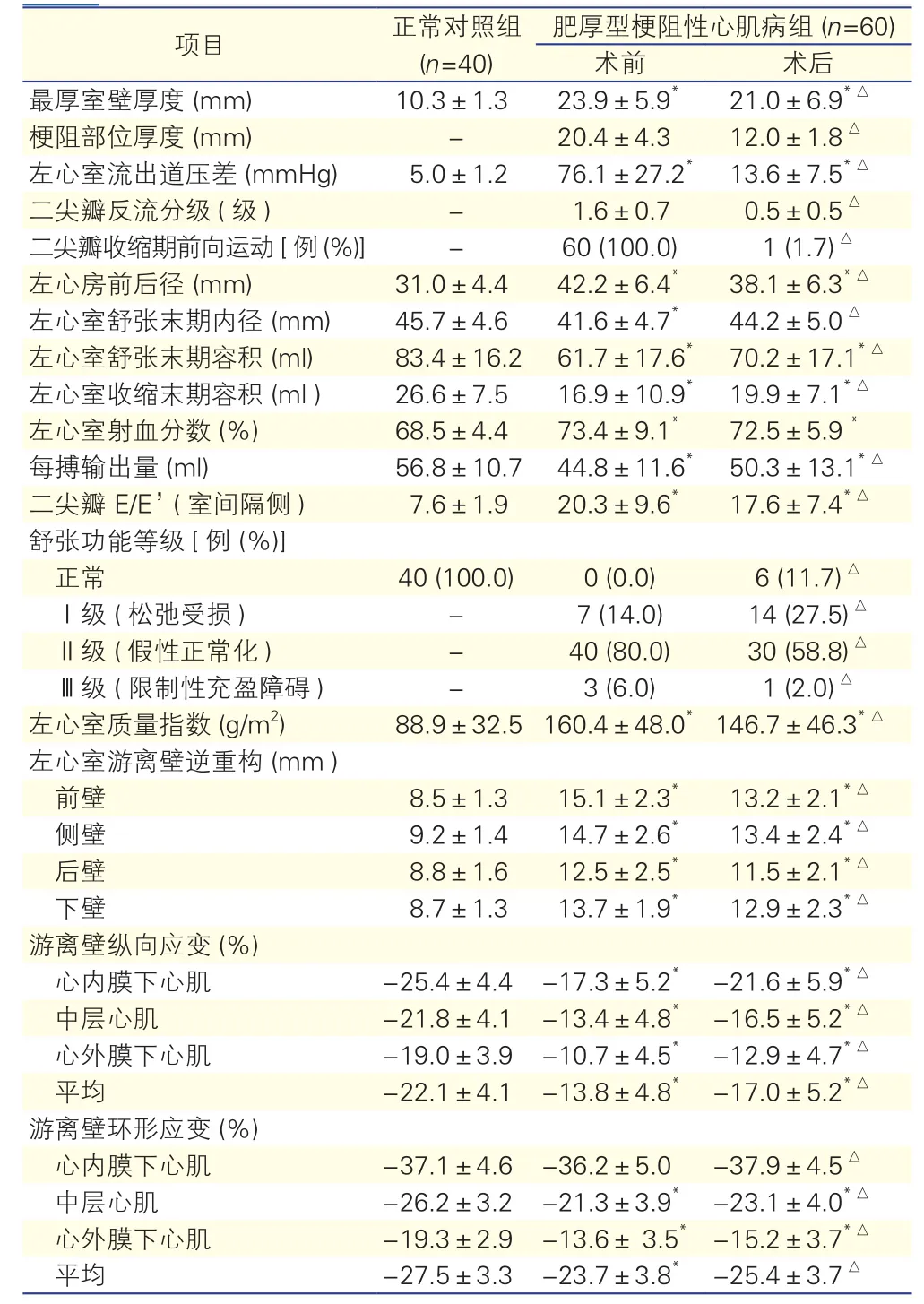
表1 两组患者手术前后超声心动图检测指标的比较(?±s)
HOCM组患者术前与术后的超声心动图检测指标的比较:(1)与HOCM组术前比较,术后最厚室壁厚度、梗阻部位厚度、LVOT压差、左心房前后径、二尖瓣反流、二尖瓣收缩期前向运动的比例、二尖瓣 E/E’(室间隔侧)、舒张功能等级中Ⅱ级 (假性正常化)和Ⅲ级 (限制性充盈障碍)的比例、左心室质量指数、左心室游离壁厚度均降低,差异均有统计学意义(P<0.05);(2) 左心室舒张末期内径、左心室舒张末期容积、左心室收缩末期容积、每搏输出量、舒张功能等级中正常和Ⅰ级 (松弛受损)的比例、游离壁纵向应变和游离壁环形应变均增加,差异均有统计学意义(P<0.05,表1)。(3)游离壁各层心肌△纵向应变的△心内膜下肌层(4.2±3.6)%、△中层肌层(3.1±3.1)%和△心外膜下肌层(2.1±2.8)%,三者之间逐渐降低,差异有统计学意义(P<0.05,ANOVA);游离壁各层心肌△环形应变的上述三者之间差异无统计学意义[(2.2±5.7)% vs(1.8±4.0)%vs(1.8±3.4)%,P = 0.895, Welch]。(4)△超声左心室质量指数大于外科切除质量指数[(13.5±30.9)g/m2vs (3.4±2.0 )g/m2,P<0.05]。
增厚左心室游离壁节段数与术后左心室游离壁纵向应变的比较(表2):60例患者根据增厚左心室游离壁节段数不同分为四组,四组患者间增厚左心室游离壁节段数越多者,纵向应变越低,差异均有统计学意义(P<0.05)。
表2 60例患者增厚左心室游离壁 (厚度≥15mm)的节段数与术后左心室游离壁纵向应变的比较(%,s)
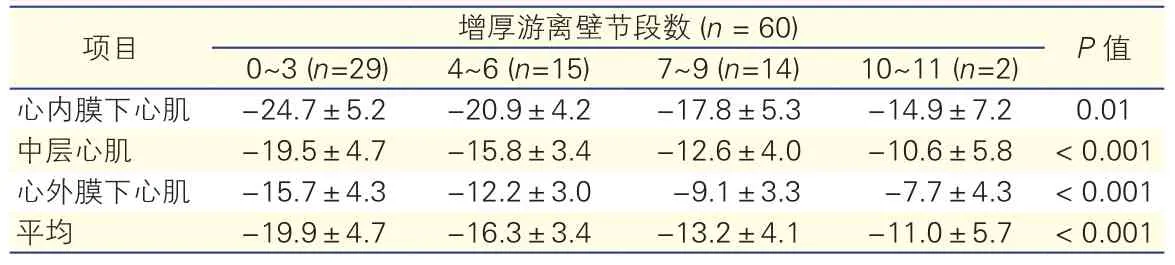
表2 60例患者增厚左心室游离壁 (厚度≥15mm)的节段数与术后左心室游离壁纵向应变的比较(%,s)
项目 增厚游离壁节段数 (n = 60) P值0~3 (n=29) 4~6 (n=15) 7~9 (n=14) 10~11 (n=2)心内膜下心肌 -24.7±5.2 -20.9±4.2 -17.8±5.3 -14.9±7.2 0.01中层心肌 -19.5±4.7 -15.8±3.4 -12.6±4.0 -10.6±5.8 < 0.001心外膜下心肌 -15.7±4.3 -12.2±3.0 -9.1±3.3 -7.7±4.3 < 0.001平均 -19.9±4.7 -16.3±3.4 -13.2±4.1 -11.0±5.7 < 0.001
左心室游离壁逆重构预测影响因素:应用相关分析,年龄与增厚左心室游离壁节段数呈负相关(r=-0.310,P=0.024)。应用线性回归分析,影响术后左心室游离壁纵向应变的独立因素是术前增厚左心室游离壁节段数(r=-0.680, P<0.001)和年龄(r=0.638,P<0.001)。影响术后左心室游离壁环形应变的独立因素是△LVOT压差(r=0.386, P=0.005)。
分层应变技术的重复性:同一观察者差异ICC:左心室游离壁纵向应变为0.92 (P<0.001);游离壁环形应变为 0.94 (P<0.001)。不同观察者间差异ICC:左心室游离壁纵向应变为0.83 (P<0.001);游离壁环形应变为0.85 (P<0.001) 。
3 讨论
目前,对HOCM外科改良扩大Morrow术后逆重构应用分层应变技术定量分析,本研究是国内较早报道,国外的HOCM研究仅1篇。本研究结果显示,左心室游离壁术后的逆重构发生在左心室游离壁三层心肌。增厚左心室游离壁节段数、年龄以及△LVOT压差与左心室游离壁逆重构相关。
本研究结果显示,超声心动图测量的术后左心室整体质量的减少大于手术切除室间隔的质量,说明左心室的逆重构。此外,左心室前壁、侧壁、后壁和下壁厚度较术前变薄,这些节段手术并未切除,说明左心室游离壁术后的逆重构。
超声心动图分层应变技术优于传统二维斑点追踪技术,其优势主要有:可分层分析心肌应变,可手动调整追踪边界,可完整包裹室壁,更适合非对称性肥厚的HOCM,可提高测量重复性和准确性。有研究表明,斑点追踪技术测得的应变指标与HOCM组织学上心肌肥大、肌纤维排列紊乱、纤维化明显相关[10]。
HOCM外科改良扩大Morrow术后,左心室游离壁纵向应变和环形应变均较术前明显增加。改良扩大Morrow术降低LVOT压差,从而促使左心室游离壁逆重构,改善左心室游离壁结构和功能[11,12]。同时,纵向应变和环形应变的增加可能与游离壁心肌纤维排列紊乱的改善有关,也可能与左心室腔压力下降导致室壁血管供血改善有关。McLeod等[13]研究表明,室间隔扩大切除术可大大降低埋藏式心脏复律除颤器(ICD)的植入率和术后猝死发生率。因为猝死可能与室壁厚度、心肌纤维化和心肌纤维排列紊乱有关。本研究发现,增厚左心室游离壁节段数多的患者术后逆重构较差。
本研究的结果显示,术后左心室逆重构与年龄呈正相关,年龄越大的患者,术后逆重构越好,与Maron等[14-18]的研究一致。Maron等选取不同年龄段患者进行研究,发现年龄较大的患者HOCM相关的死亡率较低, HOCM相关的并发症较少;同时,青年阶段诊断HOCM的患者往往预后较差。此外,我们的研究中患者年龄与增厚左心室游离壁节段数负相关,进一步说明年轻患者增厚左心室游离壁节段数多,术后左心室游离壁纵向应变低,是我们术后不容易发生逆重构的原因。本研究的结果也为今后的临床和科研工作提供了可靠的实验依据。本研究的局限性:尚有一些问题需深入探讨,如年龄与长期预后的关系等。
本研究结果显示,HOCM外科改良扩大Morrow术后,左心室游离壁发生逆重构,应用新技术—分层应变技术发现逆重构发生在左心室游离壁的三层心肌。增厚左心室游离壁节段数、年龄和△LVOT压差与左心室逆重构相关。LVOT压差缓解越好,增厚左心室游离壁节段数越小、年龄较大的患者术后逆重构较好。
参考文献
[1] Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med, 2003, 348: 295-303.
[2] Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline forthe diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. Circulation, 2011, 124: 2761-2796.
[3] Cannon RR, McIntosh CL, Schenke WH, et al. Effect of surgical reduction of left ventricular outflow obstruction on hemodynamics, coronary flow, and myocardial metabolism in hypertrophic cardiomyopathy. Circulation, 1989, 79: 766-775.
[4] Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation, 2007, 116: 196-206.
[5] Wang S, Luo M, Sun H, et al. A retrospective clinical study of transaortic extended septal myectomy for obstructive hypertrophic cardiomyopathy in China. Eur J Cardiothorac Surg, 2013, 43: 534-540.
[6] Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr, 2005, 18: 1440-1463.
[7] Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr, 2009, 22: 107-133.
[8] Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with twodimensional and Doppler echocardiography. J Am Soc Echocardiogr, 2003, 16: 777-802.
[9] Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation, 2002, 105: 539-542.
[10] Almaas VM, Haugaa KH, Strom EH, et al. Noninvasive assessment of myocardial fibrosis in patients with obstructive hypertrophic cardiomyopathy. Heart, 2014, 100: 631-638.
[11] 王巍, 马维国, 孙寒松, 等. 肥厚型梗阻性心肌病合并冠心病的外科治疗效果. 中国循环杂志, 2007, 22: 296-298.
[12] 然鋆, 宋云虎, 胡盛寿, 等. 163例肥厚型梗阻性心肌病的外科治疗及疗效评价. 中国循环杂志, 2013, 28: 136-139.
[13] McLeod CJ, Ommen SR, Ackerman MJ, et al. Surgical septal myectomy decreases the risk for appropriate implantable cardioverter defibrillator discharge in obstructive hypertrophic cardiomyopathy. Eur Heart J, 2007, 28: 2583-2588.
[14] Maron BJ, Rowin EJ, Casey SA, et al. Risk stratification and outcome of patients with hypertrophic cardiomyopathy≥60 years of age. Circulation, 2013, 127: 585-593.
[15] Maron BJ, Rowin EJ, Casey SA, et al. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol, 2015, 65: 1915-1928.
[16] Maron BJ, Dearani JA, Ommen SR, et al. Low mperative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol, 2015, 66: 1307-1308.
[17] Maron MS, Maron BJ. Clinical impact of caontemporary cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy. Circulation, 2015, 132: 292-298.
[18] Maron BJ, Casey SA, Chan RH, et al. Independent Assessment of the European Society of Cardiology Sudden Death Risk Model for Hypertrophic Cardiomyopathy. Am J Cardiol, 2015, 116: 757-764.
(编辑:曹洪红)
临床研究
收稿日期:(2015-06-29)
中图分类号:R541
文献标识码:A
文章编号:1000-3614(2016)01-0060-05
doi:10.3969/j.issn.1000-3614.2016. 01.013
作者简介:王婧金 博士研究生 主要从事肥厚型心肌病超声研究 Email:jingjin0305@gmail.com 通讯作者:王浩 Email: fwwanghao@gmail.com
目的:采用超声心动图分层应变技术,评价肥厚型梗阻性心肌病(HOCM)改良扩大Morrow术后左心室游离壁逆重构及其预测影响因素。
方法:本研究入选我院2014-06到2014-12期间成功接受改良扩大Morrow术式的HOCM患者60例(HOCM组),男性 41例(68.3%),平均年龄(39.1 ± 15.2)岁,采集术前和术后6~24个月临床和超声心动图资料;同期选取健康人40例作为正常对照组。用超声分层应变技术分析术前和术后左心室游离壁三层心肌的(心内膜下、中层和心外膜下心肌)纵向应变和环形应变的变化,用线性回归法识别左心室游离壁逆重构的影响因素。左心室游离壁厚度≥15 mm的节段定义为增厚左心室游离壁节段。
结果: HOCM组患者术后左心室游离壁的前壁、侧壁、后壁和下壁厚度与术前比较均变薄;术后游离壁纵向应变[(-13.8 ± 4.8)% vs(-17.0 ± 5.2)%]和环形应变[(-23.7± 3.8)% vs(-25.4±3.7)%]均增厚;差异有统计学意义(P<0.05)。△(术前值-术后值) 超声左心室质量指数大于外科切除质量指数[(13.5±30.9)g/m2vs (3.4±2.0 )g/m2, P<0.05]。线性回归分析显示,影响术后左心室游离壁纵向应变的独立因素是术前增厚左心室游离壁节段数(r=-0.680, P<0.001)和年龄(r=0.638, P<0.001),影响术后左心室游离壁环形应变的因素是△左心室流出道(LVOT) 压差(r=0.386, P=0.005)。
结论 :对于HOCM患者,(1) 改良扩大Morrow术后,LVOT梗阻解除引起左心室游离壁的逆重构(室壁厚度变薄,质量减低,功能改善);(2)左心室游离壁的三层心肌均发生逆重构;(3) LVOT压差缓解越好、增厚左心室游离壁节段数越小、年龄较大的患者术后逆重构较好。

