失血性休克不同时相sorcin转位及其与血管反应性变化的相关性分析*
周 荣 丁小丽 刘良明
liangmingliu@yahoo.com
失血性休克不同时相sorcin转位及其与血管反应性变化的相关性分析*
周荣丁小丽刘良明#
liangmingliu@yahoo.com
【摘要】目的:观察失血性休克后不同时相大鼠肠系膜动脉平滑肌sorcin蛋白表达及其对去甲肾上腺素(NE)的反应性,初步探讨sorcin是否参与失血性休克血管反应性的调节。方法:36只SD大鼠,12只用作非手术对照组,其余采用经股动脉放血法制成失血性休克(动脉压40 mmHg)模型。分别将成模大鼠再分为休克早期(30min)组及晚期(2h)组;各组开腹分离肠系膜动脉,采用离体器官张力测定技术检测肠系膜动脉对NE的反应性;采用Western Blotting(WB)检测肠系膜动脉平滑肌sorcin的表达及其分布;采用免疫共沉淀联合WB检测sorcin-肌浆网2型雷诺定受体(RyR2)表达,并分析休克大鼠sorcin水平与肠系膜动脉对NE反应的相关性。结果:失血性休克早期组大鼠肠系膜动脉对NE的收缩反应较对照组明显增强(P<0.05),而晚期组对NE较对照组明显降低(P<0.05)。早期组和晚期组大鼠肠系膜动脉血管平滑肌sorcin总蛋白表达无显著差异(P>0.05),但发生了明显转位现象,即早期组sorcin在胞浆及质膜内表达无明显差异(P>0.05),但晚期组sorcin在胞浆内的表达明显增强,而在质膜的表达显著降低(均P<0.05);免疫共沉淀发现,早期组大鼠肠系膜动脉平滑肌sorcin-RyR2复合子表达与对照组无显著性差异(P>0.05),而晚期组较对照组明显减少(P<0.05);相关性分析显示,晚期组sorcin由质膜向胞浆移位与其血管反应性呈显著负相关(P<0.01)。结论:失血性休克后2 h,大鼠肠系膜动脉平滑肌sorcin从RyR2解离,导致sorcin从肌浆网向胞浆转位,至少部分参与肠系膜动脉对NE低反应性的形成。
【关键词】失血性休克;血管反应性;sorcin蛋白;肌浆网2型雷诺定受体;大鼠
严重创伤失血常引起外周血管对血管活性物质如去甲肾上腺素(NE)、精氨酸加压素等的反应性异常[1]。我们的前期工作证实,血管平滑肌肌浆网2型雷诺定受体(Type Ⅱ Ryanodine Receptor, RyR2)的过度激活与失血性休克晚期血管低反应性形成有关[2,3],但其通过何种机制导致RyR2的过度激活目前尚未阐明。
基础研究表明, sorcin是参与调节肌浆网RyR2介导Ca2+释放通道活性的重要蛋白分子,其通过与RyR2胞浆段sorcin相应位点结合,抑制RyR2活性及其介导的肌浆网Ca2+释放[4];sorcin与RyR2间亲和力的减弱可能引起血管平滑肌膜超极化及血管舒张,如F112L-sorcin突变可导致sorcin与RyR2间亲和力减弱6倍,触发血管平滑肌细胞(Vascular Smooth Muscle Cell,VSMC)钙火花及瞬时外向钾电流(STOCs)的形成[4,5]。 STOCs通过激活大电导钙激活钾通道(Large Conductance Calcium Activated Potassium Channel,BKCa),促成休克晚期血管低反应性的形成[6, 7]。由此推测,失血性休克时外周血管可能发生sorcin与RyR2解离,并可能与休克晚期血管低反应性的发生有关。
本研究通过建立大鼠失血性休克模型,观察失血性休克不同时期sorcin在VSMC胞浆及质膜的表达变化、sorcin-RyR2复合子形成和解离情况及其与休克血管异常反应性之间的关系,为寻求休克时血管低反应性恢复剂提供实验依据。
1材料与方法
1.1实验动物和试剂
SD大鼠(体重200±20g,雌雄不限)由第三军医大学大坪医院野战外科研究所实验动物中心提供(动物合格证号:SYXK2002-032);NE购自远大医药(中国)有限公司,批号140507(2 mg/mL),采用0.01mol/L HCl配制;戊巴比妥钠、抗β-actin单抗购自Sigma-Aldrich公司(美国);Supersignal发光增强试剂盒购自Pierce公司(美国);Protein G、抗RyR2抗体、抗sorcin抗体购自Santa Cruz公司(美国)。其它相关试剂为国产分析纯。
1.2分组及失血性休克模型
采用随机数字表法将实验大鼠分为对照组(n=12)和模型组(n=24)。模型组参照文献[8]建立失血性休克大鼠在体模型:实验前,大鼠禁食12h,自由饮水,建模当日戊巴比妥钠(30mg/kg)腹腔注射麻醉后,右侧股动脉插管并接血压计,肝素钠500U/kg抗凝,术毕血压稳定10min后开始放血,在10min内将平均动脉压(MAP)降至40mmHg为失血性休克成模,并在此水平分别维持30min(n=12,休克早期组)和2h(n=12,休克晚期组)。对照组不行手术,普通饲养。
1.3观察指标及其检测方法
1.3.1离体血管张力:各组大鼠开腹取出整个肠组织,在解剖显微镜下剥离肠系膜上动脉主干及其1-2级分支,并清除周围结缔组织。动脉主干切取1-2个血管环,余下组织用于蛋白提取(见后述)。将血管环置于Krebs-Henseleit(K-H)液(mmol/L:NaCl 118、 KCl 4.7、 NaHCO325、 KH2PO41.03、
MgSO4·7H2O 0.45、CaCl22.5、EDTA 0.001、glucose 11.1,pH 7.4)。在解剖显微镜下将血管环悬挂于一对不锈钢丝上,一端置于固定柱,另一端与张力换能器(PowerLab,澳大利亚)相连,浸于注有K-H液的离体器官灌流浴槽中(37℃恒温)孵育,持续通氧。待血管张力平衡后,记录NE诱导血管环收缩曲线。血管环对NE(10-9-10-5mol/L)的收缩反应采用浓度累计法测定,即依次加入终浓度为10-9、10-8、10-7、10-6、10-5mol/L的NE,记录早、晚休克组不同NE浓度下血管环收缩反应的变化,以加NE后血管环的收缩力与血管环初始张力的差值作量-效曲线,以量-效曲线及10-5mol/L NE诱导的最大收缩力(Emax,g)评价血管对NE的收缩反应性[1]。
1.3.2胞浆及质膜蛋白的提取:参照本实验室方法[9]进行血管平滑肌胞浆及质膜蛋白的提取。将去内皮血管组织置于预冷蛋白裂解液中(mM: Tris-HCl 20、EDTA 2、EGTA 1、β-巯基乙醇 0.1%、NaF 10、Na3VO41、protein kinase inhibitor colktail pit),于冰浴中剪碎组织并匀浆,收集上清置冰浴中冻融1h;上清经1 000g离心10min(4℃),取上清再16 000g离心30 min (4℃),收集上清即为胞浆总蛋白;将沉淀用质膜蛋白裂解液(mM: Tris-HCl 20、EDTA 2、EGTA 1、β-巯基乙醇 0.1%、NaF 10、Na3VO41、TritonX 100 1%、SDS 0.1%)溶解后,经16 000g离心20 min(4℃),收集上清即为质膜总蛋白。采用Brad-ford法进行蛋白定量。
1.3.3胞浆和质膜sorcin蛋白表达: 采用Western blotting(WB)检测各组大鼠血管平滑肌sorcin的表达。参照文献[10],取等量样品蛋白(120μg/道)经15% SDS-PAGE凝胶电泳分离后转移至硝酸纤维素膜,转移完毕后用5% 脱脂奶粉室温封闭4 h后加入抗sorcin单抗(1∶800)室温孵育4 h,再加入辣根酶标记二抗(1∶10 000)室温孵育1h,将待测硝酸纤维素膜置于supersignal发光增强试剂中反应2min-3min后,迅速用塑料膜包裹硝酸纤维素膜,与X-ray胶片一同放入暗盒中曝光2min-5min,再将胶片显影、定影,确定蛋白条带的相应分子量。将胶片置于图像分析仪(Bio-Rad Gel 2000型,美国)中,对目标条带进行扫描及灰度分析。
1.3.4Sorcin-RyR2复合子检测: 采用免疫共沉淀联合免疫杂交检测Sorcin-RyR2复合子的形成及解离情况。收集各组总蛋白(500μl),加入抗RyR2单抗(5μg),4℃振摇(over-night),继续加入protein G (20 μl) 4℃振摇5 h后,2 500 g离心10 min(4℃),弃上清;采用RIPA洗涤3次后,加入RIPA和上样缓冲液(4∶1),100℃煮5 min。常规SDS-PAGE电泳后转移至硝酸纤维素膜,加入抗sorcin单抗(1∶1 000),室温孵育4 h,再加入辣根酶标记二抗(1∶10 000),室温孵育1 h,将待测硝酸纤维素膜置于supersignal发光增强试剂中反应2min-3min后,迅速用塑料膜包裹硝酸纤维素膜,与X-ray胶片一同放入暗盒中曝光2min-5min,再将胶片显影、定影,确定蛋白条带的相应分子量。将胶片置于图像分析仪中,对目标条带进行扫描及灰度分析。
1.4统计学处理
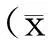
2结果
2.1各组大鼠肠系膜动脉对NE的反应性
结果显示,休克早期组大鼠肠系膜动脉对NE的收缩反应性显著高于对照组,表现为NE的量-效曲线明显左移,10-5mol/L NE引起血管Emax由0.72±0.15g升至1.11±0.28g(t=1.931,P<0.05);休克晚期组肠系膜动脉对NE的收缩反应性较对照组明显降低,表现为NE的量-效曲线明显右移,10-5mol/L NE引起血管Emax由0.72±0.15g降至0.50±0.11g(t=1.874,P<0.05),与我们以往的报道[1]一致。见图1。
注:与对照组比较,*P<0.05
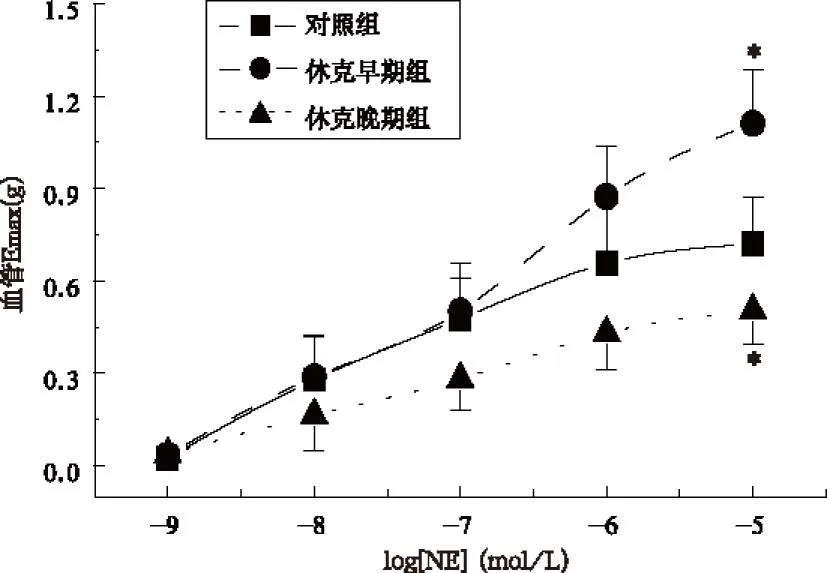
2.2各组大鼠肠系膜动脉平滑肌sorcin在胞浆及质膜的表达
结果显示,各组sorcin总表达量差异无统计学意义(F=5.607,P>0.05);失血性休克早期组,sorcin在血管平滑肌胞浆及质膜中的表达无显著差异(t=1.131,P>0.05),而晚期组胞浆sorcin表达升高,质膜sorcin表达降低,两者差异有统计学意义(t=1.868,P<0.05)。提示失血性休克晚期,sorcin可从肌浆网向胞浆转位。见图2。
注:与休克早期组比较,*P<0.01
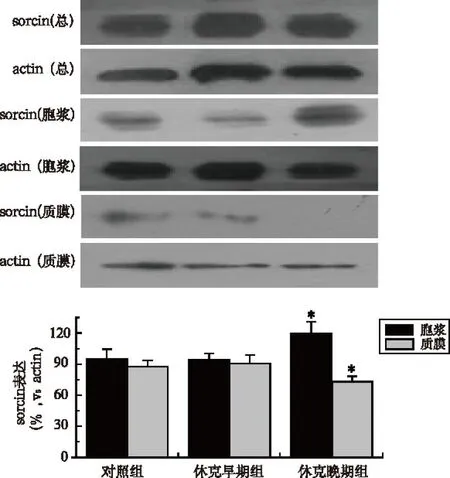
2.3休克早期组和晚期组sorcin-RyR2复合子表达
结果显示,失血性休克早期组与对照组大鼠肠系膜动脉平滑肌sorcin-RyR2复合子表达差异无统计学意义(t=1.319,P>0.05),晚期组sorcin-RyR2复合子表达较对照组显著减少,差异有统计学意义(t= 2.067,P<0.05),见图3。
2.4休克晚期组大鼠Sorcin胞浆水平与肠系膜动脉对NE反应性的相关性分析
相关分析结果显示,休克晚期组大鼠sorcin在胞浆的表达与肠系膜动脉对NE反应性(收缩力)呈显著负相关(r=-0.983,P<0.01);休克早期组大鼠sorcin在胞浆的表达与肠系膜动脉对NE反应性未见显著相关性(r=0.087,P>0.05)。见图4。
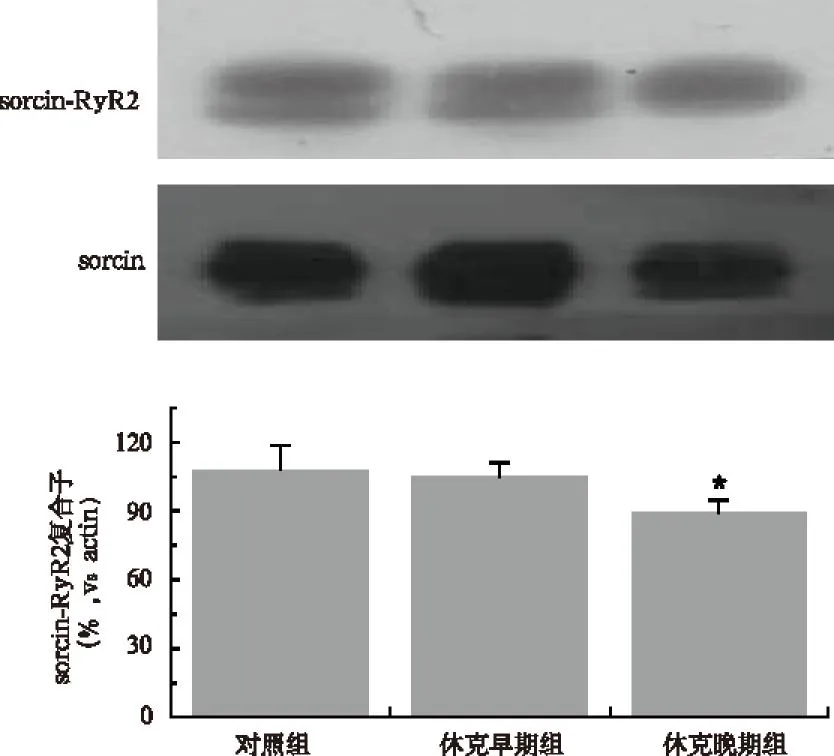
注:与对照组比较,*P<0.05
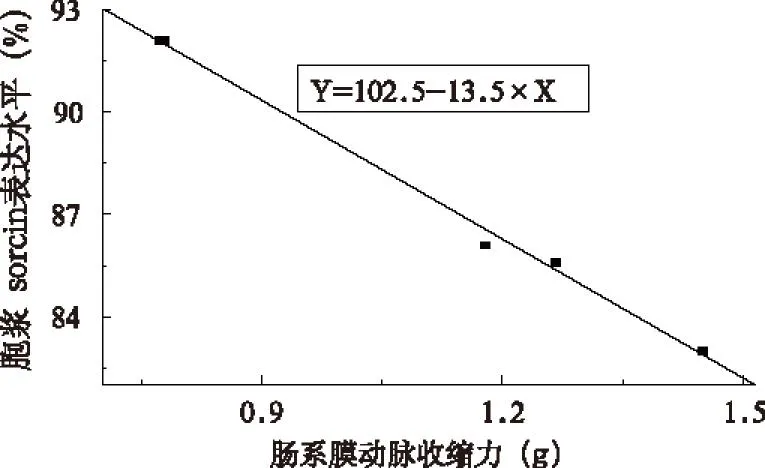
图4 失血性休克晚期组大鼠肠系膜动脉平滑肌胞浆sorcin
3讨论
严重创伤/失血性休克后血管功能障碍的发生是导致临床患者血压持续下降、器官灌流不足及机体死亡的重要原因之一。肌浆网由RyR介导的内钙释放是调控血管平滑肌舒-缩反应的重要机制之一,其在失血性休克血管反应性异常发生中的作用研究甚少。
分布于血管平滑肌的sorcin调节着血管平滑肌细胞兴奋-收缩偶联。生理状态下,位于胞浆的sorcin选择性结合RyR2并维持RyR2/钙释放通道处于闭合状态,从而抑制RyR2的基础活性[11]。本研究结果显示,在失血性休克大鼠肠系膜动脉去内皮后,sorcin的表达发生明显转位,即在失血性休克晚期(2h),sorcin在胞浆的表达明显升高,而在质膜的表达显著降低;同时发现,失血性休克晚期大鼠肠系膜动脉血管平滑肌sorcin-RyR2复合子表达水平较对照组显著降低。表明在失血性休克晚期发生了sorcin与RyR2解离及从肌浆网向胞浆转位;而且sorcin在胞浆中的表达升高与血管反应性降低呈显著负相关。而在失血性休克早期(30min),sorcin在胞浆及质膜的表达、sorcin-RyR2复合子水平以及血管对NE的收缩反应性均与对照组差异无统计学意义。
已有研究显示,sorcin从RyR2的解离可刺激RyR2通道活性及RyR2介导肌浆网内钙释放的增加,通过触发钙火花及STOCs形成[4,5],导致VSMC膜BKCa通道的激活[6];我们以往的研究也表明,BKCa通道的过度激活促成了休克晚期血管低反应性的形成。因而认为sorcin从RyR2解离可能与失血性休克后血管低反应性的形成有关。至于sorcin从RyR2解离在触发休克血管低反应性形成中的可能机制,以及休克晚期触发sorcin与RyR2解离的上游调控分子,均有待进一步探讨。
综上,失血性休克晚期,大鼠肠系膜动脉sorcin与肌浆网RyR2解离所导致的sorcin从肌浆网质膜向胞浆转位,可能是促成失血性休克晚期血管低反应性形成的重要原因。
◀
周荣(1971-),女,汉族,医学博士,副研究员,研究方向为休克血管功能障碍发生机制及防治
参考文献
1Li T, Liu L, Xu J, et al. Changes of Rho kinase activity after hemorrhagic shock and its role in shock-induced biphasic response of vascular reactivity and calcium sensitivity[J]. Shock,2006,26(5):504-509.
2Zhou R, Chen F, Li Q, et al. Stimulation of the adenosine A3receptor reverses vascular hyporeactivity hyporeactivity after hemorrhagic shock in rats[J]. Acta Pharmacol Sin,2010,31(4):413-420.
3Zhou R, Ding XL, Liu LM. Ryanodine receptor 2 contributes to hemorrhagic shock-induced bi-phasic vascular reactivity in rats[J]. Acta Pharmacol Sinic,2014,35(11):1 375-1 384.
4Franceschini S, Ilari A, Verzili D, et al. Molecular basis for the impaired function of the natural F112L sorcin mutant: X-ray crystal structure, calcium affinity, and interaction with annexin VII and the ryanodine receptor[J]. FASEB J,2008,22(1):295-306.
5Okamoto Y, Chaves A, Chen J, et al. Transgenic mice with cardic-specific expression of activating transcription factor 3, a stress-inducible gene, have conduction abnormalities and contractile dysfunction[J]. Am J Phathol,2001,159(2):639-650.
6Rueda A, Song M, Toro L, et al. Sorcin modulation of Ca2+sparks in rat vascular smooth muscle cells[J]. J Physiol,2006,576(Pt 3):887-901.
7Zhou R, Liu L, Hu D. Involvement of BKCaalpha subunit tyrosine phosphorylation in vascular hyporesponsiveness following hemorrhagic shock in rat[J]. Cardiovasc Res,2005,68:327-335.
8Xu J, Lan D, Li T, et al. Angiopoietins regulate vascular reactivity after haemorrhagic shock in rats through the Tie2-nitric oxide pathway[J]. Cardiovasc Res,2012,96(2):308-319.
9Yang G, Xu J, Li T, et al. Role of V1a receptor in AVP-induced restoration of vascular hyporeactivity and its relationship to MLCP-MLC20 phosphorylation pathway[J].J Surg Res,2010,161(2):312-320.
10Yamagishi N, Nakao R, Kondo R, et al. Increased expression of sorcin is associated with multidrug resistance in leukemia cells via up-regulation of MDR1 expression through cAMP response element-binding protein[J]. Biochem Biophys Res Commun,2014,448(4):430-436.
11Farrell EF, Antaramian A, Rueda A, et al. Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart[J]. J Biol Chem,2003,278(36):34 660-34 666.
欢迎订阅欢迎投稿
18064018058传真:027-88075389E-mail:micch@sina.com;308903786@qq.com
网址:http://WXHX.chinajournal.net.cn
The Translocation of Sorcin and the Correlating Analysis with the Changes of Vascular Reactivity after Hemorrhagic Shock
ZHOU Rong,DING Xiao-li,LIU Liang-ming#
State Key Laboratory of Trauma, Burns and Combained Injury, Second Department of Research Institute of Surgery, Daping Hospital, Third Military Medical University, Chongqing 400042, China;#Corresponding author
【Abstract】Objective: To explore the expression and distribution of sorcin in superior mesenteric artery (SMA) during different stage after hemorrhagic shock and its role in the regulation of vascular reactivity after hemorrhagic shock. Method: 30 SD rats were divided into two groups, twelve as a nonsurgical control group and the rest of the rats were classified as hemorrhagic shock model group that suffered from femoral artery bleeding method (arterial pressure is 40mmHg). The model group rats were divided into the early stage of shock group (30min) and the late stage of shock group (2h). Isolated mesenteric arteries were isolated from each group. The reactivity of mesenteric artery to NE was detected by using in vitro organ tension test. The expression and distribution of sorcin protein in mesenteric artery smooth muscle was detected by using Western Blotting (WB). The expression of sorcin-RyR2 was detected by using Co-immunoprecipitation combined with WB. Then analyze the correlation between the level of sorcin protein and the reactivity of mesenteric artery to NE in the shock rats.Results: During the early stage (30min) after hemorrhagic shock, the vascular reactivity to NE increased significantly, while the vascular reactivity to NE decreased during late stage (2h), which suggested that there was bi-phasic vascular reactivity to NE in SMA during different stage after hemorrhagic shock. The total expression of sorcin had no significant changes but the distribution of sorcin in cytoplasm and plasmalemma fraction of VSMC changed significantly, characterized with the expression of sorcin in cytoplasm and in plasmalemma had no significant changes during the early stage (30min) after hemorrhagic shock, while the expression of sorcin in cytoplasm fraction was upregulated but its expression in plasmalemma fraction decreased significantly during the late stage (2h) after hemorrhagic shock. Immunoprecipitation analysis showed that formation of sorcin-RyR2 complex decreased during the late stage but not during the early stage after hemorrhagic shock. The correlation analysis showed that the increased expression of sorcin in cytoplasm closely associated with the blunted vascular hyporeactivity to NE during the late stage after hemorrhagic shock. Conclusion: At late stage after hemorrhagic shock, sorcin translocated from cytoplasm to plasmalemma in superior mesenteric artery in rat. The dissociation of sorcin from RyR2 at least partly associated with the development of vascular hyporeactivity to NE during the late stage after hemorrhagic shock.
【Key words】Hemorrhagic shock; Vascular reactivity; Sorcin; Ryanodine receptor Ⅱ; Rat
通讯地址:430060 湖北省武汉市张之洞路9号《微循环学杂志》编辑部电话:027-88075389;
作者简介:本文第一
[中图分类号]R541.6+4
[文献标识码]A
[文章编号]1005-1740(2016)01-0001-05
*[基金项目]国家自然科学基金(81100227、81370427)
本文2015-07-09收到,2015-12-20修回

