An Oxidative Desulfurization Catalyst Based on Bimodal Mesoporous Silica Containing Quaternary Ammonium Heteropolyphosphamolybdenum
Li Jian; Wu Haishun; Yang Lina; Ma Bo
(1. School of Chemistry and Materials Science, Shanxi Normal University, Linfen 041004; 2. School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
An Oxidative Desulfurization Catalyst Based on Bimodal Mesoporous Silica Containing Quaternary Ammonium Heteropolyphosphamolybdenum
Li Jian1,2; Wu Haishun1; Yang Lina2; Ma Bo1
(1. School of Chemistry and Materials Science, Shanxi Normal University, Linfen 041004; 2. School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
A bimodal mesoporous silica (BMMS) modi fi ed with amphiphilic compound (C19H42N)3(PMo12O40) (CTA-PMO) was prepared by the two-step impregnation method. Firstly, H3PMo12O40was introduced into the bimodal mesoporous silica via impregnation, then C19H42NBr (CTAB) was grafted on the surface of BMMS containing H3PMo12O40based on the chemical reaction between quaternary ammonium compound and the phosphomolybdic acid, and then the catalyst CTAPMO/BMMS was obtained. The samples were characterized by XRD, N2adsorption and desorption, FTIR,31P-NMR,29Si-NMR and TEM analyses. It is shown that the catalyst has a typical bimodal mesoporous structure, in which the small mesopore diameter is about 3.0 nm and the large mesopore diameter is about 5.0 nm. The chemical interaction happens between the Keggin structure and silica group of BMMS. Compared with the mono-modal porous Hβ and SBA-15 zeolites modi fi ed with CTA-PMO, CTA-PMO/BMMS showed better catalytic activity in the oxidative conversion of dibenzothiophene (DBT), and the desulfurization rate can reach about 94% with the help of extraction, and the catalyst can be separated by fi ltration and reused directly. The catalytic oxidative desulfurization mechanism on CTA-PMO/BMMS was proposed and veri fi ed.
(C19H42N)3(PMo12O40), amphiphilic, bimodal mesoporous silica, oxidation, dibenzothiophene
1 Introduction
The aromatic sulfur compounds such as benzothiophene, dibenzothiophene and their derivates are difficult to be directly removed by the conventional hydrodesulfurization (HDS) methods because of their steric hindrance. Oxidative desulfurization (ODS) technology is an effective and attractive method to completely convert such compounds under mild reaction conditions into highly polar products that can be separated with extraction or adsorption by virtue of the polarity differences of the products[1-3]. Therefore ODS is considered to be a promising supplementary method of HDS to achieve the deep desulfurization of fuels. It was reported that the ODS system including H2O2serving as oxidant and polyoxometalates (PMOs) functioning as catalyst showed excellent activity[4-5]. In such system, the mass transfer across the interface of aqueous phase and oil phase is the rate-limiting step. PMOs and H2O2are immiscible in the fuel, which makes the oxidant and sulfur compounds keep only in contact at the interface of organic phase and the aqueous phase, and the poor contact can slow down the reaction rate[6].
In order to overcome this shortcoming the amphiphilic oxidation catalysts have been explored. Typically, the heteropolyacid quaternary ammonium (HPAQA) compound can improve the ODS reaction rate owing to its amphiphilic structure, because it is a phase transfer catalyst in nature. Forms of HPAQA can be composed of phosphotungstic acid/quaternary ammonium salt with different carbon chains (C8, C12, C14, C16and C18)[7-8]or phosphomolybdic acid modi fi ed with different quaternary ammonium salts including tetramethyl ammonium chloride (TMAC), dodecyl trimethyl ammonium chloride (DTAC) and hexadecyl trimethyl ammonium chloride (HTAC)[9]. HPAQA is insoluble in fuel before thereaction, however, it becomes soluble when reacting with hydrogen peroxide, and it is more interesting to know that it becomes an insoluble solid after the oxidation reaction is over[10]. Such catalyst indeed promotes the ODS by decreasing the mass transfer limits and it seems quite easy to recover the catalyst by fi ltration. However, it still should be noted that the loss of such fi ne particulate catalyst is inevitable when it is recovered through filtration. Besides the recovery efficiency it should also be noted that the less porous structure of HPAQA cannot offer high surface area and suitable pore size accessible to the large molecules of sulfur compounds like DBT and its derivatives, which would affect the activity and selectivity of the catalyst and could increase the cost of the catalyst correspondingly.
Some efforts to immobilize HPAQA on different supports have been made. HPAQA was immobilized within the silica framework through the sol-gel process of tetraethyl orthosilicate (TEOS). The structure of the catalyst looks like a reverse micelle and it is stable and can be recovered by simple filtration and reused directly[11]. HPAQA was immobilized in mesoporous silica by the sol-gel method and the catalyst had an interconnected pore-network structure[12-13]. HPAQA was also covalently anchored to silica gel, and such organic-inorganic hybrid material was proved as an ef fi cient and robust catalyst for the oxidative reaction of sulfide to sulfoxide[14]. When HPAQA was encapsulated in the channels of silica, a micro reactioncontrolled phase-transfer system was set up for oxidative desulfurization[15]. Poly(lacry-lamide) microgels covered with HPAQA have also been synthesized, and this material is quite promising in developing new catalytic materials used in phase-transfer catalytic reaction such as ODS[16].
At present, studies on the supports for HPAQA are mostly focused on silica gel or mono-modal mesoporous silica (MMMS), while the study on the hierarchical porous catalyst is rarely reported. Bimodal mesoporous silica (BMMS) is a type of hierarchical porous material based on MMMS silica. Besides all the typical characteristics of MMMS, BMMS possesses a double-peak pore size distribution in the mesopore range. Its large mesopores can make the large molecule reactants and products pass through the channels easily and freely, and its small mesopores can offer high surface area[17-18]. For large molecular sulfur compounds such as BT, DBT and their derivatives, the bimodal mesoporous silica (BMMS) should be a good selection to function as the ODS catalyst support. It can provide more chances for sulfur compounds to approach catalytic sites and increase the reaction rate.
Herein, we report a catalytic system based on the bimodal mesoporous silica containing quaternary ammonium heteropolyphosphamolybdenum. The BMMS has large mesopores (about 5.0 nm) and small mesopores (about 3.0 nm), and its large mesoporous structure is favorable for the dispersion of reactants and products. At the same time its small mesopores can offer high surface area so as to maintain enough effective active sites for adsorption or reaction. Phosphomolybdic acid (HPMO) is loaded on BMMS through impregnation, and then CTAB reacts with HPMO group on the surface of BMMS, the catalytic active composites are assembled in the channel of BMMS. The hydrophilic PMO group is the active center of the catalyst and the hydrophobic CTA functions as a phase transfer agent. During the reaction, the CTAPMO gets the active oxygen from H2O2and transfers it to the sulfur compound. The recovered CTA-PMO/BMMS sample can be reused directly.
2 Experimental
2.1 Materials
H3PMo12O40·14H2O (AR), cetyltrimethyl ammonium bromide (CTAB, AR), tetraethylorthosilicate (TEOS, AR), hydrogen peroxide (H2O2, 30%), dibenzothiophene (DBT, 98%), hydrochloric acid (2.0 mol/L), dodecane (AR) and Na2SO4·10 H2O (AR) were all purchased from the China Pharmaceutical Group. P123 (AR) and F127 (AR) were obtained from the Sigma-Aldrich Corporation. The Hβ zeolite was purchased from the catalyst plant of Fushun Petrochemical Company. All the reagents were used directly without further puri fi cation.
2.2 Synthesis of bimodal mesoporous silica
The gel A was prepared based on the sol-gel process, with pluronic P123 serving as the template, and the mass ratio of P123:H2O:hydrochloric acid: TEOS was equal to1.0:24.5:6.3:2.12. The reaction was carried out at 40 ℃, after the reaction mixture was subject to stirring for 24 h, the gel A was obtained. The gel B was obtained in a similar way, with pluronic P127 serving as the template, and the mass ratio of F127: H2O: hydrochloric acid: TEOS: Na2SO4·10H2O was equal to 1.0:22.5:7.5:4.2:4.85, while the reaction time and temperature were the same as those used for the preparation of gel A. Gel A had the same amount of surfactant as gel B. Gel B at first was mixed with gel A and the mixed gel was stirred at room temperature for 2 h. Then, the mixture was transferred into a Te fl on-lined autoclave for co-hydrothermal crystallization at 100 ℃ for 24 h. After the resulting solid was subject to filtration and washing with distilled water until the discharged solution showed a neutral reaction, the obtained fi lter cake was dried at 140 ℃ for 12 h, and then was calcined in air at 550 ℃ for 5 h, with the fi nally obtained silica labeled as BMMS.
2.3 Synthesis of SBA-15
According to the published synthesis procedure[19], the SBA-15 zeolite was synthesized using P123 as the template and TEOS as the silica source in an acidic medium.
2.4 Preparation of catalysts
HPMO and C19H42NBr (CTAB) in a mole ratio of 1:4 were put into a 30% alcohol solution (1 mol of HPMO: 40 L of alcohol solution) to enter into reaction at 60 ℃ under stirring, when a precipitate was immediately formed. After continuously stirring for 2 h the resulting precipitate was fi ltered and washed with alcohol until a neutral reaction was obtained, fi nally the sample was dried at 110 ℃ for 12 h, and the obtained sample was labeled as CTA-PMO.
The bimodal mesoporous silica containing CTA-PMO was synthesized by means of the two-step impregnation method. Based on the incipient-wetness impregnation method, 1.0 g of bimodal porous silica and a certain calculated amount of HPMO according to their required mass concentrations in the catalyst were put into a 30% alcohol solution at ambient temperature, after 2 h of stirring and further stewing overnight, the mixture was filtrated and dried at 100 ℃ for over 12 h, and the bimodal mesoporous silica containing HPMO was obtained and noted as HPMO/BMMS.
The obtained bimodal mesoporous silica containing HPMO was impregnated in a solution of C19H42NBr (HPMO: CTAB=1:4 in the mole ratio) at 60 ℃ under stirring, after continuously stirring for 2 h and further stewing overnight, the resulting mixture was filtered, washed with alcohol until a neutral reaction was identi fi ed, and dried at 110 ℃ for 12 h. The fi nally obtained bimodal mesoporous catalysts was named as CTA-PMO/ BMMS (X%), with X% representing the loaded mass percent of CTA-PMO in the sample.
The Hβ and SBA-15 zeolites modified with CTA-PMO were prepared with the same method and CTA-PMO loading as those used in the case for modifying the catalytic materials with CTA-PMO/BMMS (28.6%), and they were named as CTA-PMO/ Hβ (28.6%) and CTAPMO/ SBA-15 (28.6%), respectively.
2.5 Characterization
X-ray diffraction (XRD) patterns were determined on a D/ max-IIIA X-ray diffractometer (Rigaku, Japan) using Cu Kα radiation (35 kV, 30 mA) at a scanning rate of 0.5(°)/ min. Nitrogen adsorption and desorption measurements were performed with an ASAP 2420 (Micrometrics, USA) system at 77K. The31P and29Si cross polarizationmagic angle spinning (CP-MAS, Germany) NMR spectra were recorded on a Bruker AV300 spectrometer. Tetraethylorthosilicate (δ=82.4 from TMS) was used as the reference for29Si chemical shifts and 0.81 NH4H2PO4was referenced for31P chemical shifts. Transmission electron microscope (TEM) images were collected on a JEM-2010CX (JEOL, Japan) electron microscope operating at 200 kV.
2.6 Oxidation of model sulfur-containing fuels
In a typical reaction run, catalyst (1%, calculated in terms of the ratio of catalyst mass to model fuel mass), hydrogen peroxide (O/S=10:1 mole ratio), 25 mL of model fuel (500 μg/g of DBT in dodecane solution) and 25 mL of DMF were put in an Erlenmeyer flask under stirring. This mixture was heated to 70 ℃ for reaction under vigorous stirring for 1.5 h, the reaction system was separated with centrifugation and the upper products were subjected to sulfur measurement with an Agilent-6890plus gas chromatograph equipped with a FID detector and a HP-5 capillary column (with a precision of detection in μg/g). The catalyst on the bottom of the flask was recovered with fi ltration and reused after drying for more than 12 h at 100 ℃.
CTA-PMO/BMMS (33.3%) was chosen to make a study on the mechanism of the catalytic ODS of DBT. The catalyst was filtered after adding hydrogen peroxide for 10 min at 70 ℃ and the catalyst was noted as [O]CTAPMO/BMMS (33.3%). The catalyst after the ODS of DBT without extraction at 70◦C under vigorous stirring for 1.5 h was also fi ltered and this catalyst was noted as [R]CTA-PMO/BMMS (33.3%). [O]CTA-PMO/BMMS (33.3%) and [R]CTA-PMO/BMMS (33.3%) were both dried at room temperature for more than 24 h.
2.7 Hot catalyst fi ltration test
This procedure is based on the methodology suggested by Sheldon and co-workers[20], the steps of treatment were the same as ODS just without the addition of DBT, and after treatment the hot catalyst was fi ltrated prior to addition of DBT and the ODS procedures were repeated, and fi nally the products were sent for analysis.
3 Results and Discussion
3.1 XRD
XRD patterns of dried CTA-PMO/SBA-15 zeolite (28.6%), CTA-PMO/Hβ zeolite (28.6%) and CTA-PMO/ BMMS (9.1%—33.3%) are shown in Figure 1. In Figure 1(a), small angle XRD patterns of CTA-PMO/SBA-15 (28.6%) (2θ=0.88°, 1.53° and 1.78°) indicate the long range ordered arrangement of 2D hexagonal channels. For CTA-PMO/BMMS the obvious double-peaks are found in the range of 2θ=0.89°—1.0°, which suggests two kinds of ordered mesopores and the peaks become weak with the introduction of more catalytic active composite, which is ascribed to the filling of channels and covering of surface by the loading of CTA-PMO. No orderly arranged mesopores exist in the Hβ zeolite, therefore small angle XRD pattern was not necessary to be measured.
In Figure 1(b) no characteristic patterns of CTA-PMO exist in the visual field for CTA-PMO/ BMMS (9.1%), while with the growth of CTA-PMO amount, peaks at 2θ= 8.44° (Keggin structure of heteropolyacid salt) appear and show correspondingly a slight increase. Clear wideangle XRD patterns of Hβ zeolite are observed for CTAPMO/Hβ (28.6%), peaks for the Keggin structure can be observed on CTA-PMO/Hβ zeolite (28.6%) and CTAPMO/ SBA-15 zeolite (28.6%).
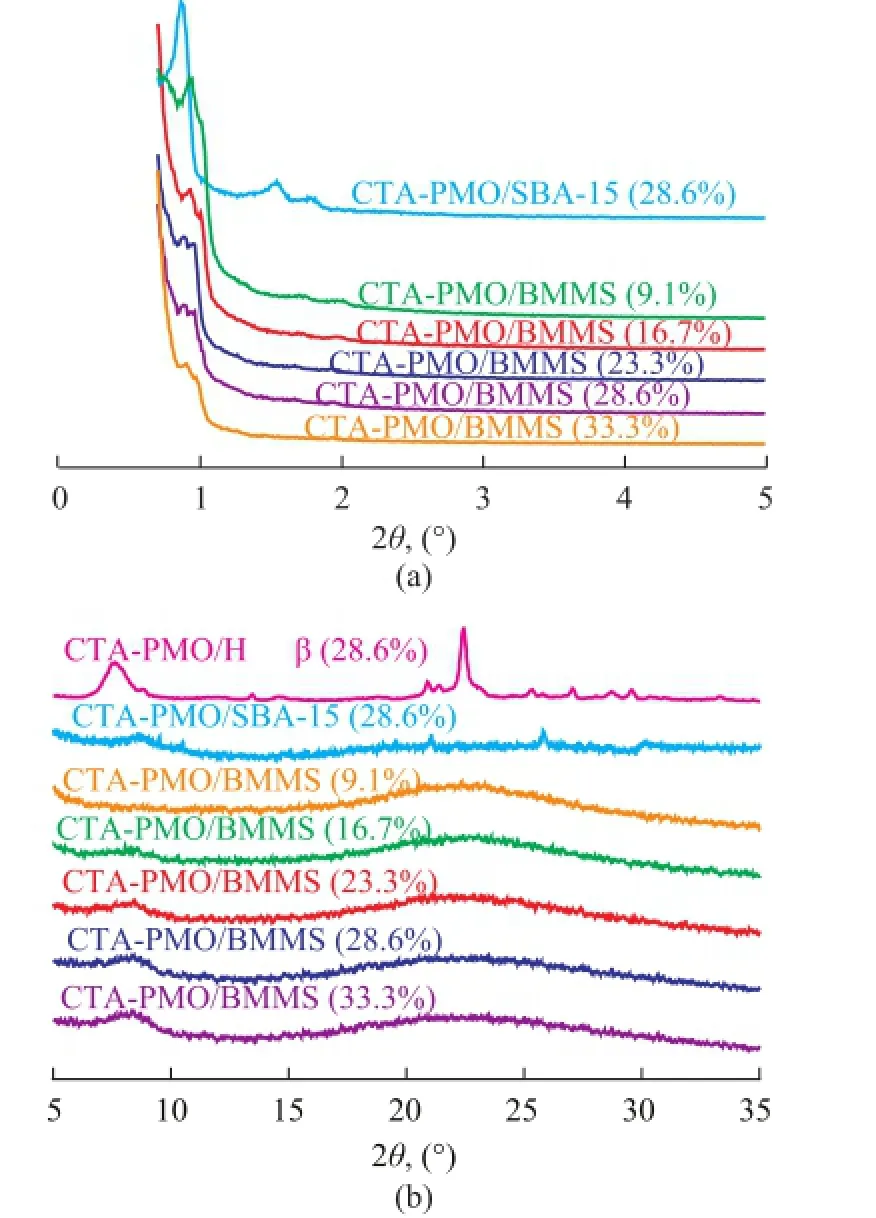
Figure 1 (a) Small angle XRD and (b) wide angle XRD of CTA-PMO-SBA-15 (28.6%), CTA-PMO-Hβ (28.6%) and CTA-PMO /BMMS (9.1%—33.3%)
3.2 N2adsorption and desorption
The N2adsorption and desorption isotherms are listed in Figure 2. The isotherm of CTA-PMO/SBA-15 (28.6%) has a typically single H1 hysteresis loop, which denotes the existence of mono-modal cylindrical mesopores in this sample. The isotherm of CTA-PMO/Hβ has a H4 hysteresis loop in de Boer’s classification, which is characteristic of the slit-shaped pores[21]. Isotherms of CTA-PMO/BMMS samples have typically double hysteresis loops, the lower one is a type H2 corresponding to the bottle-shape mesopore and the higher one is a type H1 relating to the cylindrical mesopore. Besides XRD results the appearance of double hysteresis loops in isotherms is another valid evidence for the existence ofbimodal mesopores in CTA-PMO/BMMS.
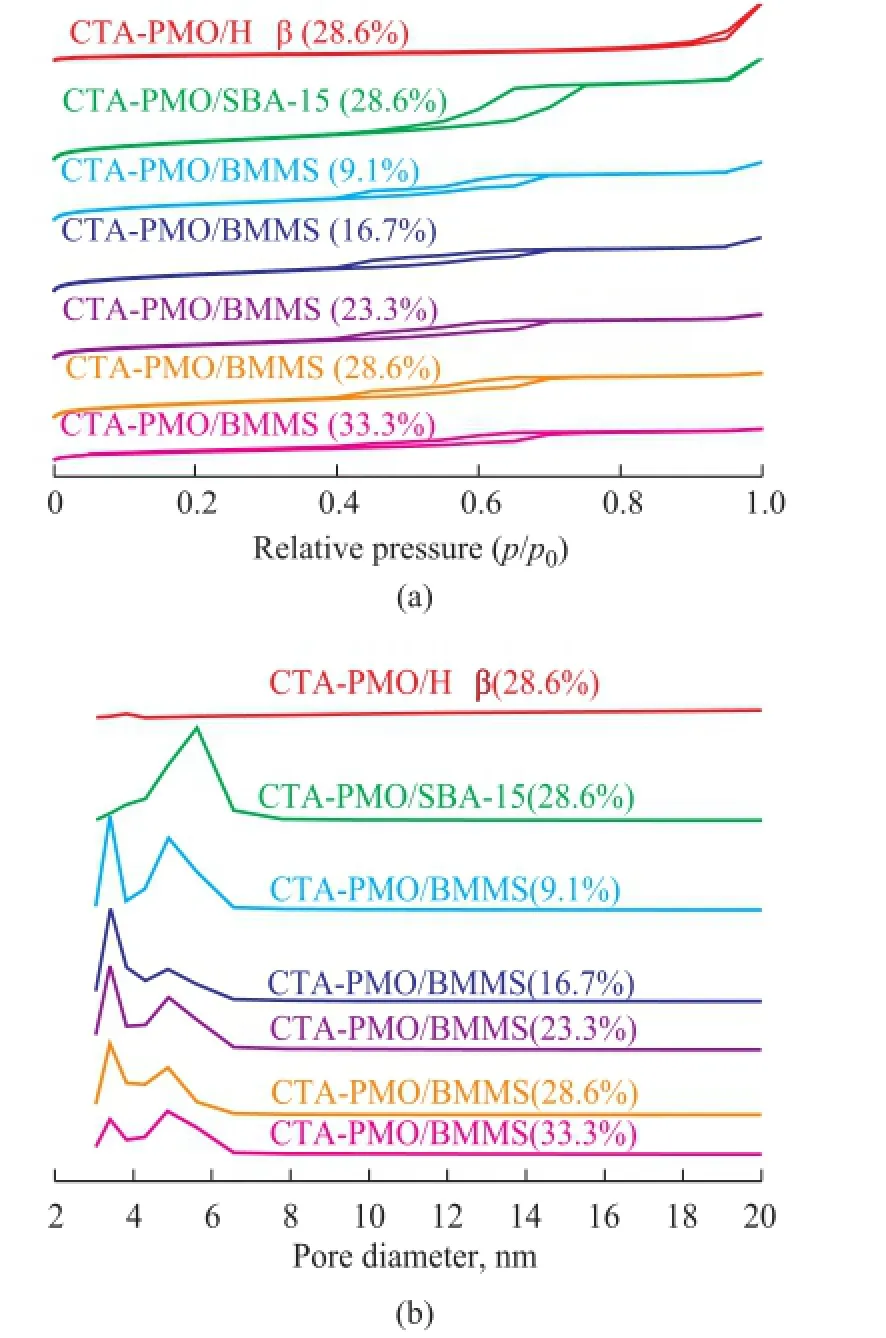
Figure 2 (a) N2adsorption-desorption isotherms and (b) pore diameter distribution of CTA-PMO-SBA-15 (28.6%), CTA-PMO-Hβ (28.6%) and CTA-PMO/BMMS (9.1%-33.3%)
The pore size distribution of the samples is also listed in Figure 2. CTA-PMO/SBA-15 (28.6%) showed a characteristic single peak, indicating to the existence of the mono-modal pores, however, CTA-PMO/Hβ (28.6%) does not show such an apparent narrow pore size distribution peak as CTA-PMO/SBA-15 (28.6%). Double peaks pore size distribution of CTA-PMO/BMMS can further prove the bimodal mesoporous structure. In Table 1 the physical structure parameters including the surface area and pore volume denote a regular decrease when the CTA-PMO amount grows gradually in the samples. Most probable pore diameters of samples do not have many changes, the pore size at the lower end is about 3.4 nm and the pore size at another end is about 4.9 nm. Compared with CTA-PMO/SBA-15 (28.6%) sample, CTA-PMO/BMMS (28.6%) has a lower surface area, pore volume and smaller pore size. Compared with CTAPMO/Hβ (28.6%) sample, it has a higher surface area and a lower pore volume.

Table 1 Physical pore structure parameters of samples
3.3 FTIR spectrometry
FTIR spectra of samples are shown in Figure 3. The bands at 1 086 cm-1, 960 cm-1, 802 cm-1and 468 cm-1for BMMS are assigned to the asymmetric stretching vibrations of Si—O—Si, the vibrations of Si—OH and the symmetric stretching and bending vibrations of Si—O—Si. As regards HPMO/BMMS, the bands at 1 086 cm-1(asymmetric stretching vibrations for central P—O), 963 cm-1(symmetric stretching vibrations for Mo=O), 879 cm-1(stretching vibrations for Mo—Ob—Mo) and 800 cm-1(stretching vibrations for Mo—Oc—Mo) indicate the existence of Keggin structure in the samples. All the samples loaded with 28.6% CTA-PMO have bands at 1 086 cm-1(overlapping the bands of SiO2), 960 cm-1(overlapping the bands of Si—OH), 873 cm-1and 802 cm-1, the existence of these bands means that the Keggin structures are retained in samples. After the introduction of CTAB the obviously decreased intensity of the band at 963 cm-1and the shift of band from 879 cm-1to 873 cm-1suggests the chemical interaction between CTAB and HPMO. Two adjacent peaks at 2 926 cm-1and 2 857 cm-1are characteristic of symmetric and asymmetric stretching vibrations of —CH2radicals[22]. Compared with the reported wave number for bulk CTAB (at 1 472 cm-1), peaks at 1 403—1 465 cm-1have had red shifts. Based on the above FTIR results it canbe concluded hereby that CTA-PMO has been successfully introduced in BMMS, and the Keggin unit is retained in the samples, while CTAB has chemical interaction with HPMO. The two-step impregnation method had introduced H3PMo12O40into bimodal mesoporous silica firstly and then CTAB was grafted on the surface of BMMS containing H3PMo12O40based on the chemical reaction. The catalytically active CTA-PMO particles are insoluble in normal solvent; however, this process made it possible to introduce the particles into the bimodal mesoporous pores via impregnation. CTA-PMO is dispersed on the surface of the pore wall and the obtained catalyst has high surface area.
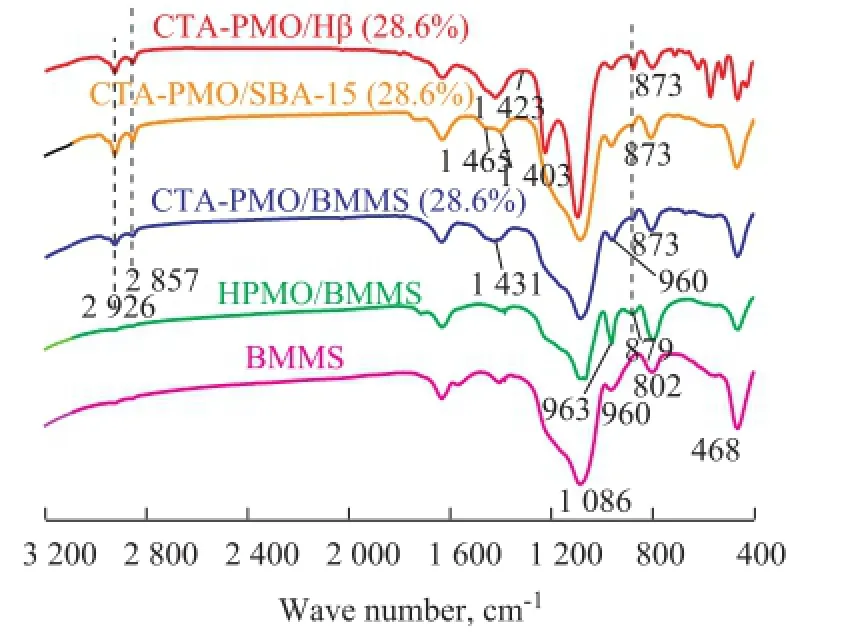
Figure 3 FTIR of CTA-PMO-SBA-15 (28.6%), CTA-PMOHβ (28.6%), CTA-PMO/BMMS (28.6%), HPMO/BMMS and BMMS
3.431P CP-MAS NMR spectrometry
Although the above-mentioned FTIR spectrometric results gave a clear presentation of the interaction between HPMO and CTAB, no valid evidence was found in FTIR spectra on the chemical interaction between CTA-PMO and BMMS. In order to make a detailed research on the chemical interaction of CTA-PMO and BMMS,31P and29Si CP-MAS NMR spectrometric analyses were carried out. The31P CP-MAS NMR spectrometric results are listed in Figure 4, in which CTA-PMO shows a resonance signal at -5.4 related to the Keggin structure of PMO, and the composite CTA-PMO/BMMS (28.6%) exhibits another signal at -7.38 that may be attributed to the chemical interaction of lacunary Keggin structure with silica groups, while the highly nucleophilic monovacant lacunary Keggin units tend to saturation by grafting with electrophilic —Si—OH groups[23].
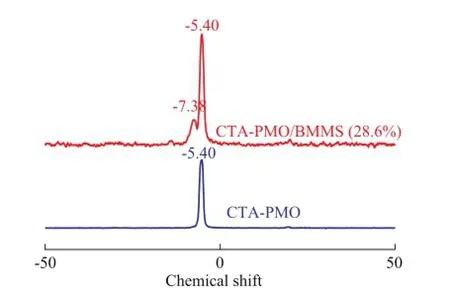
Figure 431P CP-MAS NMR spectra of CTA-PMO and CTA-PMO/BMMS (28.6%)
3.529Si CP-MAS NMR spectrometry
29Si CP-MAS NMR spectrometric analysis was accomplished to make further studies on the chemical interaction between P groups and silica groups (Figure 5). Silica groups can exist in various forms at crystallographically unequivalent sites in silica, so usually do not show the single sharp peak other than serial peaks. Peaks at -94.2, -104.8 and -112.8 are ascribed to BMMS, these peaks are close to the Si chemical shifts in SBA-3 (-90, -100 and-110) as described by Zhao Ruiyu, et al.[24], and such results are also similar to the case of SBA-15, since its Si chemical shifts are at -92, -101 and -110, corresponding to Si(OH)2(OSi)2, Si(OH)3(OSi) and Si(OSi)4, which are Q2, Q3 and Q4 surface silanol groups, respectively[25-26]. After introduction of CTAPMO the intensity of the peak for Q2 group almost disappears. The loss of intensity for Q2 group after the modification of BMMS occurs thanks to this group’s reactivity. Based on the31P CP-MAS NMR and29Si CPMAS NMR analysis results, it can be concluded herewiththat the chemical interaction exists between BMMS and the Keggin structure of CTA-PMO.
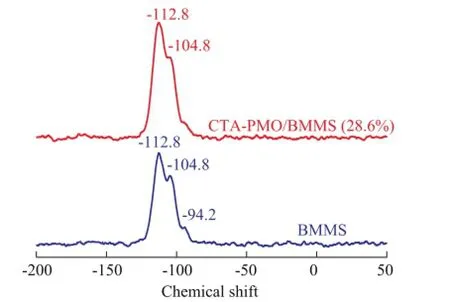
Figure 529Si CP MAS NMR spectra of CTA-PMO and CTA-PMO/BMMS (28.6%)
3.6 TEM analysis
In order to further clarify the pore structure of CTAPMO/BMMS and the situation of dispersed CTA-PMO in BMMS, the TEM analysis of CTA-PMO/BMMS (28.6%) was carried out, with the results listed in Figure 6. Representative ordered regular hexagonal cylindrical pores and cubic bottle-shaped pores are clearly shown in the image. It can be seen that different pore sizes (ca. 3.0 nm and 5.0 nm, respectively) exist in the sample. Such bimodal mesoporous structure is accordant with the formal XRD and N2adsorption-desorption results. The darker part in the fi eld can be attributed to the existence of CTA-PMO in the channels.

Figure 6 TEM of CTA-PMO/BMMS (28.6%)
3.7 Catalytic activity
The CTA-PMO/BMMS samples with different loadings were applied in the ODS of model fuel containing DBT with the same catalyst dosage, with the results presented in Figure 7. It can be found that with the increase of loadings the desulfurization rate showed a parabolic curve with its upper extreme point located at 28.6%. The catalytic activity went up along the fi rst half of the parabola because the increase of loadings offered more amount of catalytically active composites and the catalytic activity then fell off because of the agglomeration of too much CTA-PMO which remarkably decreased the surface area, pore volume and pore size of the samples. This explanation conformed with the XRD and N2adsorption desorption characterization results. Thus further researches on the catalytic performance comparisons were made among the catalysts with the same loading of 28.6%.
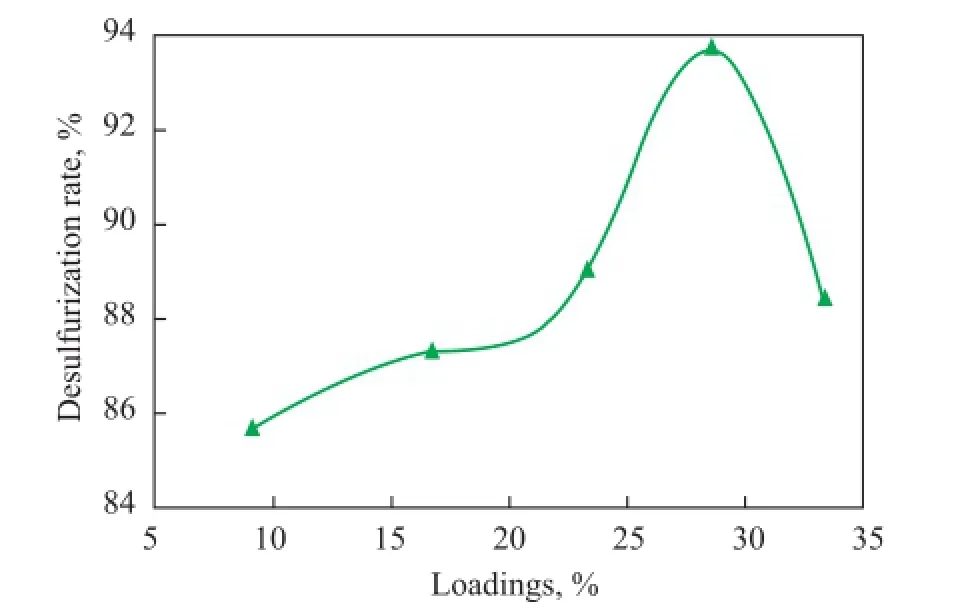
Figure 7 Effects of loadings on the catalytic activity
The catalytic performance values of bulk CTA-PMO, CTA-PMO/BMMS (28.6%), CTA-PMO/SBA-15 (28.6%) and CTA-PMO/Hβ (28.6%) were compared in Figure 8. Results showed that the supported catalysts containing much less amount of active composite than the bulk catalysts displayed significantly higher activity. This is the charm of supported catalyst, and the support BMMS can afford high surface area and suitable porous channels, since CTA-PMO is dispersed finely on the surface of mesopores. Differences of supports can also be revealed fully by comparing the catalytic performance of CTAPMO/BMMS (28.6%) with CTA-PMO/SBA-15 (28.6%) and CTA-PMO/Hβ (28.6%) during ODS of DBT. In general, the catalytic activity decreases in the following order: CTA-PMO/BMMS (28.6%)> CTA-PMO/SBA-15 (28.6%)> CTA-PMO/Hβ (28.6%), and for CTA-PMO/ BMMS (28.6%) the sulfur concentrationCAcan be as low as about 30 μg/g with extraction. CTA-PMO/Hβ (28.6%) has a lowest catalytic activity due to its low surfacearea, pore volume and pore size, and CTA-PMO/SBA-15 (28.6%) has higher catalytic activity possibly because of its preferable pore structure. Such results are quite expected, and an interesting thing denotes that despite its highest catalytic activity, CTA-PMO/BMMS (28.6%) has not shown a highest surface area and pore volume among three catalysts. It is reasonable hereby to attribute the catalytic performance to its bimodal pore size distribution, since during ODS of DBT such pore size distribution has a relatively high prominent effect on the catalytic performance than pore size and pore volume of the samples.
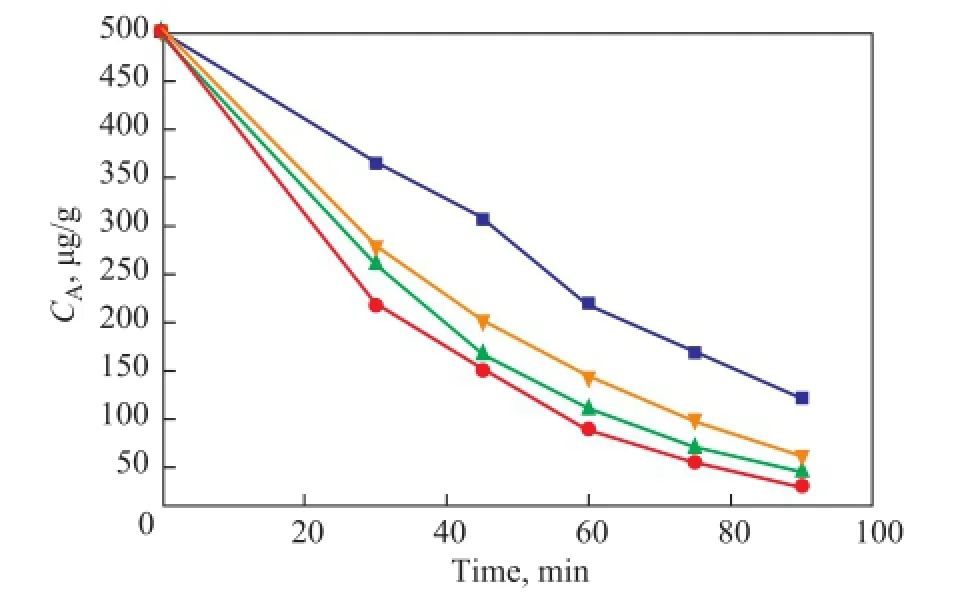
Figure 8 Comparison of catalytic performance of the bulk and supported CTA-PMO catalysts (loading 28.6%) in ODS
Experiments for studying kinetic parameters of the oxidation of DBT were performed, with the obtained dynamic data listed in Figure 9. For all the samples -ln(CA/CA0) displays a linear increase with the reaction time, and consequently ODS of DBT on these catalysts fi ts the normal fi rst order kinetic equation, while the slope of the lines are the reaction rate constants (min-1), and the reaction rates decreased in the following order: CTAPMO/BMMS (28.6%)> CTA-PMO/SBA-15 (28.6%)>CTA-PMO/Hβ (28.6%)> CTA-PMO.
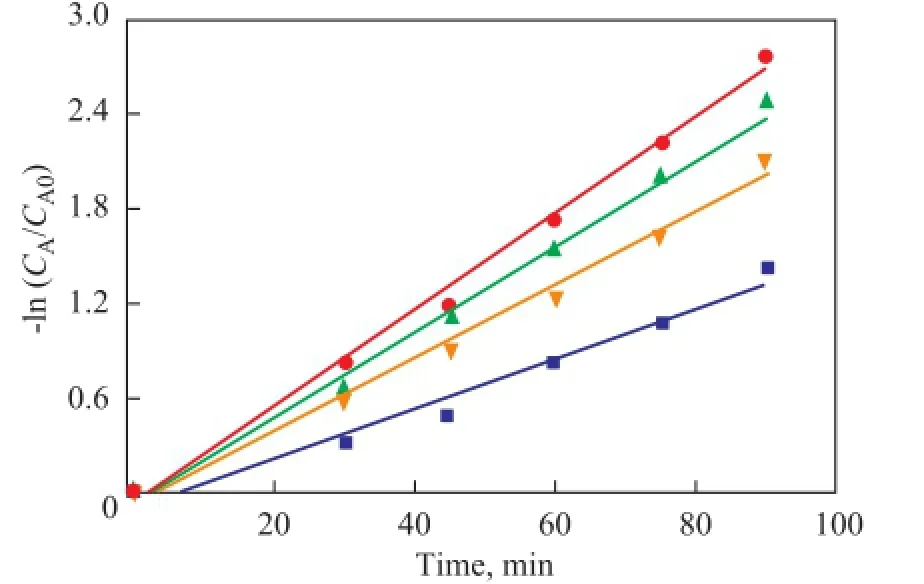
Figure 9 Pseudo- fi rst-order kinetics for ODS of DBT on bulk and supported phase transfer catalysts
3.8 Catalytic stability
Based on the above-mentioned ODS results we can say that CTA-PMO/BMMS has an improved performance in ODS of DBT, however, the stability of the catalyst is also a critical issue. In order to check the stability of the catalyst, CTA-PMO/BMMS was subject to the next turn of ODS reactions just after its simple fi ltration and drying, and the results for testing the reused catalyst sample in Figure 10 showed that no signi fi cant decrease of catalytic activity was found. The hot catalyst fi ltration test was also performed and no conversion of DBT was found after the removal of catalyst, con fi rming no leaching of CTA-PMO during the ODS, because the oxidation happened on the catalyst surface and did not take place in the solution.
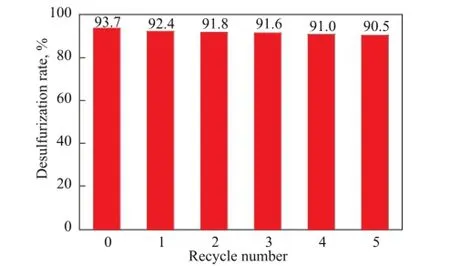
Figure 10 Catalytic stability of the catalyst
3.9 Mechanism of ODS on CTA-PMO/BMMS
Scheme of the ODS on CTA-PMO/BMMS is proposed in Figure 11. Based on the interaction of the Keggin structure with the Si(OH)2(OSi)2group, CTA-PMO is grafted on the pore-wall of BMMS. With respect to CTAPMO, the long alkyl chain of CTA is a hydrophobic nonpolar part, PMO is a hydrophilic polar part and CTAPMO is a kind of surfactant in nature. PMO acts as the catalytic active site and is orderly distributed on the pore wall of BMMS. According to the solubilization principle for surfactants, peroxide can pass through the interval between the barriers formed by the long alkyl chain of CTA thanks to its small molecule size. The active oxygen group can be transferred to PMO, since after obtaining the active oxygen groups PMO is converted to the intermediate PMO [O]. The product H2O can also easily go out through the barrier. The long alkyl chain is cordial with non polar dibenzothiophene (DBT) for the similar miscibility principle so that DBT has more opportunities to approach PMO [O]. The active oxygen is transferred successfully to DBT and then DBT sulfone is formed after oxidation. In this process the large mesopores can afford wide channels for DBT and corresponding products to be transferred in or out of the channels. The small mesopores have high surface area, which is good for the fine dispersion of CTA-PMO, and consequently the original objective to increase the conversion effectiveness of DBT can be achieved in this way.
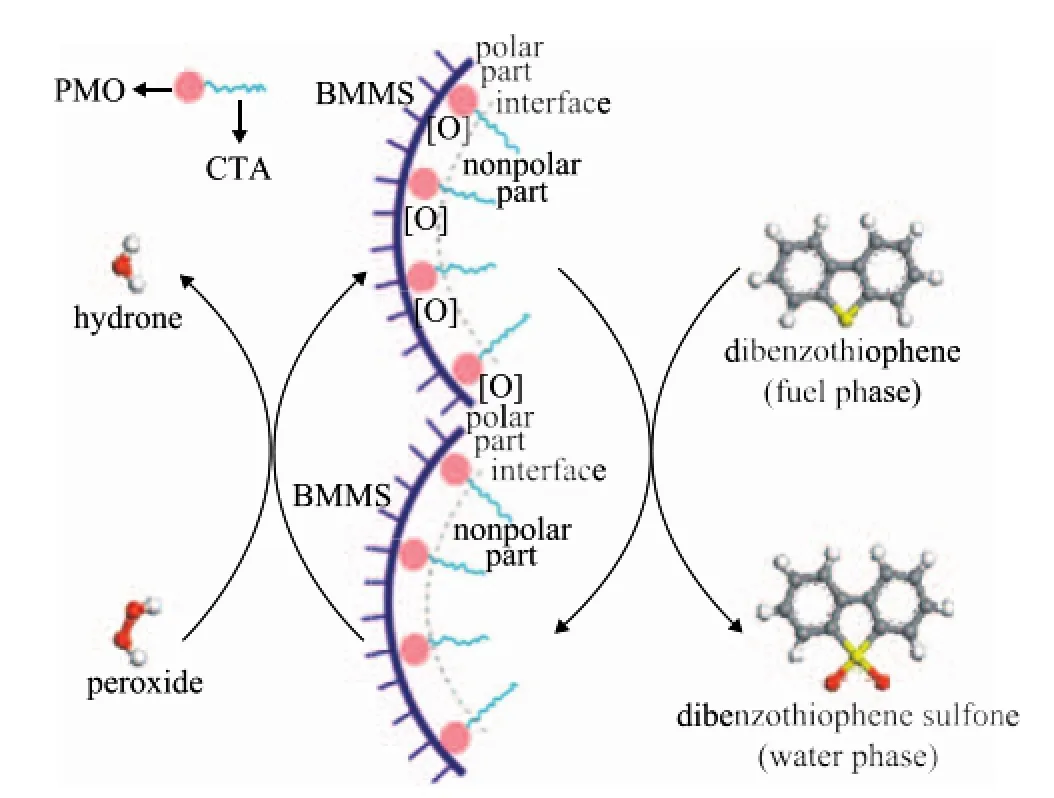
Figure 11 Proposed scheme of the catalytic oxidative desulfurization on CTA-PMO/BMMS
In order to verify the mechanism of oxidative desulfurization on PMO/BMMS (33.3%), FTIR characterization of [O] CTA-PMO/BMMS (33.3%) and [R]CTA-PMO/BMMS (33.3%) was carried out, with the results shown in Figure 12. After oxidation the band at the 873 cm-1for the Mo-Ob-Mo functional group of CTA-PMO/BMMS has a shift to 884 cm-1which can be attributed to the existence of the functional group of Mo-Ob-O-Mo in [O]CTA-PMO/ BMMS. After ODS the band at 1 166 cm-1appears for [R]CTA-PMO/BMMS which is assigned to —SO2—functional group of dibenzothiophene sulfones[27], which are the products of the oxidation of DBT and some of them can be adsorbed on the catalyst before extraction. It can also be found out in Figure 12 that the band at 884 cm-1shifts back to 873 cm-1after the depletion of active oxygen species. Hereby it can be said that the mechanism proposed in Figure 11 is reasonable.
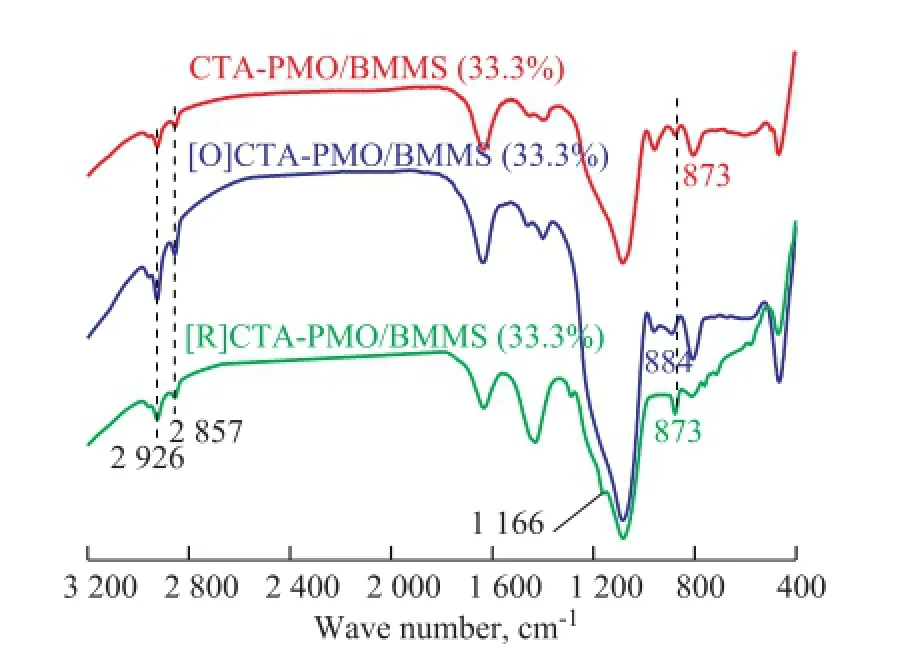
Figure 12 FTIR of the CTA-PMO/BMMS (33.3%) during the process of catalytic oxidation desulfurization
4 Conclusions
In summary, characterization of catalyst samples, including XRD, N2adsorption and desorption, FTIR,31P CP-MAS NMR,29Si CP-MAS NMR and TEM, has proved that the two-step impregnation method can successfully introduce CTA-PMO units into bimodal mesoporous silica channels, while the chemical interaction happens between the Keggin units and the silica group Si(OH)2(OSi)2. A catalytic micro-reactor for ODS of DBT in the mesopores was set up. The large mesopores could provide wide passage for DBT and its products in the reaction system, while at the same time because of the high surface area of the small mesopores the CTA-PMO is fi nely distributed on the surface of pore wall. The ODS of DBT on CTA-PMO/BMMS (28.6%) meets the first order kinetic equation and the reaction rate constant is significantly higher than that for the monomodal porous materials. The desulfurization rate can reach around 94.0%, and a more important advantage is that the catalyst is reusable, while the conversion of DBT on CTA-PMO/BMMS can still reach over 90.0% after 5 reaction cycles.
Acknowledgments: This work was financially supported by the Program for Liaoning Excellent Talents in Universities (LJQ2015062) and the Fushun Science Project (FSKJHT201376).
[1] Babich I V, Moulijn J A. Science and technology of novel processes for deep desulfurization of oil re fi nery streams: A review[J]. Fuel, 2003, 82(6): 607-631
[2] Wang Danhong, Zhang Jianyong, Liu Ni, et al. Hydrothermal synthesis of MoO2and supported MoO2catalysts for oxidative desulfurization of dibenzothiophene[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 19-23
[3] Liu Ni; Zhang Minghui; Wang Danhong. Solvothermal synthesis of V2O3catalysts for oxidative desulfurization of dibenzothiophene[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(3): 26-32
[4] Li Huaming, He Lining, Lu Jidong, et al. Deep oxidative desulfurization of fuels catalyzed by phosphotungstic acid in ionic liquids at room temperature[J]. Energy Fuels, 2009,23(3): 1354-1357
[5] Te M, Fairbridge C, Ring Z. Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2and formic acid/H2O2systems[J]. Appl Catal A, 2001, 219 (1/2): 267-280
[6] Imtiaz A, Waqas A, Muhammad I. Desulfurization of liquid fuels using air-assisted performic acid oxidation and emulsion catalyst[J]. Chin J Catal, 2013, 34 (10): 1839-1847
[7] Huang D, Wang Y J, Yang L M, et a1. Chemical oxidation of dibenzothiophene with a directly combined amphiphilic catalyst for deep desulfurization[J]. Ind Eng Chem Res, 2006, 45(6): 1880-1885
[8] Huang D, Zhai Z, Lu Y C, et al. Optimization of composition of a directly combined catalyst in dibenzothiophene oxidation for deep desulfurization[J]. Ind Eng Chem Res, 2007, 46 (5): 1447-1451
[9] Qiu J H, Wang G H, Zeng D L, et al. Oxidative desulfurization of diesel fuel using amphiphilic quaternary ammonium phosphomolybdate catalysts[J]. Fuel Proc Technol, 2009, 90(12): 1538-1542
[10] Gao J B, Wang S G, Jiang Z X, et al. Deep desulfurization from fuel oil via selective oxidation using an amphiphilic peroxotungsten catalyst assembled in emulsion droplets[J]. J Mol Catal A: Chem, 2006, 258 (1/2): 261-266
[11] Qi W, Wang Y Z, Li W, et al. Surfactant-Encapsulated Polyoxometalates as Immobilized Supramolecular Catalysts for Highly Efficient and Selective Oxidation Reactions[J]. Chem: Eur J, 2010, 16(3): 1068-1078
[12] Zaki E A A, Li Baoshan, Asma Tufail. Direct synthesis of mesoporous (C19H42N)4H3(PW11O39)/SiO2and its catalytic performance in oxidative desulfurization[J] Colloid Surface A, 2009, 341(1/3) 86-92
[13] Yu X D, Xu L L, Yang X, et al. Preparation of periodic mesoporous silica-included divacant Keggin units for the catalytic oxidation of styrene to synthesize styrene oxide[J]. Appl Surf Sci, 2008, 254 (15): 4444-4451
[14] Bigi F, Corradini A, Quarantelli C, et al. Silica-bound decatungstates as heterogeneous catalysts for H2O2activation in selective sul fi de oxidation [J]. J Catal, 2007, 250 (2): 222-230
[15] Zheng H W, Sun Z, Chen X L, et al. A micro reactioncontrolled phase-transfer catalyst for oxidative desulfurization based on polyoxometalate modified silica[J]. Appl Catal A: Gen, 2013, 467: 26-32
[16] Zhou T, Li H, Liu G Y, et al. Syntheses of PAM Microgel Surfacely Covered with Alkyl Quaternary Ammonium Surfactant/Keggin-Type Polyoxometalate Complexes[J]. J Appl Polym Sci, 2009, 114(6):4000-4010
[17] Zhang Y, Masahiko K, Noritatsu T. Preparation of alumina-silica bimodal pore catalysts for Fischer-Tropsch synthesis[J]. Catal Lett, 2005, 99(3): 193-198
[18] Sato S, Ryoji T, Toshiaki S, et al. Bimodal porous Pd-silica for liquid-phase hydrogenation[J]. Appl Catal A: Gen, 2005,284(1/2): 247-251
[19] Zhao D Y, Huo Q, Feng J, et al. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures[J]. J Am Chem Soc, 1998, 120 (24) : 6024-6036
[20] Sheldon R A, Wallau M, Arends I W C E, et al. Heterogeneous Catalysts for Liquid-Phase Oxidations: Philosophers' Stones or Trojan Horses?[J]. Acc Chem Res, 1998, 31(8): 485-493
[21] Seaton N A. Determination of the connectivity of porous solids from nitrogen sorption measurements[J]. Chem Eng Sci, 1991 (8): 1895-1909
[22] Li M, Zhang M, Wei A, et al. Facile synthesis of amphiphilic polyoxometalate-based ionic liquid supported silica induced efficient performance in oxidative desulfurization[J]. J Mol Catal A: Chem, 2015, 406: 23-30
[23] Yang Y, Guo Y H, Hu C W, et al. Lacunary Keggin-type polyoxometalates-based macroporous composite films: preparation and photocatalytic activity[J]. Appl Catal A: Gen, 2003, 252 (2): 305-314
[24] Zhao R Y, Xu Y Q, Yin C L, et al. Effects of silica source on structure and properties of mesoporous molecular sieve[J]. J Chin Univ Petro, 2008, 32(6):142-146
[25] Sindorf D W, Maciel G E. Silicon-29 CP/MAS NMR studies of methylchlorosilane reactions on silica gel[J]. J Am Chem Soc, 1981,103 (14): 4263-4265
[26] Yasmin T, Müller K. Synthesis and characterization of surface modified SBA-15 silica materials and their application in chromatography[J]. J Chromatogr, 2011, 1218 (37): 6464-6475
[27] Vacque V, Sombret B, Huvenne J P, et al. Characterisation of the O-O peroxide bond by vibrational spectroscopy[J]. Spectrochim Acta A, 1997, 53 (1): 55-66
Received date: 2016-01-08; Accepted date: 2016-03-23.
Ma Bo, E-mail: bomasx@163.com.
- 中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
- Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease

