Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
(School of Chemistry and Chemical Engineering, Huangshan University, Huangshan 245041)
Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
Wang Yonglei; Li Haiyun; Fang Hongxia; Xu Tao; Lu Lulu
(School of Chemistry and Chemical Engineering, Huangshan University, Huangshan 245041)
This article describes a method for synthesizing an excellent oil soluble calcium isostearate detergent using isostearic acid and calcium hydroxide as main starting materials. Reaction conditions, including the molar ratios of calcium hydroxide to isostearic acid, methanol to calcium hydroxide and water to calcium hydroxide, the carbonation temperature, the CO2flow rate, and the volume of injected CO2, were optimized. Under the optimized conditions, a high alkalinity calcium isostearate detergent with a total base number (TBN) of 358 mgKOH/g and an overbased calcium isostearate detergent with a value of TBN equating to 406 mgKOH/g could be obtained. Finally, the properties of the calcium isostearate detergent were evaluated by the size distribution analysis, the thermogravimetric analysis (TGA) and the coking tendency tester.
synthesis, environmentally friendly, calcium isostearate, lubricant detergent, excellent oil solubility
1 Introduction
In the course of operation, lubricating oil is an indispensable material in the internal combustion engine. However, internal combustion in engines produces a range of acidic degradation products that can attack and corrode engine parts and catalyze the formation of sludge. At present, adding alkaline lubricant detergents to lubricating oil is the most commonly used method[1-3]. Alkaline lubricant detergent contains excess alkaline substances, which can neutralize those acidic products and prevent the formation of varnish lacquer and other deposits in the engine. The total base number (TBN) and the viscosity of the product are the two primary parameters among all property indicators of lubricant detergents. The degree of alkalinity of the product depends on the amount of CaCO3contained therein, which shows the capability of the product to neutralize acidic compounds.
In order to adapt to the development of environmentally friendly lubricants and meet the requirements of sustainable development[4-6], a series of fatty acids[7-9](such as oleic acid, stearic acid, palmitic acid, etc.) were used as starting materials to synthesize high alkalinity lubricant detergents. However, in the industrial application process, we found that the overbased calcium oleate and calcium stearate detergents were both less compatible with the base oil, and in the course of storage and reuse, they also gave rise to surface skinning and mobility deterioration, which could bring about a great deal of inconvenience for industrial applications of products. Compared to oleic acid and stearic acid, isostearic acid[10-12]as a branched-chain organic acid, has good fl ow property at low temperatures and excellent miscibility with various base oils. Meanwhile, isostearic acid can be produced from renewable materials. In this aspect, the isostearic acid is often used as an environmentally friendly lubricant because of its excellent lubricity and potentially good biodegradability[12-13]. However, at present the lubricant detergent using isostearic acid as the starting material is still uncommon. Consequently, we use isostearic acid to synthesize high alkalinity overbased calcium isostearate detergents with excellent oil solubility. The properties of the calcium isostearate detergent were evaluated by size distribution analysis, thermogravimetric analysis (TGA) and coking tendency tester.
2 Experimental
2.1 Materials
Isostearic acid was of the technically pure grade and provided by the Croda Chemicals Ltd.; diluent oil (trimethylolpropane trioctanoate) was of the technically pure grade and provided by the Liyang Ruipu Chemical Technology Research Center. Calcium hydroxide, xylene and methanol were analytically pure and were provided by the Xilong Chemical Co., Ltd. CO2was of the technically pure grade and was received from the Huangshan Industrial Air Company. All other materials were obtained from commercial sources.
2.2 Analytical methods
The total base number (TBN, mgKOH/g) was determined according to the ASTM method D2896-11 and viscosity (mm2/s, 100℃) was determined according to the ASTM D445 method. The size distribution of colloidal carbonate particles was tested using the Malvern Zetasizer of Nano-ZS90 (Malvern Instruments Ltd, UK), adopting toluene as the dispersant, at a temperature of 25℃ with the sample concentration equating to 0.2%. The TGA 4000 thermal gravimetric analyzer (PerkinElmer Instruments Co., Ltd., China) was used to test the heat resistance of products in the nitrogen atmosphere at a heating rate of 5℃/min. The detergency and oxidation stability of products were measured according to the method SH/T 0300—1992 (150 ℃×320 ℃×6 h) using the C9coke tendency tester (Shanghai Modi Instruments Co., Ltd., China).
2.3 Synthesis of calcium isostearate detergent with excellent oil solubility
Measured quantities of isostearic acid and diluent oil were dissolved in xylene and methanol solution. The calcium hydroxide then was added and stirring was initiated. The reaction mixture was held at 50—55℃ until the neutralization reaction completed. The mixture was then heated to 65℃ and the water was added to the mixture and gaseous CO2was then introduced into the reactor via a gas fl owmeter for conducting the carbonation reaction. Finally, the waste residue was removed by centrifugation and fi ltration, and the solvents (such as xylene, methanol, and water) were evaporated to obtain the calcium isostearate detergent with excellent oil solubility.
The feed for the synthesis of high alkalinity calcium isostearate solution was composed of: 28.4 g of isostearic acid +40 g of diluent oil +66.5 g of calcium hydroxide +8 g of water +23 g of methanol+CO2and xylene. The final product of this synthesis had the following properties, viz.: a TBN of 345 mgKOH/g and a viscosity of 32 mm2/s. The feed for the synthesis of overbased calcium isostearate solution consisted of: 28.4 g of isostearic acid +40 g of diluent oil +111 g of calcium hydroxide +13.5 g of water +38 g of methanol +CO2and xylene. The final product of this synthesis had the following properties, viz.: a TBN of 406 mgKOH/g, and a viscosity of 68 mm2/s.
3 Results and Discussion
3.1 Molar ratio of calcium hydroxide to isostearic acid
In the synthesis process for synthesis of calcium salt lubricant detergents, the calcium hydroxide[2]was most commonly used and its amount had an important effect on the TBN of the product. We investigated the effects of the molar ratio of calcium hydroxide to isostearic acid on both the TBN and viscosity of the calcium isostearate detergent and the results obtained thereby are shown in Figure 1.
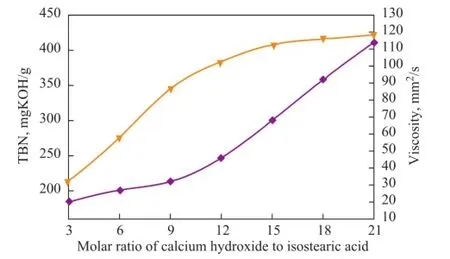
Figure 1 Effects of molar ratio of calcium hydroxide to isostearic acid on both the TBN and viscosity of the calcium isostearate detergent
The results (Figure 1) demonstrated that with an increasing molar ratio of calcium hydroxide to isostearicacid, the TBN of the product increased at first rapidly and then rose slowly, while the viscosity of the product increased continuously. However, when the molar ratio of calcium hydroxide to isostearic acid was beyond 15, the trend of TBN changes was not obvious with a further increasing molar ratio. This phenomenon demonstrated that when the amount of colloidal calcium carbonate was insuf fi cient, it was dif fi cult to obtain a product with high TBN. Then by increasing the amount of calcium hydroxide, the TBN of the product could increase rapidly. When the amount of colloidal calcium carbonate was suf fi cient, upon further increasing the amount of calcium hydroxide, the TBN did not increase obviously and the residue increased rapidly. Meanwhile, when the TBN was close to 400 mgKOH/g,the viscosity of the product increased dramatically, which was consistent with the trend of change in the calcium oleate detergent[14]. An appropriate molar ratio of calcium hydroxide to isostearic acid is essential to produce an alkaline calcium isostearate detergent. Upon considering a satisfactory quality of products and a high utilization rate of calcium hydroxide, a molar ratio of calcium hydroxide to isostearic acid in the range of 9-15 was feasible.
3.2 Molar ratio of methanol to calcium hydroxide
Because of the multiphase reaction process, some promoters[1-2,15]needed to be used to improve the reaction efficiency during the synthesis of lubricant detergent. Among these promoters, methanol often is used as a solubilizing agent for the conversion of calcium hydroxide to colloidal calcium carbonate. The effects of molar ratio of methanol to calcium hydroxide on both the TBN and viscosity of the calcium isostearate detergent are shown in Figure 2.
As shown in Figure 2, when the molar ratio of methanol to calcium hydroxide was increased, the TBN of the product at first increased and then decreased, whereas the viscosity of the product increased gradually. This occurred because[1,15]the amount of methanol would affect the formation and dispersion of colloidal calcium carbonate in the diluent oil. A highest TBN was obtained at a methanol to calcium hydroxide molar ratio of 0.8. Thus, we chose 0.8 as the optimal molar ratio of methanol to calcium hydroxide.
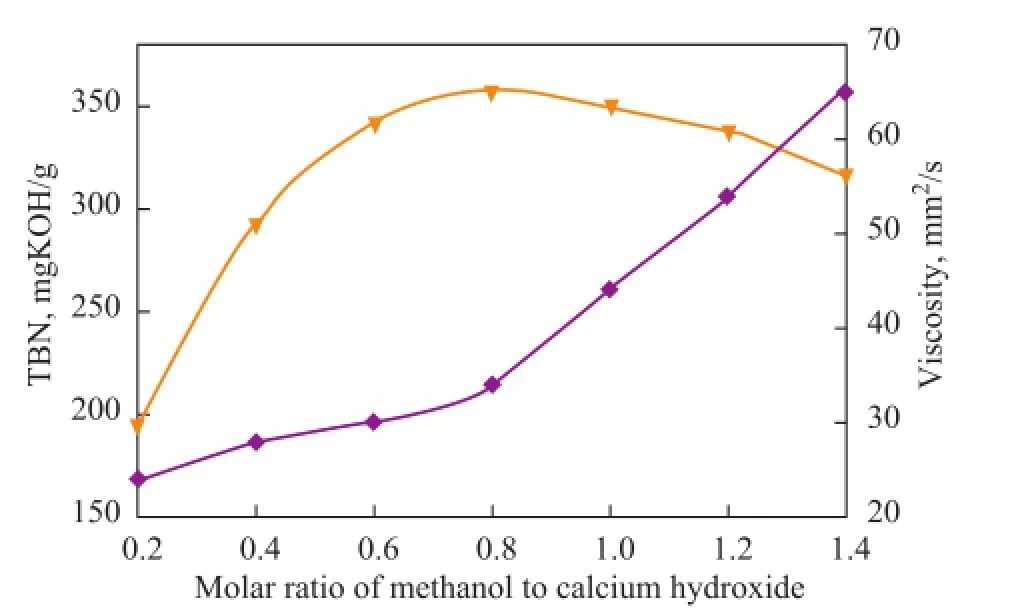
Figure 2 Effects of molar ratio of methanol to calcium hydroxide on both the TBN and viscosity of the calcium isostearate detergent
3.3 Molar ratio of water to calcium hydroxide
During the carbonation reaction, water is necessary[16]. Although a small amount of water is added, it has a very important role in forming and dispersing the colloidal carbonate. The effects of the molar ratio of water to calcium hydroxide on both the TBN and viscosity of the calcium isostearate detergent are shown in Figure 3.
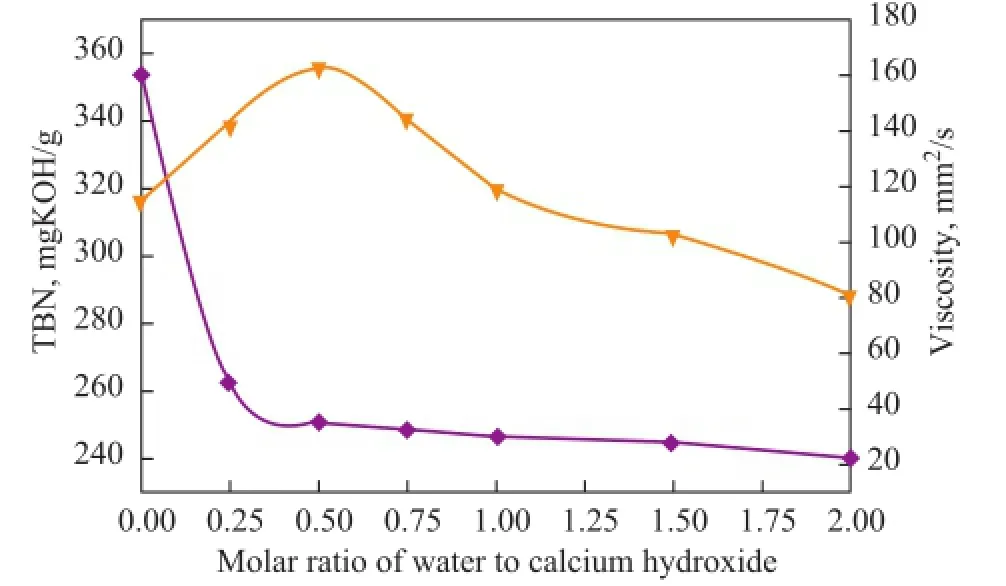
Figure 3 Effects of molar ratio of water to calcium hydroxide on both the TBN and viscosity of calcium isostearate detergent
As shown in Figure 3, when the molar ratio of water to calcium hydroxide was increased, the viscosity of theproduct decreased gradually, whereas the TBN of the product at fi rst increased and then decreased. The absence of water would cause the formation of larger crystalline calcium carbonate particles during carbonation reaction, which could result in a too viscous final product to be readily handled. When a certain quantity of water was added, the TBN of the product increased, and meanwhile the viscosity of the product decreased sharply, which also con fi rmed the important role of water in carbonation reaction. The highest TBN was attained at a molar ratio of water to calcium hydroxide equating to 0.5, thus, this fi gure was the optimal molar ratio.
3.4 Carbonation temperature
The carbonation temperature has an important effect on the quality of the calcium isostearate detergent. The effects of carbonation temperature on the TBN and viscosity of the calcium isostearate detergent are shown in Table 1.

Table 1 Effects of different carbonation temperature on the property of calcium isostearate detergent
As shown in Table 1, with an increasing carbonation temperature, the viscosity of the product increased gradually, whereas the TBN of the product at first increased rapidly and then decreased slightly. When the carbonation temperature was higher than 70 ℃(including 70 ℃), the final product would contain crystalline material, resulting in a poor quality of the product. This phenomenon demonstrated that an appropriate temperature could promote the formation and dispersion of colloidal carbonate. Thus, according to these results, we selected 65 ℃ as the optimal carbonation temperature.
3.5 CO2fl ow rate
Generally, during the synthesis of lubricant detergent, CO2fl ow rate depends on the needs for the formation rate of colloidal carbonate and dispersiong rate of surfactant micellae, etc. For different reaction systems, the optimal CO2flow rate often is different. The effects of the CO2flow rate on both the TBN and viscosity of the calcium isostearate detergent are shown in Figure 4.
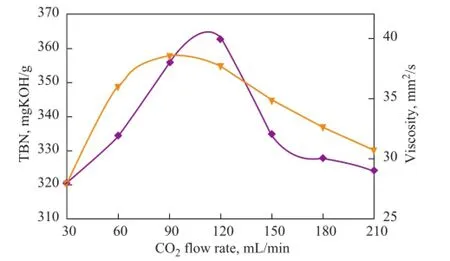
Figure 4 Effects of CO2fl ow rate on both the TBN and viscosity of calcium isostearate detergent
As shown in Figure 4, with an increasing CO2fl ow rate, the TBN and viscosity of the product at first increased and then decreased. It could be found out that a high gas flow rate of CO2could increase the product alkalinity moderately and shorten the time of carbonation. However, a too high CO2fl ow rate could also induced a decreased TBN of the calcium isostearate detergent. Overall, when the CO2fl ow rate was less than 210 mL/min, the effects of the CO2fl ow rate on both TBN and viscosity of calcium isostearate detergent were not obvious. The TBN of the product attained a highest value at a CO2fl ow rate of 90 mL/min. Therefore, a value of 90 mL/min was chosen as the optimal CO2fl ow rate.
3.6 Volume of injected CO2
When CO2is used as a carbonation material, its amountshould meet the needs for conducting the reaction with calcium hydroxide used. In the carbonation experiments, the volume of injected CO2was calculated by gas fl owmeter with its unit expressed in milliliters. The effects of the volume of injected CO2on the TBN and viscosity of calcium isostearate detergent are shown in Figure 5.

Figure 5 Effects of volume of injected CO2on both the TBN and viscosity of calcium isostearate detergent
The results (Figure 5) demonstrated that with an increasing volume of injected CO2, the TBN of the product at fi rst increased and then decreased, whereas the viscosity of the product increased gradually. When the amount of CO2was insufficient, the calcium hydroxide could not be converted completely to colloidal calcium carbonate, which would lead to a low TBN of the product along with a high residue ratio. Contrarily, an excessive CO2would give rise to overcarbonation reaction products, which could induce a decreased TBN of the product and a deteriorated product quality. To achieve a satisfactory quality of the product and a high utilization rate of raw materials in our experiments, an injected CO2volume of 5 400 mL is feasible.
3.7 Storage stability of calcium isostearate detergent
The storage stability of calcium isostearate detergent was investigated. Results are shown in Table 2.
As shown in Table 2, the storage stability of calcium isostearate detergent was significantly better than that of the calcium oleate detergent and calcium stearate detergent. As regards the high alkalinity and overbased calcium oleate (or calcium stearate) detergents, after60 days of storage the surface skinning and viscosity increase of the product all occurred, demonstrating that the mobility of the product after storage showed a trend of deterioration. Compared to the high alkalinity calcium oleate (or calcium stearate) detergents, the overbased calcium oleate (or calcium stearate) detergents contained more colloidal calcium carbonate, hence their viscosity increase was more than that of the high alkalinity calcium oleate (or calcium stearate) detergents after storage. However, with respect to the high alkalinity and overbased calcium isostearate detergents, they displayed almost no surface skinning phenomenon with their viscosity increasing less after 60 days of storage, which was mainly attributed to its excellent oil solubility.
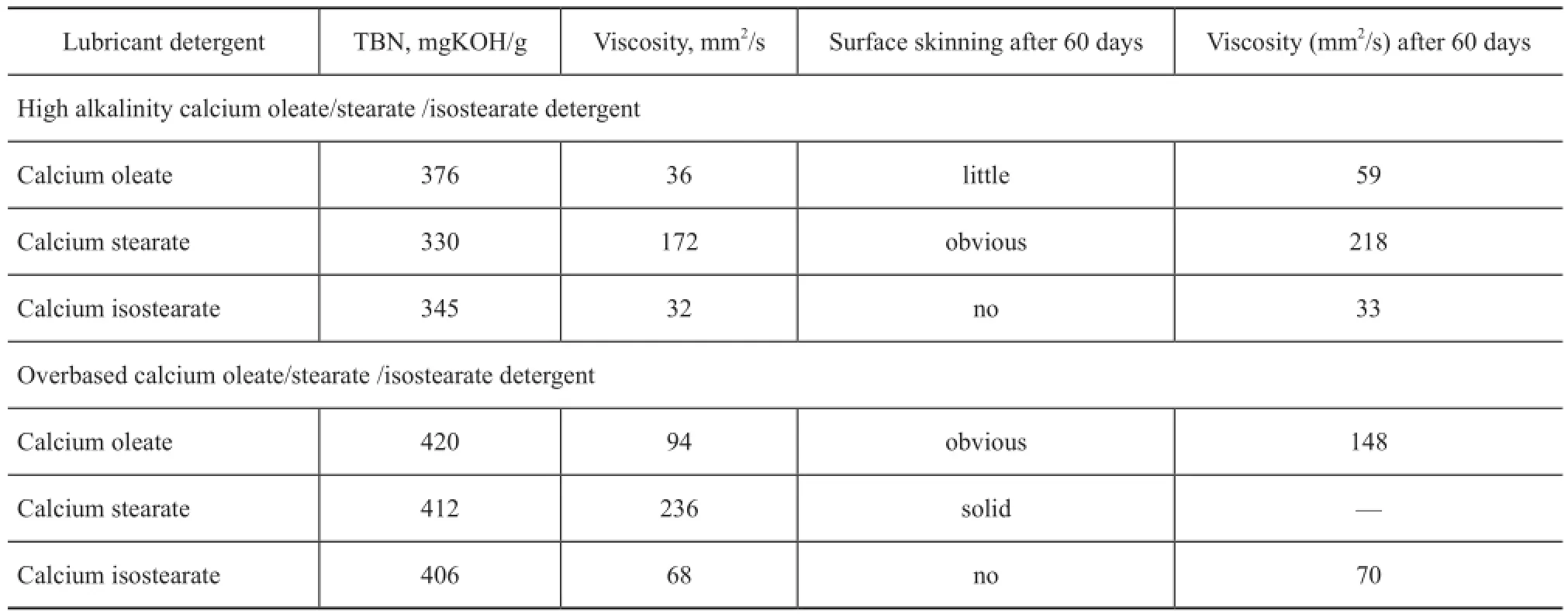
Table 2 Storage stability of calcium oleate/stearate /isostearate detergents
3.8 Size distribution analysis
For comparing the properties between the high alkalinity calcium isostearate detergent, the overbased calcium isostearate detergents, and a commercially available overbased calcium sulfonate, the size distribution of the colloidal carbonate particles in these detergent samples are shown in Table 3.

Table 3 Size distribution of the calcium isostearate detergent
As shown in Table 3, in comparison with the overbased calcium isostearate detergent, the size of colloidal carbonate particles in the high alkalinity calcium isostearate detergent was smaller. This is because in comparison with the high alkalinity calcium isostearate detergent, the overbased calcium isostearate detergent contained more colloidal calcium carbonate, and hence more colloidal calcium carbonate was prone to aggregate into large particles. Overall, compared to the commercially available overbased calcium sulfonate, the size of colloidal carbonate particles of the calcium isostearate detergent was relatively small and the size distribution was relatively uniform. This phenomenon also demonstrated that these parameters of the calcium isostearate detergent could fulfill the commercial requirements for lubricant detergent. Thus, the isostearic acid was feasible to be adopted as raw material for synthesizing the calcium salt lubricant detergent.
3.9 Thermal stability analysis
To examine the thermal stability of the calcium isostearate detergent,the overbased calcium isostearate detergent (with its TBN equating to 406 mgKOH/g) was used as an example for TGA measurements (Figure 6). It was observed that the initial weight loss temperature of the overbased calcium isostearate detergent was 127 ℃, and then the weight loss increased gradually with an increasing temperature. When the temperature reached 308 ℃, the weight loss of the calcium isostearate detergent was less than 20%. Meanwhile, the weight loss reached 50% when the temperature was 461 ℃. Thus, it can be concluded that the calcium isostearate detergent has an excellent thermal stability.
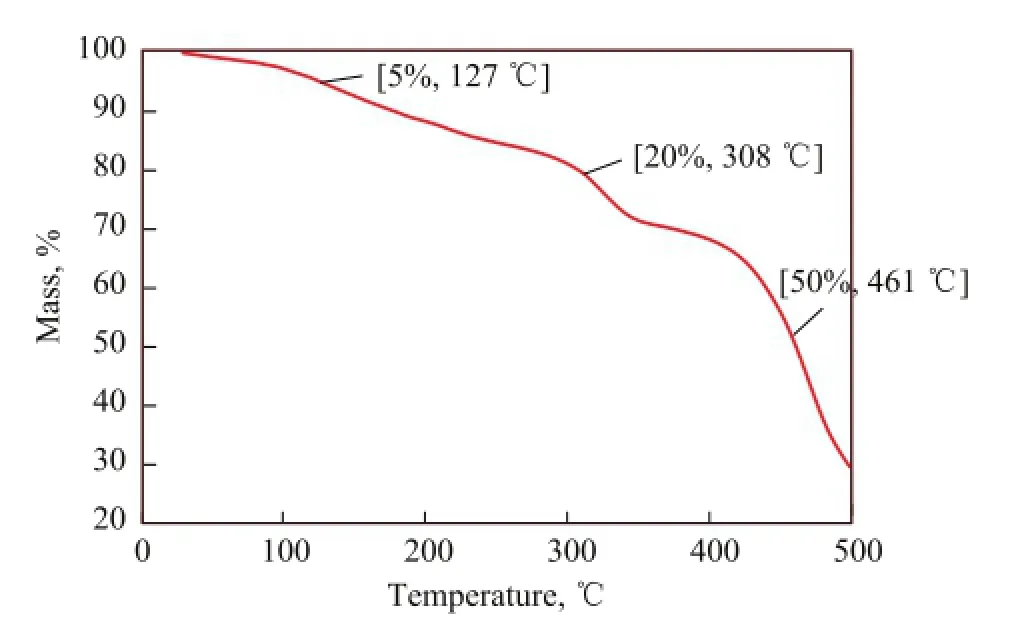
Figure 6 TGA of the overbased calcium isostearate detergent
3.10 Detergence and oxidation stability
The detergency and oxidation stability of the high alkalinity calcium isostearate detergent and the overbased calcium isostearate detergent were investigated according to the method SH/T 0300—1992 with the results depicted in Table 4.
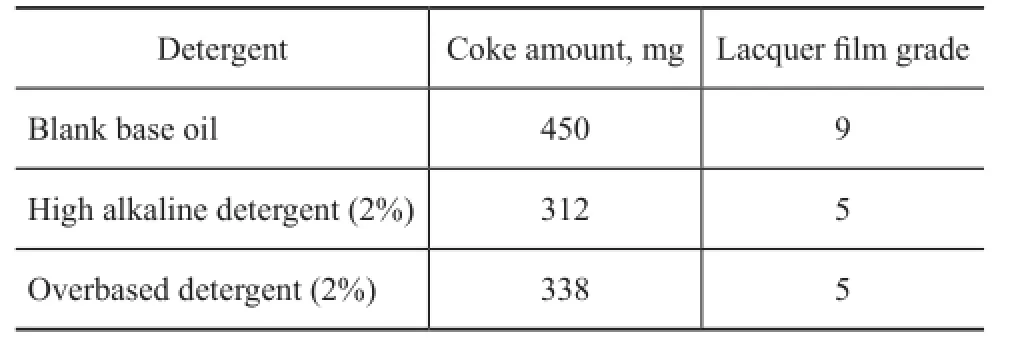
Table 4 Detergency and oxidation stability of the calcium isostearate detergent
As shown in Table 4, in comparison with the blank base oil, the coke amount and lacquer film grade of the oil samples added with 2% of the high alkalinity isostearate detergent (or 2% of overbased calcium isostearate detergent) all decreased, which demonstrated that the calcium isostearate detergent could improve the high temperature detergency and oxidation stability of the baseoil greatly.
4 Conclusions
A high alkalinity calcium isostearate detergent and an overbased calcium isostearate detergent were synthesized using isostearic acid as the starting material. Upon using the isostearic acid feed for the synthesis of high alkalinity calcium isostearate solution under optimized conditions, a calcium isostearate detergent with a TBN of 345 mgKOH/g was obtained. Similarly, an overbased calcium isostearate detergent with a TBN of 406 mgKOH/g could be obtained using the same feed for the synthesis of overbased calcium isostearate solution. The storage tests showed that the storage stability of the calcium isostearate detergent was better than that of the calcium oleate detergent and calcium stearate detergent. The TGA and coking tendency analysis showed the excellent thermal stability and high temperature detergency of calcium isostearate detergent. Thus, this method for synthesizing an environmentally friendly calcium isostearate detergent was feasible and it shows great potential for commercialization.
Acknowledgements: This work was supported by the Anhui Province Students’ Innovative Training Program (No.201510375015) and the Scienti fi c Research Foundation of Huangshan University (No. 2015xkjq002). We also appreciate the Analysis and Testing Center of Huangshan University for their technical and instrumental support.
[1] Hudson L K, Eastoe J, Dowding P J. Nanotechnology in action: overbased nanodetergents as lubricant oil additives[J]. Adv Colloid Interface Sci, 2006, 123-126(21): 425-431
[2] Besüergil B, Akin A, Celik S. Determination of synthesis conditions of medium, high, and overbased alkali calcium sulfonate[J]. Ind Eng Chem Res,2007, 46(7): 1867-1873
[3] Greenall A, Neville A, Morina A, et al. Investigation of the interactions between a novel, organic anti-wear additive, ZDDP and overbased calcium sulphonate[J], Tribo Int, 2012, 46(1): 52-61
[4] Erhan, S Z, Asadauskas S. Lubricant basestocks from vegetable oils[J]. Ind Crop Prod, 2000, 11(2): 277-282
[5] Cermak S C, Isbell T A. Physical properties of saturated estolides and their 2-ethylhexyl esters[J]. Ind Crop Prod,2002, 16(2): 119-127
[6] Agamuthu P, Abioye O P, Aziz A A. Phytoremediation of soil contaminated with used lubricating oil using Jatropha Curcas[J]. J Hazard Mater, 2010, 179(1/3): 891-894
[7] Wang Y, Eli W, Zhang L, et al. Synthesis of environmentally friendly composite-metal (calcium and magnesium) oleate detergent[J]. Ind Eng Chem Res, 2011, 50(3): 1530-1535
[8] Mohammed A, Ahmad M R, Al-Messri Z. Synthesis, characterization and evaluation of overbased magnesium fatty acids detergent for medium lubricating oil[J]. Iraqi Journal of Chemical and Petroleum Engineering, 2013, 14(3): 1-9
[9] Wang Y, Li H, Fang H, et al. Synthesis of environmentally friendly magnesium linoleate detergent[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 96-100
[10] Ngo H L, Dunn R O, Brajendra S, et al. Synthesis and physical properties of isostearic acids and their esters[J]. Eur J Lipid Sci Technol, 2011, 113(2): 180-188
[11] Ngo H L, Hoh E, Foglia T A. Improved synthesis and characterization of saturated branched-chain fatty acid isomers[J]. Eur J Lipid Sci Technol, 2012, 114(2): 213-221
[12] Ngo H L, Dunn R O, Hoh E. C18-unsaturated branchedchain fatty acid isomers: Characterization and physical properties[J]. Eur J Lipid Sci Technol, 2013, 115(6): 676-683
[13] Ngo H L, Yee W C, McAloon A J, et al. Process and cost modeling of saturated branched-chain fatty acid isomer production[J]. Ind Eng Chem Res, 2012, 51(37): 12041-12045
[14] Wang Y L, Eli W, Liu Y, et al. Synthesis of environmentally friendly calcium oleate detergent[J]. Ind Eng Chem Res, 2008, 47(22): 8561-8565
[15] Galsworthy J, Hammond S, Hone D. Oil-soluble colloidal additives[J]. Curr Opin Colloid Interface Sci, 2000, 5(5/6): 274-279
[16] Tavacoli J W, Dowding P J, Steytler D C, et al. Effect of water on overbased sulfonate engine oil additives[J]. Langmuir, 2008, 24 (8): 3807-3813
Received date: 2016-01-28; Accepted date: 2016-04-10.
Li Haiyun, Telephone: +86-559-2546612; E-mail: lhy@hsu.edu.cn.
- 中国炼油与石油化工的其它文章
- Study on Preparation and Properties of Grease Based on Ultra fi ne Bentonite Powder
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease
- Preparation and Tribological Behavior of Hydrophobic Lanthanum Borate Nanosheets in Rapeseed Oil
- Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor

