Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
Li Jin; Hu Zexiang; Chen Guoxu; Tao Zhiping; Zhao Jie
(1. Logistic Engineering University of PLA, Department of Oil Application and Management Engineering, Chongqing 401311; 2. Academy of Naval Logistic Technology and Equipment, Beijing 100072; 3. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
Li Jin1; Hu Zexiang2; Chen Guoxu1; Tao Zhiping3; Zhao Jie3
(1. Logistic Engineering University of PLA, Department of Oil Application and Management Engineering, Chongqing 401311; 2. Academy of Naval Logistic Technology and Equipment, Beijing 100072; 3. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Since the 1990s, the kerosene fuel (code: JP-8) had been applied in the ground equipment provided with direct injection compression ignition engines in the U.S. Army, resulting in increased occurrence of injection pump failures. Anti-wear additives must be used in the single fuel due to its poor lubricity. In the present work, lubricity improvers were selected on the basis of molecular simulation theoretically and these agents were evaluated to improve the lubricity of jet fuel using the high frequency reciprocating rig (HFRR) apparatus and the ball-on-cylinder lubricity evaluator (BOCLE). It was revealed that dimer acid with higher value of adsorption energy on the Fe (110) plane surface had more ef fi cient lubricity promoting properties than that of naphthenic acid. The experimental results suggested that the dimer acid had a better tribological behavior compared with that of naphthenic acid used as lubricity improver of jet fuel. And addition of anti-wear additives at a dosage of 15 μg/g was able to promote the lubricity of jet fuel to a required level on BOCLE, while a higher concentration over 80 μg/g was needed to improve the lubricity to a demanded value of diesel on HFRR.
jet fuel; diesel; lubricity; dimer acid; naphthenic acid; molecular simulation
1 Introduction
Most components within the fuel-injection system rely on the fuel for effective lubrication and wear resistance. Mandated use of low-sulfur fuel including the low-sulfur diesel and aviation fuels has to adopt hydro-treating at the refineries for removing sulfur compounds, which would also result in the removal of trace amounts of polar compounds necessary for effective lubrication and wear prevention[1]. After the World War II, the U.S. Department of Defense was moving toward the use of a single fuel in ground military aircraft, vehicles and equipment employed in the European battlefield[2-3]. Nonetheless, recurrent problems had been reported with the Stanadyne rotary fuel injection pump, such as mild scuffing, oxidative corrosion and wear failure[4], and especially in the 1990—1991 Operation “Desert Shield/ Storm” in Saudi Arabia a sharp rise of the occurrence of injection pump failures was experienced[5]. In order to improve the lubricity characteristics of diesel or kerosene fuel, a number of anti-wear additives quali fi ed under the MIL-I-25017 standard were evaluated by U.S. Army[6].
These lubricity enhancers were commonly composed of dimeric organic acid, usually dilinoleic acid, as well as solvents and other additives[7-8].
In recent years the molecular simulation was effectively applied to the research on the adsorption behavior of antiwear agents at the microscopic molecular level and the boundary lubrication nature of the thin protective metalmetal contact fi lm provided by lubricity additives[9-10]. The molecular flexibility calculated with the Kier flexibility calculation method could be used as the characteristic parameter to quantitatively assess the influence of alkyl chain of friction modifier on its friction-reducing and anti-wear ability[11]. The density functional theory (DFT) and molecular dynamics (MD) method were used to con fi rm that the lubricity enhancing properties of diesel lubricity improvers were mainly determined by the cohesive energy of adsorbed fi lms on the iron surface[12]. There were a few papers which reported that molecularsimulation was applied to the friction-reducing and antiwear properties of oil lubricants and anti-wear additives of diesel[13-18], but few domestic reports were related with the lubricity improver of jet fuel .
The objective of this study attempts not only to simulate the adsorption of molecules of lubricity improver in jet fuel on metal surface theoretically, but also to evaluate the lubricity of jet fuel with the addition of lubricity enhancer by HFRR and BOCLE.
2 Experimental
According to the ISO 12156-1-2006 standard and the ASTM D5001 method, all tribological measurements were carried out by using the high frequency reciprocating rig (HFRR) and the ball-on-cylinder lubricity evaluator (BOCLE), with their test conditions presented in Table 1. The wear scars were checked using the photo microscope, and the results of lubricating property were referred to the corrected wear scar (WS1.4) values and wear scar diameters (WSD). The jet fuel was provided by the Maoming Petrochemical Company with its major properties listed in Table 2.
A speci fi ed amount of oleic acid and water were added into the reactor, followed by introduction of activated clay and chemical catalyst to enter into reaction for several hours. After being treated by several laboratory chemical processes, the product was fi nally puri fi ed toobtain the synthesized dimer acid, which was veri fi ed to contain more than 95% of dipolymer. Naphthenic acid additive provided by the SINOPEC Corporation was qualified to meet the industry product standard specification SH/T 0766—2005. Both naphthenic acid and dimer acid were dissolved in the base fuel at different concentration levels.
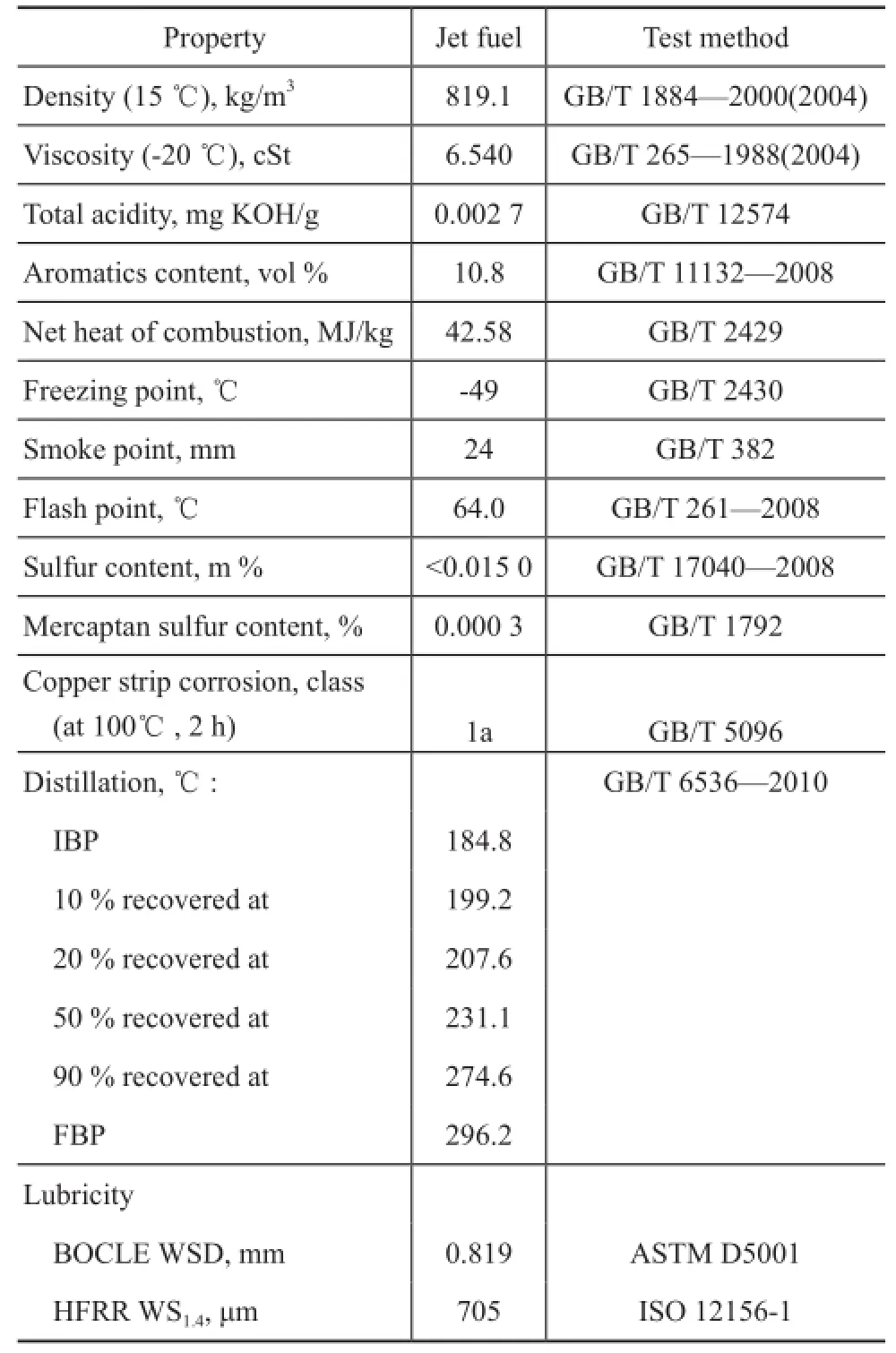
Table 2 Properties of jet fuel
3 Results and Discussion
3.1 Molecular simulation
In this paper, the Forcite model in the software of Materials Studio 6.1 was used in molecular simulation. Both naphthenic acid and dimer acid were optimized with its parameters determined after 500 iterative calculations. The optimized model is shown in Figure 1, in which (a) stands for the molecular structure of naphthenic acid with a molecular weight of 226, and (b) represents the molecular structure of hydrogenateddimer acid with its molecular weight equating to 565.
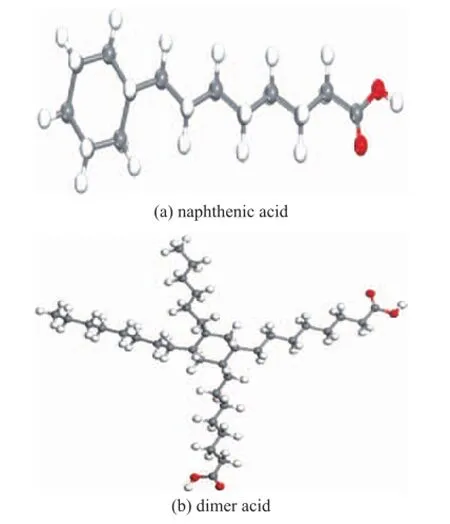
Figure 1 Optimized model of lubricity improvers
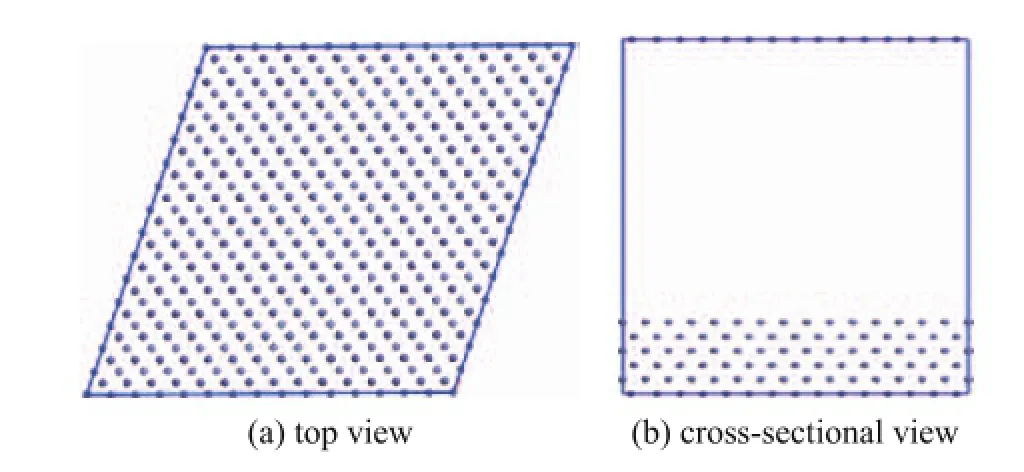
Figure 2 Optimized model of the surface of Fe (110) plane
The friction surface of Fe (110) plane was established by simulating the friction materials of tested ball, with its volume consisting of 3.724 nm×3.724 nm×5.134 nm (as shown in Figure 2)[19].
The calculation formulas of molecular adsorption and its fl exibility[20]were as follows:
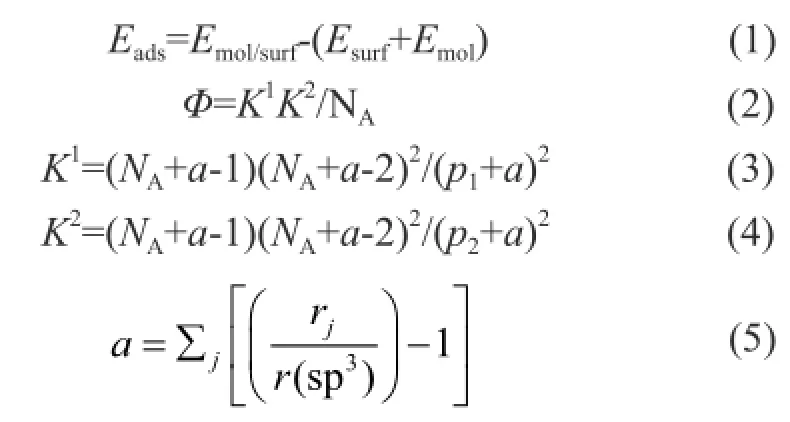
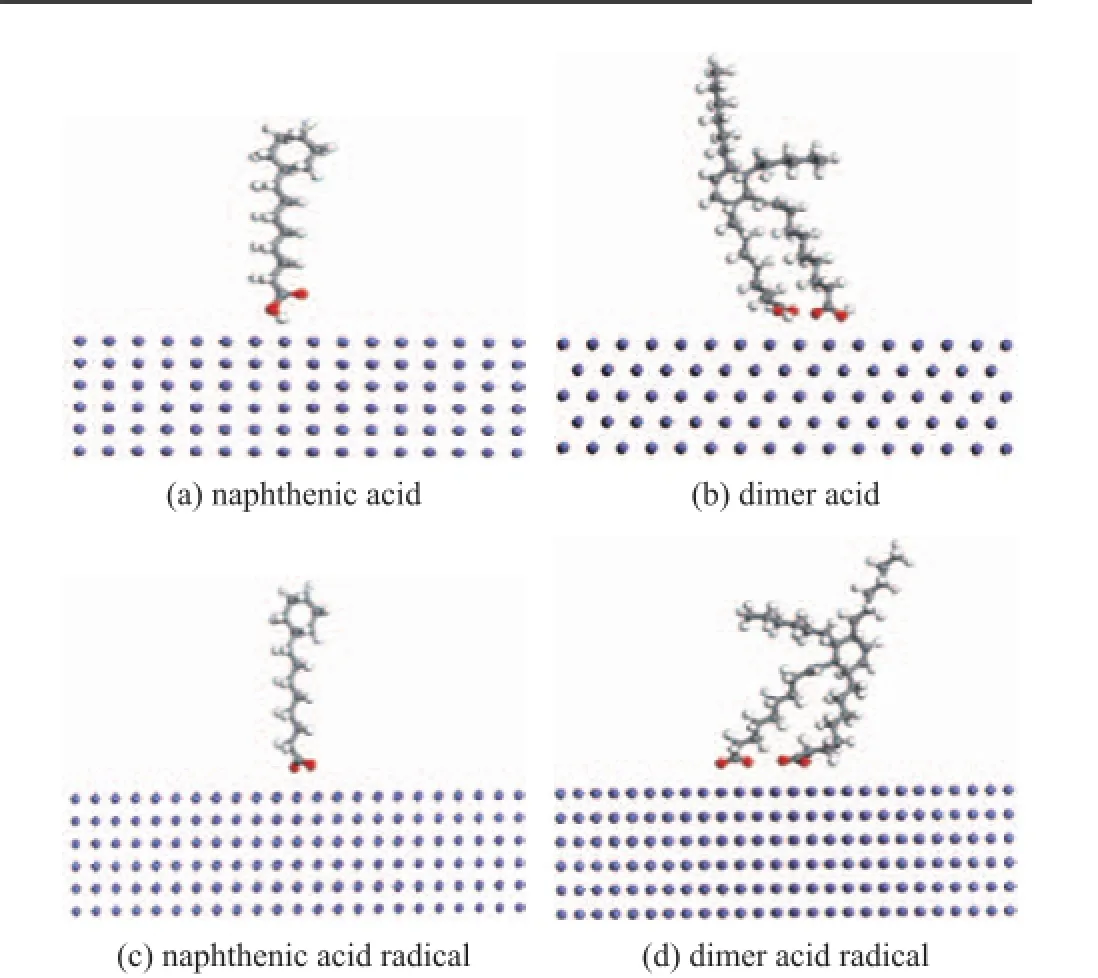
Figure 3 Optimized adsorption model of anti-wear agents on the friction surface
where:Eadsrepresents adsorption energy, andEmol/surfstands for total energy.Esurfrepresents the surface energy, andEmolis the molecular energy.Φis the flexibility parameter, andNAis the total number of atoms. BothK1andK2represent the derived index of molecular size, andr(sp3) represents the covalent radius of sp3bonding carbon. Thep1is the number of carbon-carbon single bonds, andp2stands for the amount of two continuous C—C single bonds. Therjrepresents the covalent radius ofjatom, andais the derived index of covalent atom.
Based on the above model, the adsorption energy of lubricants and the molecular flexibility were obtained from the calculation of Material Studio 6.1. When the adsorption energy value is negative, it is an exothermic process. To some extent, the value is greater and the adsorption process is more stable. According to adsorption energy of lubricity improver and its corresponding acid radical as well as its molecular flexibility provided in Table 3, it is evident that the dimer acid has a better lubricity performance than naphthenic acid in theory.
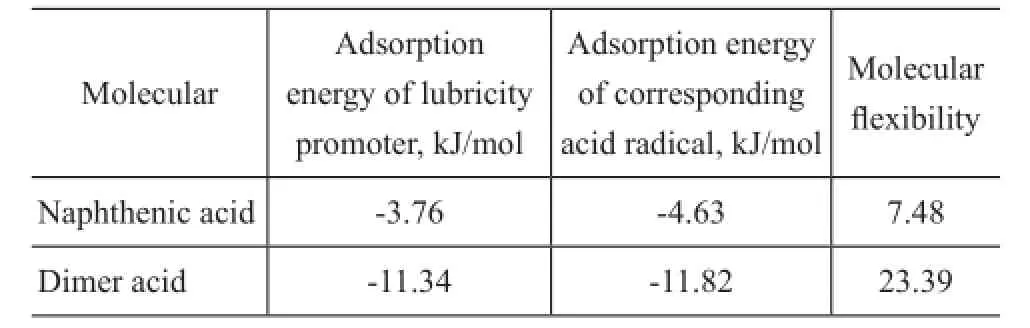
Table 3 The molecular adsorption energy and molecular flexibility of lubricity improver
3.2 Tribological behavior
The base fuel was tested to determine its lubricityperformance repeatedly for confirming the results. The lubricity performance of WS1.4and WSD is given in Table 2. It was obvious that the base fuel had poor lubricating properties.
The impact of anti-wear agents on the lubrication properties of the base fuel on HFRR is outlined in Figure 4. Lower concentration of anti-wear agents ranging from 5 μg/g to 20 μg/g practically contributes no changes on the WS1.4of base fuel. Increasing the concentration from 40 μg/g to 80 μg/g can effectively improve the lubricity of the base fuel. When the concentration of anti-wear agent reaches 80 μg/g, the WS1.4value limit of 460 μm is easily satis fi ed to meet the requirements for commercial diesel fuels. In comparison with the naphthenic acid, the dimer acid showed a relatively good lubricating performance which was consistent with the calculated data listed in Table 3. The reason could be attributed to the two-carboxyl structure of dimer acid.
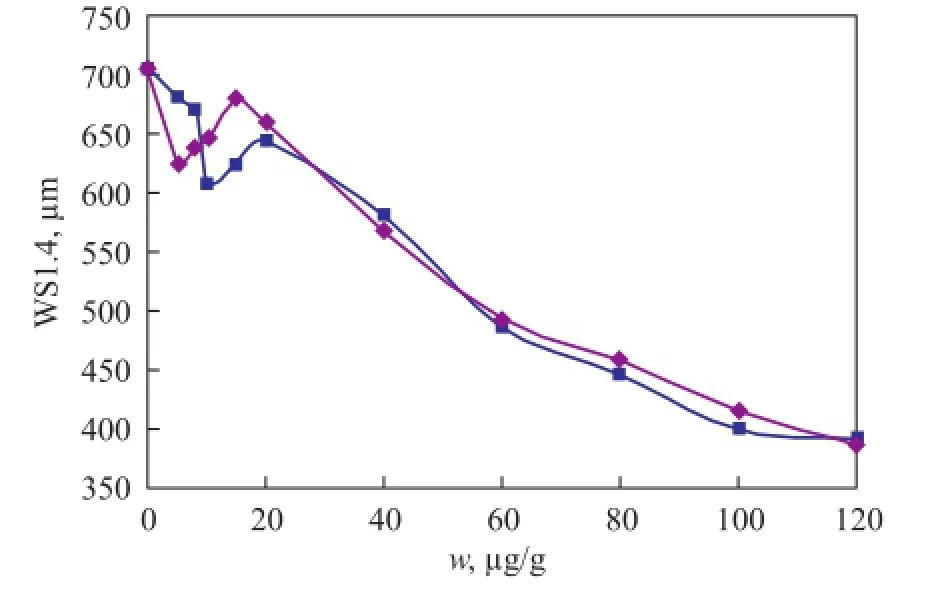
Figure 4 Impact of lubricity improver addition on the lubrication properties of jet fuel on HFRR
Diversified curves showing the influence of lubricity promoters addition on wear scar diameter on BOCLE are given in Figure 5. The anti-wear additives showed great effect on improving the lubricity of jet fuel, and the WSD value limit of 0.65 mm could be satis fi ed when the additive concentration exceeded 15 μg/g. At an additive concentration of less than 20 μg/g, the lubricating property of dimer acid showed almost the same tribological behaviors as naphthenic acid. It is evident that the dimer acid has a better lubricity at concentration ranging from 20 μg/g to 80 μg/g, which complies with the calculated molecular adsorption energy listed in Table 3.

Figure 5 Impact of lubricity improver addition on the lubrication properties of jet fuel on BOCLE
It is verified that the addition of both naphthenic acid and dimer acid at concentrations exceeding 80 μg/g is necessary to keep the WS1.4value within the required limit of diesel fuel. In the BOCLE tests, both dimer acid and naphthenic acid at concentrations above 15 μg/g are apparently effective in promoting lubricity to the WSD value within the demanded limit of jet fuel. The lubrication experimental result con fi rming that dimer acid has a better tribological property than naphthenic acid is consistent with the outcome of molecular simulation theoretically. In conclusion, the evaluated lubricity improvers have a beneficial impact on the anti-wear properties of the base fuel at the proper concentration recommended.
4 Conclusions
The effect of synthesized dimer acid and naphthenic acids added to the fuel at different concentration levels on the tribological properties of jet fuel was evaluated on HFRR and BOCLE. The following conclusions could be drawn from this paper:
1) According to both the WS1.4and WSD values of base fuel, the jet fuel without the lubricity promoter could not satisfy the lubrication needs of diesel fuel.
2) Tests of fuel samples on HFRR and BOCLE have revealed that the dimer acid serving as the lubricity improver of jet fuel has a better lubricating performance as compared to naphthenic acid, which could be attributed to their different adsorption energy on the iron surface as calculated by molecular simulation.
3) The concentration of anti-wear agents should beabove 80 μg/g to meet the lubricity requirements of diesel fuel in HFRR test, while the lubricity of jet fuel could be satis fi ed at an additive concentration of 15 μg/g determined during the BOCLE tests.
[1] Wei D, Spikes H A. The lubricity of diesel fuels[J]. Wear, 1986, 111(2): 217-235
[2] Lacey P I. Wear analysis of diesel engine fuel injection pumps from military ground equipment fueled with Jet A-1[R]. Virginia. The Defense Technical Information Center of USA, AD-A239022, 1991
[3] Lacey P I. The relationship between fuel lubricity and diesel injection system wear[R]. Virginia: The Defense Technical Information Center of USA. AD-A247927, 1992, 1
[4] Lacey P I, Lestz S J. Failure analysis of fuel injection pumps from generation sets fueled with Jet A-1[R]. Virginia. The Defense Technical Information Center of USA, ADA234930, 1991, 1
[5] P.I. Lacey. S J Lestz. Fuel lubricity equipment for diesel injection systems[R]. Virginia. The Defense Technical Information Center of USA, AD-A235972, 1991, 2
[6] Lacey P I. Fuel lubricity additive evaluation[R]. Virginia. The Defense Technical Information Center of USA, ADA326098, 1995
[7] Black B H, Wechter M A, Hardy D R. Determination of corrosion inhibitor or lubricity enhancer additives in jet fuels by size-exclusion chromatography[J]. Journal of Chromatography, 1988, 437(1): 203-210
[8] Black B H. Determination and use of isothermal adsorption constants of jet fuel lubricity enhancer additives[J]. Ind Eng Chem Res, 1989, 28(5): 618-622
[9] Hsu S M, Gates R S. Boundary lubricating fi lms: formation and lubrication mechanism[J]. Tribol Int, 2005, 38(3): 305-312
[10] Awad M K, Mustafa M R, Abo Elnga M M. Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface[J]. J Mol Struct (THEOCHEM), 2010, 959(1/3): 66-74
[11] Liu Qiong, Long Jun, Wu Zhiqiang, et al. Effect of alkyl chain characteristics of friction modifier on frictionreducing[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2014, 30(2): 189-193
[12] Luo Hui, Fan Weiyu, Li Yang. Effects of fatty acids on low-sulfur diesel lubricity: Experimental investigation, DFT calculation and MD simulation[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(2): 74-81
[13] Feng Lijuan, Yang Huaiyu, Wang Fuhui. Inhibition behavior of ascorbic benzoate for steel rebar in alkaline solution[J]. Acta Chimica Sinica, 2011, 69(20): 2359-2367
[14] Cruz J, Martinez R, Genesca J, et al. Experimental and theoretical study of 1-(2-ethylamino)- 2-methylimidazoline as an inhibitor of carbon steel corrosion in acid media[J]. J Electroanal Chem, 2004, 566(1): 111-121
[15] Duda Y, Govea-Rueda R, Galicia M. Corrosion inhibitors: design, performance, and computer simulations[J]. J Phy Chem, 2005, 109(47)B: 22674-22684
[16] Xia S W, Qiu M, Yu L M, et al. Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance[J]. Corrosion Sci, 2008, 50(7): 2021-2029
[17] Li W H, He Q, Pei C L, et al. Experimental and theoretical investigation of adsorption behavior of new triazole derivatives as inhibitors for mild steel corrosion in acid media[J]. Electrochem Acta, 2007, 52(22): 6386-6394
[18] Chen Boshui, Gao Lingyue, Fang Jianhua, et al. Lubricant biodegradation enhancers: Designed chemistry and engineered technology[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(3): 102-110
[19] Green fi eld M, Ohtani H. Molecular dynamics simulation study of model friction modifier additives confined between two surfaces[J].Tribology Letters, 1999, 7(2/3): 137-145
[20] Kier L B. An index of molecular flexibility from kappa shape attributes[J]. Quantitative Structure-Activity Relationships, 1989, 8(3): 221-224
Received date: 2015-12-05; Accepted date: 2016-02-28.
Professor Chen Guoxu, E-mail: chen_ guoxu@21cn.com.
- 中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
- Electrospinning Preparation and Mechanical Properties of Polymethyl Methacrylate (PMMA)/Halloysite Nanotubes (HNTs) Composite Nano fi bers
- Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease

