Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Study on the Adaptability of Etheri fi cation Feedstock to Reactor Type
Mao Junyi; Yuan Qing; Wang Lei; Huang Tao
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
A reactive C5ole fi ns and methanol etheri fi cation kinetic model based on E-R mechanism was established and three different types of reactors including the adiabatic fi xed-bed liquid reactor, the external loop reactor and the mixedphase reactor were constructed by Aspen Plus. The adaptability of reactive C5ole fi ns to these reactors was studied and simulated using various gasoline fractions with different ole fi ns content. After the theoretical model was validated by the experimental data of the etheri fi cation of three C5light cut fractions from different gasoline sources in different reactors, the simulated isoamylene conversion with reactive C5olefin contents increasing from 10% to 60% was studied in the three different types of reactors for etheri fi cation with methanol, respectively. Test results show that there is an obvious adaptability of the feedstock composition to the reactor type to achieve a high conversion.
etheri fi cation; reactor; feedstock; adaptability
1 Introduction
As an economic gasoline blending oxygenate for octane boosting,tert-amyl methyl ether (TAME) gains more and more attention in recent years[1-2], which is catalytically synthesized in the liquid phase by the reaction of methanol with reactive C5olefins, mainly 2-methyl-1-butene (2M1B) and 2-methyl-2-butene (2M2B), which are normally obtained from a light cut of gasoline. The reaction of ole fi ns with methanol is an exothermic process and the isoamylene conversion is closely influenced by temperature, which is kinetically dependent and also limited by thermodynamic equilibrium[3-4]. In order to control the reaction temperature to achieve a higher isoamylene conversion, various sorts of reactors have been developed including the adiabatic fixed bed and other types with heat extraction, such as the multitubular reactor provided with cooling water, the external loop reactor, and the mixed-phase reactor[5].
It is known that gasoline fractions obtained from different process units differ significantly from each other, especially in terms of total ole fi n content, which normally ranges from 10% to over 60% among different gasoline fractions[6], resulting in a different reactive ole fi n content in a light cut from these gasoline sources. Researchers are wondering whether there is a proper adaptability of the reactant with different ole fi n content to the reactor type, which forms the focus of this paper.
2 Model Establishment
2.1 Kinetic model
The synthesis of TAME from reactive C5olefins involves three simultaneous reversible reactions, i.e. two etherification reactions of 2M1B and 2M2B with methanol to form TAME, and the isomerization reaction between 2M1B and 2M2B. The networks and notations for the reactions are shown in Figure 1. The side reactions such as dehydration of methanol to dimethylether (DME), and water reacting with ole fi n to formtert-amyl alcohols are ignored due to the high selectivity of etherification reaction over resins used as the catalysts.
In the published kinetic models for the synthesis of TAME, strong non-ideal kinetic behavior of the reactant must be considered[7]; thus, the activity instead of the concentration of species should be used to calculate the kinetic parameters such as the reaction rate and equilibrium constant. In present studies, the activitycoefficients in etherification system were calculated from the group-contribution method such as UNIFAC, UNIQUAC and the Wilson methods. Some binary parameters were obtained from the UNIQUAC method in the Aspen Plus databank, while some missing parameters were computed theoretically by the UNIFAC method.
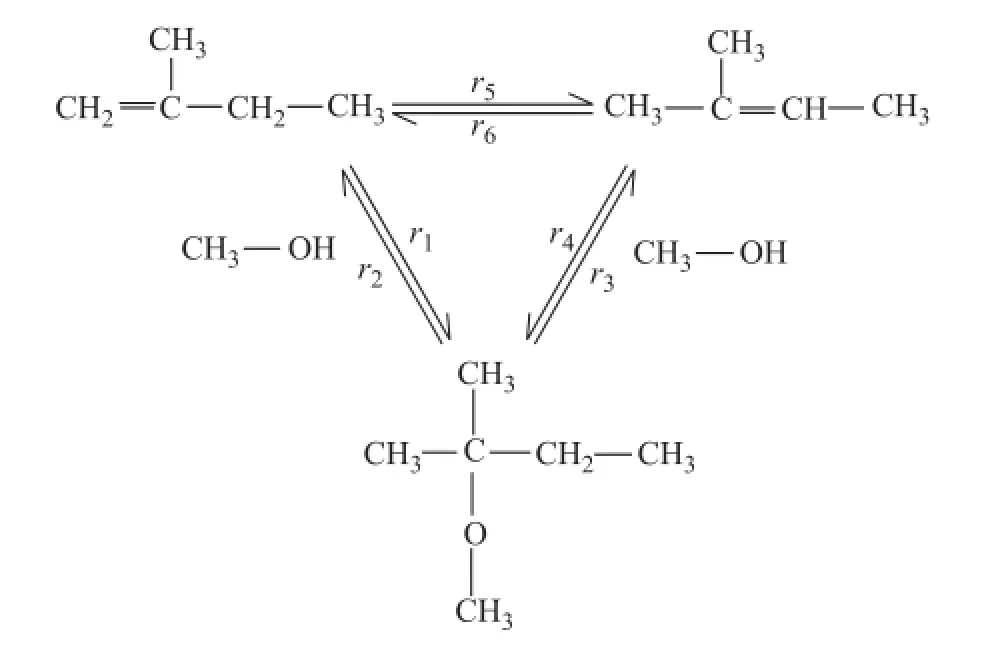
Figure 1 Scheme of the TAME reaction triangle
In the process for synthesis of TAME from C5isoamylenes and methanol, three different mechanisms were developed, including the homogeneous reaction mechanism, the Langmuir-Hinshelwood (L-H) type mechanism and the Eley-Rideal (E-R) type mechanism[8]. The homogeneous mechanism assumes that the reactions take place in the liquid phase without adsorption of reactants on the catalyst, while the L-H mechanism involves the competitive adsorption of all reactants. As regards the E-R mechanism, which is adopted in this study, it is assumed that only methanol and TAME are adsorbed on the active sites of the catalyst, while the adsorption of isoamylene is very insignificant, and the surface reaction is a controlling step[9]. The reaction rate can be written as follows:
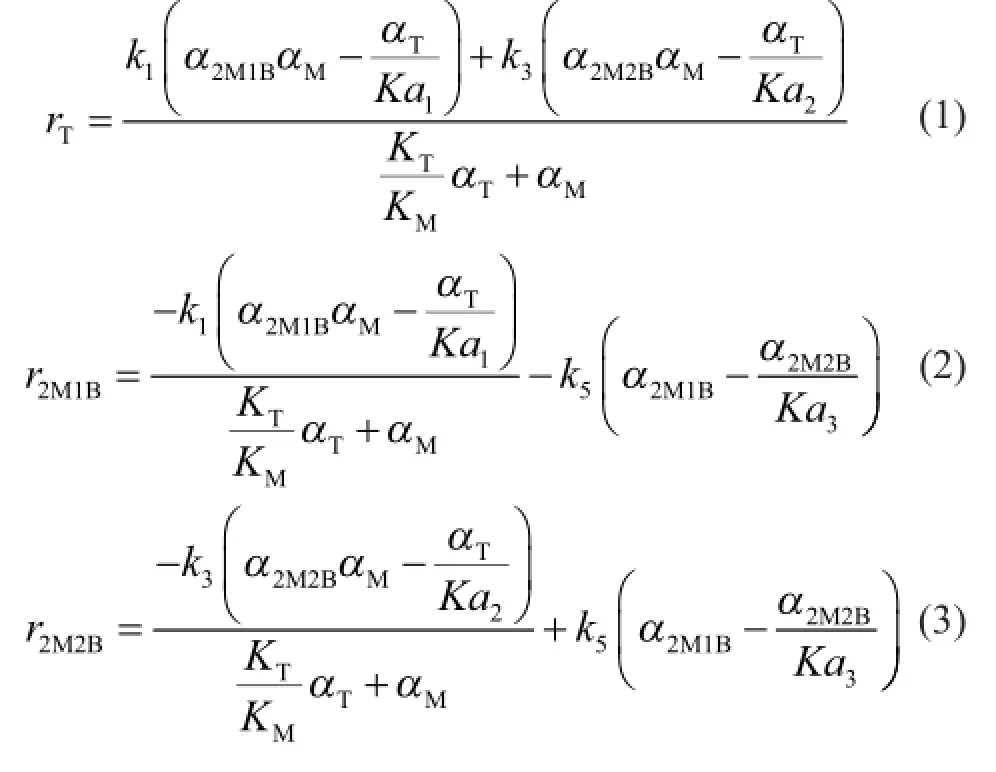
Values of the kinetic constantkiare calculated by the Arrhenius equation as follows.
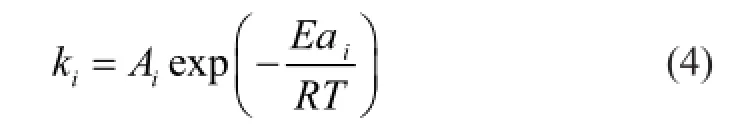
Reaction equilibrium constants for the reversible reactions shown in Figure 1 are calculated from the following equations.

The adsorption equilibrium constants for components TAME and methanol bear the following relationship:
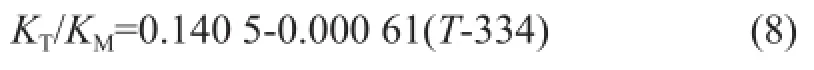
The above listed and other kinetic data such as activation energyEaiwere all obtained from Mao Wei, et al.[4]
2.2 Etheri fi cation reactor modeling
The most wildly used type of reactors for light olefin etherification includes the adiabatic fixed bed, the external loop reactor, and the mixed-phase reactor in the industrial field. Figure 2 is the simplified process flow diagram of each reactor. Feedstocks are preheated to the required temperature and then routed to the reactors. In an adiabatic fi xed bed, as shown in Figure 2 (a), pressure is set to be higher than the bubbling point of the lightest component in the feedstock to keep a liquid phase in the reactor, and the flow temperature increases along the flux direction. Since etherification is an exothermic reaction and is limited by a thermodynamic equilibrium, the temperature rise in the reactor is determined by the isoamylene conversion, while the isoamylene conversion is also limited by the outlet temperature inversely. As a result, there should be an optimum feed temperature to maximize the isoamylene conversion at a given feed rate and catalyst loading, such as adopting proper measures to decrease the temperature rise. A recycled flux from the reactor outlet is usually introduced to mix with the inlet feedstock especially in the case of using high ole fi n concentration feed, which is the so-called external loop reactor, as shown in Figure 2(b). Another way to decrease the temperature rise in the reactor is to utilizethe component vaporization under a certain temperature and pressure, with the sensible heat being transferred to the latent heat, leading to a gas-liquid mixed phase in the reactor. The most important operating parameter of the mixed-phase reactor is the system pressure, which has a great in fl uence on the amount of the vaporized fl ux and then on the fi nal temperature.
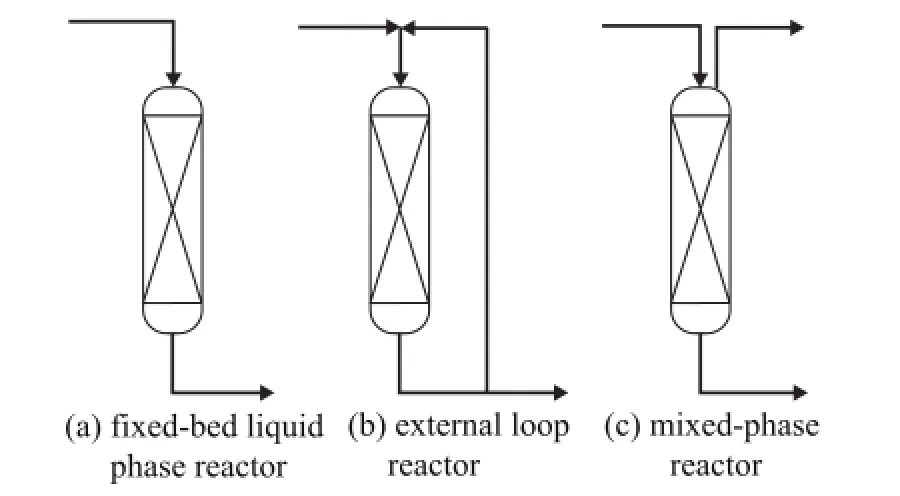
Figure 2 Different types of reactors for light ole fi n etheri fi cation
In this study, the commercial process simulator Aspen Plus was used to calculate the rate of the reactive C5ole fi n etherification reaction in different types of reactors, in which the rate equations were written in a user-supplied subroutine and put into the RPLUG model. The reaction heat and heat capacity for the energy balance was also calculated using the internal databank. The reactors were assumed to be adiabatic with an ideal plug fl ow, and the catalyst loading was given according to the actual liquid hourly space velocity of the feed with a void fraction of 0.3. The reactor inlet temperature, the system pressure and other operating parameters were given according to the experimental parameters, and the outlet temperature was calculated by the thermodynamic data of Aspen Plus.
3 Experiment Study
3.1 Materials
A light cut of gasoline is usually used in the etheri fi cation with methanol, which mainly contains C5hydrocarbon components and also some C4and C6components. The C5components in a light gasoline fraction are as follows: n-pentane (NC5), iso-pentane (iC5), 1-pentene (1C5=), 2-methyl-1-butene (2M1B), 2-methyl-2-butene (2M2B), 3-methyl-1-butene (3M1B),cis-2-pentene (c2C5=),trans-2-petene (t2C5=), cyclopetene (CYC5=), and a small amount of pentadienes, among which only the isoamylenes, viz. 2M1B and 2M2B, are reactive with methanol to form TAME.
Three C5cut feedstocks from different gasoline sources were used to react with methanol during etheri fi cation in this study; the composition and content of C5hydrocarbons in gasoline fractions are shown in Table 1, with the results obtained from an Agilent gas chromatograph (GC) 7890B.
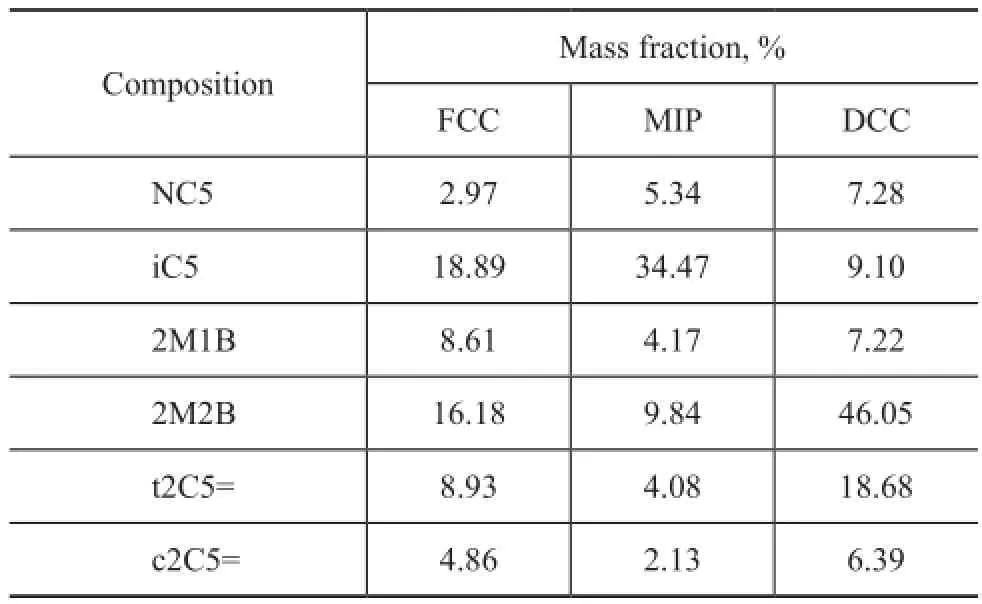
Table 1 Feed composition of different gasoline sources
It can be seen from Table 1 that the total mass fraction of reactive C5olefins (2M1B and 2M2B) varies greatly in different gasoline sources, which ranges from about 10% in a MIP gasoline to 55% in a DCC gasoline. The mass ratio of 2M2B to 2M1B in different sources also changes greatly, which ranges from about 2 to nearly 6.5.
The catalyst used for isoamylene etherification was a commercial, strong cation-exchange resin D005Ⅱ (provided by the Dandong Mingzhu Special Type Resin Co. Ltd, China), with a ion-exchange capacity of≥5.2 mmol[H+]/g, and a particle size of 0.35—1.25 mm. Before the reaction, the resin was pretreated by methanol to displace the water in the catalyst.
3.2 Experiments
The etherification process was carried out in a tube reactor, with a height to diameter ratio of catalyst loading equating to 8, which can be operated as a fixed bed, or an external loop reactor, or a mixed-phase reactor as well. The C5composition and content in feedstock are shown in Table 1, with a given methanol to reactive C5ole fi ns (2M1B+2M2B) molar ratio of 1.05. The feed fl ow, which was preheated to the required temperature before combining with the catalyst for reaction, was controlled according to a liquid hourly space velocity of 1.5 basedon the catalyst loading.
The analyzed isoamylene (IA) conversion was calculated by the following equation (9).
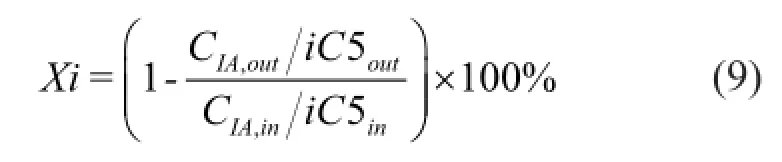
Under the fixed-bed reaction conditions, the pressure was set at 0.8 MPa to keep a pure liquid phase flow in the reactor; while making a part of the reactor effluent recycled to mix with the feed for creating an external loop reactor condition as shown in Figure 2(b), with the recycled stream to the feed mass ratio being set at 1.0; the operating pressure of a mixed-phase reactor was set to keep the vaporized amount to be less than 15% of the total fl ow, and actually the pressure value was equal to around 0.2—0.4 MPa. Finally, the temperature of gas phase at the reactor outlet was measured and the isoamylene conversion was calculated under each experimental condition.
4 Model Validation
The model simulation was carried out in accordance with the experimental conditions, and the simulated reactor outlet temperatures and isoamylene conversion values were compared with the experimental data,respectively. Comparison of the reactor outlet temperature is shown in Figure 3, and comparison between the calculated isoamylene conversion and the experimental data is presented in Figure 4.
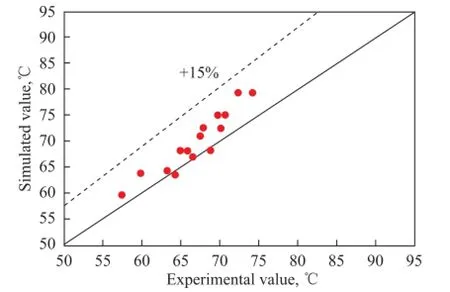
Figure 3 Comparison of the reactor outlet temperature between the simulation values and experimental ones
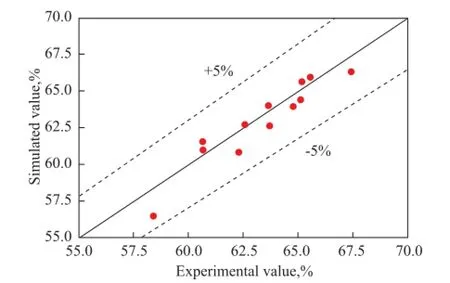
Figure 4 Comparison of the isoamylene conversion between the simulation values and experimental ones
It can be seen from Figure 3 that the simulated reactor outlet temperature was a little higher than that of the experimental results, with a maximum relative error reaching nearly 15%. This is mainly caused by the heat loss of the tube reactor as the furnace was not operated under a strict adiabatic condition. Judging from the simulated and experimental values of isoamylene conversion, the relative errors were less than 5%, indicating to the reliability of the model, which can be used for further research on C5ole fi ns etheri fi cation with methanol in a certain type of reactor.
5 Results and Discussion
As it can be concluded from Table 1, the distribution of C5hydrocarbons varies greatly with different gasoline sources, and the reactive C5olefin content ranges from around 10% up to 60%, which would have a great in fl uence on the etheri fi cation process. In this paper, the adaptability of the feed composition to reactor type was studied based on the validated model.
A virtual feedstock, with its total reactive C5olefins (2M1B+2M2B) mass fraction ranging from 10% to 60%, was adopted for this study, in which the mass ratio of 2M2B to 2M1B varied from 2 to 6, and other inert components such as the amount of iC5hydrocarbons changed in proportion. The operating conditions included: (1) a methanol to total isoamylene molar ratio of 1.05 and a preheated temperature of 55℃ for all cases, (2) a recycled flow to feed mass ratio of 1.0 for the external loop reactor, and (3) an operating pressure being speci fi ed to achieve a vaporized amount of 10% of total flow for the mixed-phase reactor.
Changes in isoamylene conversion with an increasing reactive C5ole fi n content in different reactors is shown inFigure 5.
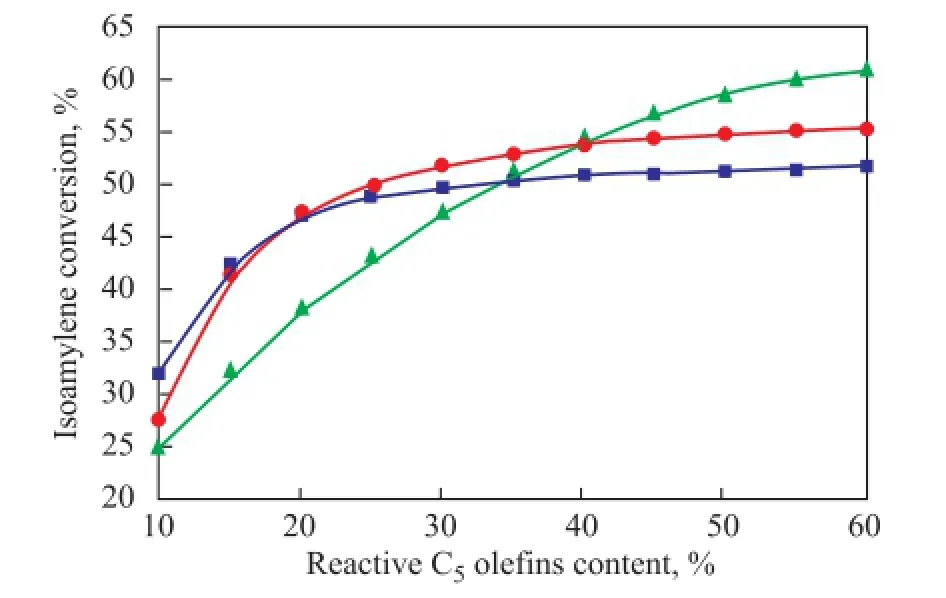
Figure 5 Comparison of isoamylene conversion with an increasing ole fi n content in different reactors
As shown in Figure 5, the isoamylene conversion increased with an increasing olefin content under all conditions, and an obvious adaptability of the feedstock composition to the reactor type was revealed.
For the C5olefin feedstock with low reactive olefin content, for example 10% to 20%, the isoamylene conversion was higher in the adiabatic fi xed-bed reactor than the other two reactor types. This was mainly caused by the interactive influence of temperature on the etheri fi cation reaction. On the one hand, the reaction rate would be very slow if the temperature was low according to the kinetic nature; on the other hand, the isoamylene conversion would be decreased if the reaction temperature was too high due to the exothermic property of isoamylene etheri fi cation. For the feedstock with low isoamylene content, the reaction rate was the controlling factor since the released heat is very small. Therefore, the adiabatic fi xed-bed reactor gave a higher isoamylene conversion since no heat extraction could result in a higher reaction rate[10].
For a middle range content of reactive olefin feedstock, both the kinetic and thermodynamic effects had their influence on the final isoamylene conversion. If the exothermic reactions occurred in the adiabatic fi xed-bed with an appropriate initial temperature, the reaction rate was high enough to achieve an equilibrium conversion of the reactants, and then the final conversion would be determined by the outlet temperature of the reaction zone. If a certain amount of light components in the fl ow was vaporized after absorbing the reaction heat, the temperature could be controlled near the bubbling point of the vaporizing components, which was determined by the system pressure. Under this condition, a gas-liquid two-phase fl ow existed in the reactor, and the isoamylene conversion was achieved at a relative low temperature in comparison with the adiabatic liquid phase reactor[11]. This is why the isoamylene conversion was higher for the mixed-phase reactor as compared to the fi xed-bed liquid reactor with a reactive olefin content being higher than 20%. However, for an external loop reactor, the reactive ole fi n content was diluted by the recycled fl ow, resulting in a lower reaction rate in comparison, and a certain amount of TAME was also recycled to the feed as well, which would decrease the total isoamylene conversion as well. This is the reason for the low conversion of the external loop reactor, when the reactive ole fi n content was lower than 40%.
When the reactive ole fi n content was higher than 40% in the feedstock, the reaction rate would be fast enough, and the heat release would be very large. Under this condition, the isoamylene conversion was greatly determined by the outlet temperature of the reaction zone. Besides, it is known that the lightest components in a C5olefins etherification feedstock are the azeotropes composed of methanol and nearly all the C5hydrocarbons, in particular the isopentane (iC5). If the heat release is large enough, the important reactant methanol would be vaporized into the gas phase massively without taking part in the etheri fi cation reaction, resulting in a decrease of reaction rate and a low isoamylene conversion. However, if the high reactive ole fi n content feed was diluted by a recycled fl ow, the reaction rate can be controlled at an appropriate level, and the outlet temperature could also be decreased due to an increased flow of heat capacity at a certain reaction heat release amount, then a higher isoamylene conversion could be achieved.
6 Conclusions
An E-R mechanism based reactive C5ole fi n etheri fi cation kinetic model was established for the reaction rate calculation using a commercial process simulator Aspen Plus, and three different types of reactors for etheri fi cation including the adiabatic fixed-bed liquid reactor, theexternal loop reactor and the mixed-phase reactor were studied in this work. Experiments were carried out with three different sources of gasoline in the above-mentioned reactors respectively, and then the model was validated by the experimental data, indicating to the reliability of the model.
Isoamylene conversion with a reactive C5ole fi n content ranging from 10% to 60% was studied in three different types of reactors for etherification with methanol. Test results show that there is an obvious adaptability of the feedstock composition to the reactor type. For a feedstock with its reactive ole fi n content being lower than 20%, the adiabatic fi xed-bed liquid reactor is quite able to achieve a good isoamylene conversion, while the mixed-phase reactor exhibits a better adaptability to a feedstock with a reactive ole fi n content in the range of between 20% and 40%; however, if the reactive ole fi n content is higher than 40%, the external loop reactor seems to be more suitable for the etheri fi cation reaction.
Nomenclature
αiliquid-phase activity of componenti
Aipre-exponential factor of reactioni, mmol/(h·g)
Cimass fraction of componenti
Eaiactivation energy of reactioni, kJ/mol
kikinetic constant for reactioni, mmol/(h·g)
Kaithermodynamic equilibrium constant of reactioni
Kiadsorption equilibrium constant of componenti
rirate of reactioni, mmol/(h·g)
Rideal gas constant, 8.314 5 J/(mol·K)
Ttemperature, K
Xiconversion of componenti
[1] Ancillotti F, Fattore V. Oxygenated Fuels: Market Expansion and Catalytic Aspect of Synthesis[J]. Fuel Processing Technology, 1998, 57(3): 163-194
[2] Muja I, Toma A, Popescu D C, et al. Thermodynamic study of the methanol addition to isoamylene[J]. Chemical Engineering and Processing. 2005, 44: 645-651
[3] Yao Wensheng, Lu Guigeng, Zhang Guangping, et al. Study on Kinetics of the Liquid-Phase Etheri fi cation of Isoamylenes with Methanol[J]. Petrochemical Technology, 1998, 27(6): 391-395 (in Chinese)
[4] Mao Wei, Wang Xiaolei, Wang Hua, et al. Thermodynamic and kinetic study oftert-amyl methyl ether (TAME) synthesis[J]. Chemical Engineering and Processing, 2008, 47: 761-769
[5] Liu Chengjun, Zhang Xiangling, Wen Shichang, et al. A discussion on the process design of light FCC gasoline etheri fi cation[J]. Petroleum Processing and Petrochemicals, 2011, 42: 13-17 (in Chinese)
[6] Wang Tianpu, Wang Yingchun, Wang Wei, et al. Upgrading FCC naphtha by etheri fi cation[J]. Petroleum Processing and Petrochemicals, 2001, 32(8): 64-66 (in Chinese)
[7] Verevkin S P. Thermochemistry of branched ethers: experimental study of chemical equilibrium in the reacting system oftert-amyl alkyl ether synthesis[J]. Journal of Chemical & Engineering Data, 2004 (49): 576-581
[8] Parra D, Tejero J, Cunill F, et al. Kinetic Study of MTBE Liquid-Phase Synthesis Using C4Ole fi nic Cut[J]. Chemical Engineering Science, 1994, 49: 4563-4578
[9] Reh fi nger A, Hoffmann U. Kinetics of methyl tertiary butyl ether liquid phase synthesis catalyzed by ion exchange resin. I. Intrinsic rate expression in liquid phase activity[J]. Chemical Engineering Science, 1990, 45(6): 1605-1617
[10] Hu Yuqi, Fang Jing, Li Chunli. Simulation optimization and experimental study of cross-wall adiabatic dividing wall column used to separate hexane-heptane-octane system[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(1): 108-116
[11] Lü Chao, Zhang Zimu, Zhao Qiuyue, et al. Numerical simulation of enhanced oil-water separation in a threestage double-stirring extraction tank[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(4): 121-126
Received date: 2016-04-01; Accepted date: 2016-04-23.
Mao Junyi, E-mail: maojunyi.ripp@ sinopec.com.
- 中国炼油与石油化工的其它文章
- Synthesis and Evaluation of Environmentally Friendly Calcium Isostearate Detergent with Excellent Oil Solubility
- Electrospinning Preparation and Mechanical Properties of Polymethyl Methacrylate (PMMA)/Halloysite Nanotubes (HNTs) Composite Nano fi bers
- Modeling of Isobutane/Butene Alkylation Using Solid Acid Catalysts in a Fixed Bed Reactor
- Analysis and Modeling of Wangqing Oil Shale Drying Characteristics in a Novel Fluidized Bed Dryer with Asynchronous Rotating Air Distributor
- Experimental and Molecular Simulations for Evaluating the Effect of Lubricity Improvers on the Property of Jet Fuel
- Preparation and Tribological Properties of Lanthanumdoped Muscovite Composite Particles as Lubricant Additives in Lithium Grease

