磷酸甘油酸变位酶5与坏死样凋亡*
磷酸甘油酸变位酶5与坏死样凋亡*
佘浪,彭军△
(中南大学药学院药理学系,湖南 长沙 410078)
多年来人们认为细胞死亡方式主要包括坏死和凋亡。坏死是不受特定调控的被动死亡方式,表现为细胞器肿胀,细胞膜破裂,内容物渗出,有炎症反应[1],而凋亡则是由细胞内胱天蛋白酶(caspase)调控的一种主动死亡方式,因caspase激活导致细胞内底物裂解,破坏胞膜囊泡形成凋亡小体,死亡过程中无内容物渗出,不引起炎症反应[2]。坏死样凋亡是近年来发现的细胞死亡又一方式,由死亡受体和病原体模式识别受体介导,既有类似凋亡信号的调节通路,又呈现坏死样形态学特征,因此又称为程序性细胞坏死,在细胞生理和病理功能中发挥重要作用[3-4]。
磷酸甘油酸变位酶5(phosphoglycerate mutase 5,PGAM5)是磷酸甘油酸变位酶超家族成员之一,是一种糖酵解酶。研究表明,PGAM5能加速发动蛋白相关蛋白1(dynamin-related protein 1,DRP1)去磷酸化,促进线粒体分裂,进而促进坏死样凋亡,在炎症反应[5]、神经退行性疾病[6]和缺血再灌注损伤[7-8]中发挥重要作用。由于PGAM5在坏死样凋亡中扮演重要角色,可能会成为预防和治疗损伤相关性疾病的新靶点。
1肿瘤坏死因子诱导的坏死样凋亡
坏死样凋亡通路中以肿瘤坏死因子(tumor necrosis factor,TNF)诱导的坏死样凋亡研究最透彻。肿瘤坏死因子受体(tumor necrosis factor receptor,TNFR)与其配体结合,可以启动3种不同的细胞反应:核因子κB(nuclear factor-κB,NF-κB)介导的促细胞存活通路及炎症反应、caspase介导的凋亡途径、受体相互作用蛋白1(receptor-interacting protein 1,RIP1)和受体相互作用蛋白3(receptor-interacting protein 3,RIP3)依赖的坏死样凋亡。
TNF-α能特异性结合位于细胞表面的TNFR1和TNFR2受体,由于TNFR2不具有死亡结构域,因此主要由TNFR1介导涉及细胞死亡通路的一系列反应[9]。TNFR1激活后,招募RIP1启动死亡通路,RIP1是决定细胞生存与死亡的交叉点,其结构含有N-末端激酶结构域、中间结构域和C末端死亡结构域。RIP1的泛素化发生在中间结构域的赖氨酸377位点,其泛素化状态是由凋亡蛋白抑制剂(inhibitor of apoptosis proteins,IAP)维持[10]。RIP1 泛素化决定其促细胞死亡作用。RIP1泛素化后,通过其C末端结构域中的死亡结构域(death domain,DD)促进RIP1招募TNF受体相关死亡结构域(TNF receptor- associated death domain,TRADD),并与E3泛素连接酶形成复合物Ⅰ[11],与IκB激酶(IκB kinase,IKK)的调节亚基NF-κB必须调节蛋白(NF-κB essential modulator,NEMO)结合,激活NF-κB抑制细胞死亡及免疫反应[12]。若RIP1去泛素化,RIP1在胞质内不与TNFR1结合,通过招募TRADD、caspase-8形成复合物Ⅱ,则激活凋亡信号通路,抑制坏死样凋亡的发生[13]。通过RNA干扰抑制去泛素化酶cylindromatosis(CYLD)表达,阻止RIP1去泛素化,则可促进TNF-α诱导的坏死样凋亡[14],表明RIP1泛素化是TNF-α诱导坏死样凋亡的重要一步。坏死样凋亡一旦启动,涉及的下游通路和蛋白分子众多,其中PGAM5参与介导的线粒体裂变对坏死样凋亡的发生发展至关重要。
2磷酸甘油酸变位酶
磷酸甘油酸变位酶(phosphoglycerate mutase,PGAM)是一种糖酵解酶,能催化3-磷酸甘油酸(3-phosphoglyceric acid,3-PGA)转化为2-磷酸甘油酸(2-phosphoglyceric acid,2-PGA),在碳水化合物转运、新陈代谢、催化活性及生长发育扮演重要的作用[15]。人类磷酸甘油酸变位酶超家族至少有10种以上的蛋白编码基因[16],根据编码蛋白的功能,这些基因可分为3组:第1组包括编码PGAM的3个基因和二磷酸甘油酸变位酶(bisphosphoglycerate mutase,BPGM),编码的蛋白具有磷酸甘油酸变位酶活性或二磷酸甘油酸变位酶活性;第2组包括能编码6-磷酸果糖激酶2/果糖双磷酸酶(6-phosphofructokinase-2/fructose-2,6-bisphosphatase,PFKFB)家族同工酶的4个基因和肿瘤蛋白p53(tumor protein p53,TP53)诱导糖酵解及凋亡调节因子(TP53-induced glycolysis and apoptosis regulator,TIGAR),编码的蛋白能诱导癌细胞凋亡和糖酵解失调;第3组包括T细胞受体信号抑制蛋白1和2(suppressors of T-cell receptor signaling-1/2,STS-1/2)[17]和编码PGAM5的3个基因,编码的蛋白含有泛素相关序列和C末端PGAM结构域,在T细胞受体信号转导和调节受体酪氨酸激酶中发挥重要的作用[16,18]。
3PGAM5
PGAM5虽然属于磷酸甘油酸变位酶超家族成员,但没有甘油酸变位酶活性,而是具有磷酸酶活性。PGAM5通过其N端的线粒体导向序列定位在线粒体外膜,因此,PGAM5通常被称为线粒体磷酸酶[19]。人类PGAM5位于第12号染色体上,通过3'外显子选择性剪切产生2种不同的剪切变体,即长型PGAM5-L和短型PGAM5-S(图1)。2种剪切变体中1~239氨基酸序列相同,不同之处在于PGAM5-L的C末端包含50个氨基酸残基,而PGAM5-S则包含16个氨基酸残基,PGAM5-S比PGAM5-L的疏水性强。有趣的是,虽然PGAM5的C末端结构域与磷酸甘油酸变位酶家族其它成员的催化结构域具有同源性,并含有催化活性所必须的组氨酸,但PGAM5并不具有磷酸甘油酸变位酶活性,它主要发挥磷酸酶活性[18]。
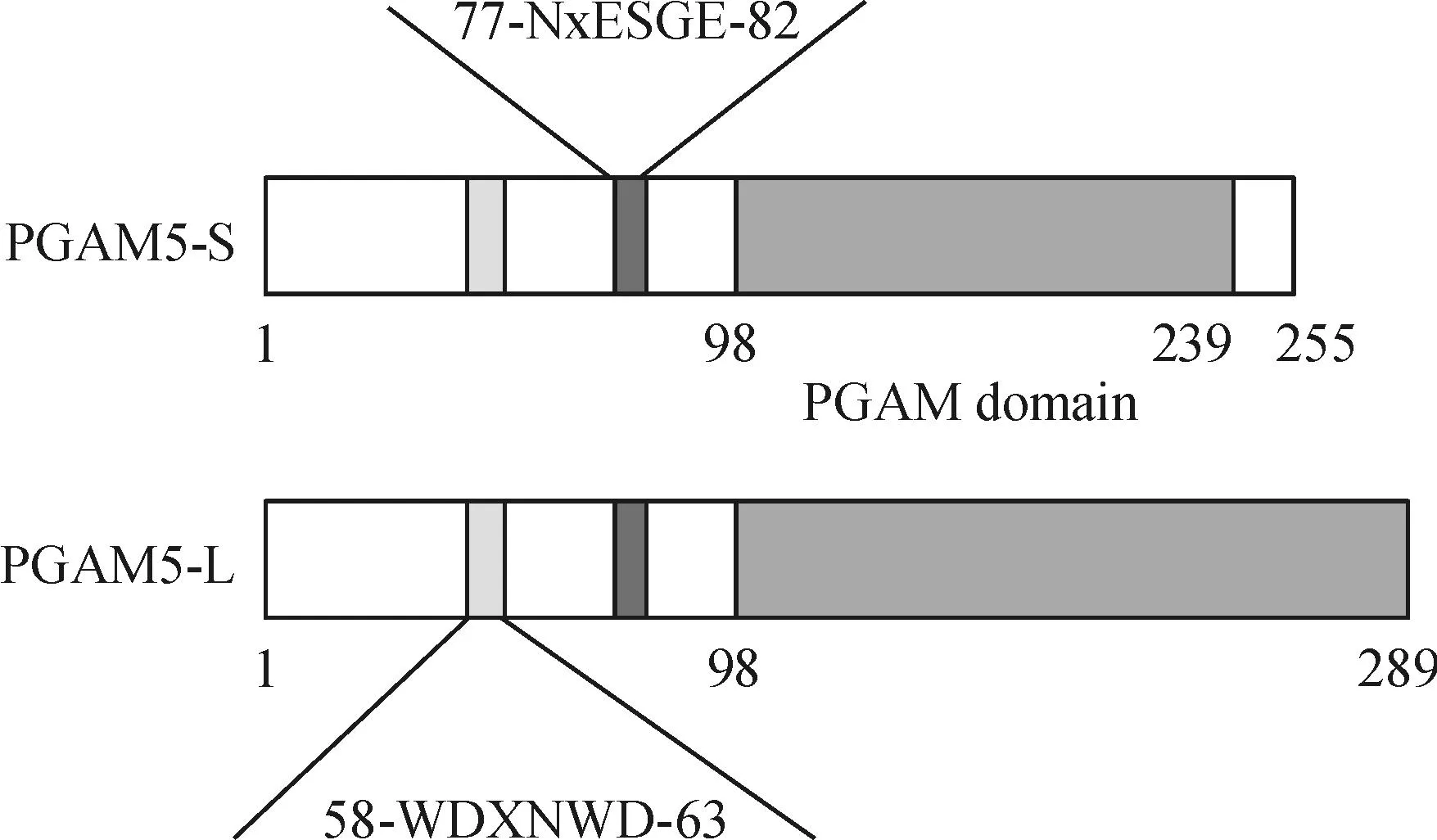
Figure 1.The structure of PGAM5-S and PGAM5-L.Relevant domains for PGAM5-S and PGAM5-L include a conserved multimerization motif WDXNWD (amino acids 58~63), a NxESGE motif that binds Keap1 (amino acids 77~82) and the PGAM domain (beginning at amino acid 98).The difference in the structure between the two isforms locates at the C-terminal starting from amino acid 239.
图1PGAM5-S和PGAM5-L结构示意图
近年来发现PGAM5存在一个高度保守的氨基酸序列WDXNWD,位于N末端的氨基酸58~63位点,对PGAM5参与多聚体复合物的组装和激活下游通路是必须的,可最大限度地提高磷酸酶的活性[20]。PGAM5-L和PGAM5-S的N末端都含有线粒体定位序列NxESGE,与核因子E2相关因子2(nuclear factor E2-related factor 2,Nrf2)的DxETGE序列非常相似,都能与Kelch样环氧氯丙烷相关蛋白1(Kelch-like ECH-associated protein-1,Keap1)结合,形成PGAM5/Keap1/Nrf2三元复合物,定位于线粒体外膜[21]。该序列位于PGAM5的77~82氨基酸位点,是PGAM5与DRP1相互作用的基础,对PGAM5参与线粒体分裂至关重要。此外,PGAM5缺乏磷酸甘油酸变位酶活性,但保留丝氨酸/苏氨酸蛋白磷酸酶活性,该特性也是其参与线粒体分裂调节的生物学基础[19]。
4PGAM5促线粒体分裂和坏死样凋亡作用
线粒体是一种高度动态变化的细胞器,在细胞中不断分裂与融合,形成紧密连接的线粒体网络。线粒体的分裂和融合过程除了对于线粒体遗传和其自身功能的维持很重要外,对于能量代谢、发育、衰老和细胞死亡等多种细胞功能也十分重要[22]。在哺乳动物中,线粒体融合受线粒体融合蛋白1(mitofusin-1,Mfn-1)、线粒体融合蛋白2(mitofusin-2,Mfn-2)和 opticatrophy 1(OPA1)等调控,而线粒体分裂主要由DRP1和它相应的配体线粒体分裂蛋白1(fission protein 1,Fis1)、线粒体分裂因子(mitochondrial fission factor,Mff)所调控[23]。
研究表明, PGAM5存在于坏死样凋亡的坏死复合体碎片中,用siRNA沉默PGAM5的2种剪切变体,证实PGAM5-L和PGAM5-S均参与坏死样凋亡。目前已合成抑制坏死样凋亡的小分子化合物necrosulfonamide(NSA),能特异性识别混合系列激酶结构域样蛋白(mixed lineage kinase domain-like protein,MLKL),阻止坏死样凋亡信号传导。有趣的是,NSA能抑制PGAM5-S与RIP1/RIP3的相互作用,而对PGAM5-L与RIP1/RIP3的相互作用无影响。此外,NSA能抑制PGAM5-S磷酸化而对PGAM5-L磷酸化无影响。利用NSA干预和基因沉默等研究手段,科研人员目前已基本阐明PGAM5参与介导坏死样凋亡的信号通路[24]。
如图2所示,TNFR介导的坏死样凋亡启动后,将形成RIP1/RIP3/MLKL坏死复合体[25-26]。作为RIP3特异性底物蛋白,MLKL是拥有激酶结构域但无激酶功能的假激酶。在坏死复合体形成的起始阶段,RIP3丝氨酸227位点发生磷酸化,并促进MLKL激酶结构域苏氨酸357位点和丝氨酸358位点双重磷酸化,MLKL的磷酸化是坏死样凋亡中不可或缺的一步[27]。虽然MLKL本身不具有激酶活性,但它与RIP3的结合并发生磷酸化后,成为适配蛋白介导 RIP3与底物蛋白PGAM5结合。PGAM5-L先与RIP1/RIP3/MLKL复合物结合,再激活位于线粒体膜上的PGAM5-S参与,进一步招募线粒体分裂因子DRP1,使DRP1丝氨酸637位点去磷酸化并激活其GTPase活性[24]。DRP1主要分布于胞浆中,是控制线粒体分裂的主要蛋白,一旦被激活,在线粒体外膜招募因子Fis1或Mff的参与下,从胞浆转移到线粒体外膜特定的分裂位点,形成螺旋样多聚体结构围绕在线粒体外膜上,借助于GTP的水解作用,收缩线粒体外膜,直到脂质双分子层失去其稳定性,形成2个线粒体,此时,DRP1已恢复其单体结构回到胞浆中[28]。线粒体分裂导致活性氧簇(reactive oxygen species,ROS)增多,膜通透性开放,ATP生成减少,是坏死样凋亡的早期特征。

Figure 2.The diagram of necroptosis signal pathway initiated by TNF-α. Once TNFR is activated, RIP1, RIP3 and MLKL will interact to form a necrosome after a series of steps.MLKL itself does not have the kinase activity, but can combine with RIP3 to mediate interaction between RIP3 and PGAM5. PGAM5-L combines with RIP1/RIP3/MLKL complex to form a new compound. Once PGAM5-S joins this process, it can recruit DRP1 through dephosphorylation of DRP1 at serine 637, which in turn to promote the mitochondrial fission and necroptosis subsequently.
图2TNFR介导的细胞坏死样凋亡通路示意图
5结语与展望
PGAM5在参与介导促细胞坏死样凋亡过程中发挥重要作用,与多种疾病(如心肌梗死、脑卒中)发展和预后密切相关。目前,PGAM5参与坏死样凋亡信号通路的环节已基本了解,但仍有许多问题,如PGAM5与RIP1/RIP3/MLKL复合物之间的相互作用机制、PGAM5-L与PGAM5-S的关系等,尚待阐明。此外,PGAM5除了参与介导TNFR介导的坏死样凋亡外,是否还参与其它死亡受体和病原体模式识别受体介导的坏死样凋亡?阐明和解决上述问题将为开发针对PGAM5的特异性抑制剂奠定基础,对于减轻相关疾病症状和改善预后具有重要临床意义。
[参考文献]
[1]Orrenius S, Gogvadze V, Zhivotovsky B. Calcium and mitochondria in the regulation of cell death[J]. Biochem Biophys Res Commun, 2015, 460(1):72-81.
[2]Sarvothaman S, Undi RB, Pasupuleti SR, et al. Apoptosis: role in myeloid cell development[J]. Blood Res, 2015, 50(2):73-79.
[3]Feoktistova M, Leverkus M. Programmed necrosis and necroptosis signalling[J]. FEBS J, 2015, 282(1):19-31.
[4]李澎,任钧国,付建华,等. Necroptosis: 一种新的程序性死亡的研究进展[J].中国病理生理杂志,2010,26(12):2487-2492, 2496.
[5]Galluzzi L, Kepp O, Kroemer G. MLKL regulates necrotic plasma membrane permeabilization[J]. Cell Res, 2014, 24(2): 139-140.
[6]Lu W, Karuppagounder SS, Springer DA, et al. Genetic deficiency of the mitochondrial protein PGAM5 causes a Parkinson’s-like movement disorder[J]. Nat Commun, 2014, 5:4930.
[7]Koshinuma S, Miyamae M, Kaneda K, et al. Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia-reperfusion injury[J]. J Anesth, 2014, 28(2):235-241.
[8]Rosentreter D, Funken D, Reifart J, et al. RIP1-depen-dent programmed necrosis is negatively regulated by caspa-ses during hepatic ischemia-reperfusion[J]. Shock, 2015, 44(1):72-76.
[9]Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death[J]. Crit Rev Oncol Hematol, 2012, 82(3):249-258.
[10]de Almagro MC, Goncharov T, Newton K, et al. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination[J]. Cell Death Dis, 2015, 6:e1800.
[11]Park Y, Jeong MS, Jang SB. Death domain complex of the TNFR-1, TRADD, and RIP1 proteins for death-inducing signaling[J]. Biochem Biophys Res Commun, 2014, 443(4):1155-1161.
[12]Christofferson DE, Li Y, Yuan J. Control of life-or-death decisions by RIP1 kinase[J]. Annu Rev Physiol, 2014, 76:129-150.
[13]Marshall KD, Baines CP. Necroptosis: is there a role for mitochondria?[J]. Front Physiol, 2014, 5:323
[14]Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis[J]. PLoS One, 2013, 8(10):e76841.
[15]Jedrzejas MJ. Structure, function, and evolution of phosphoglycerate mutases: comparison with fructose-2,6-bisphosphatase, acid phosphatase, and alkaline phosphatase[J]. Prog Biophys Mol Biol, 2000, 73(2-4):263-287.
[16]Sadatomi D, Tanimura S, Ozaki K, et al. Atypical protein phosphatases: emerging players in cellular signaling[J]. Int J Mol Sci, 2013, 14(3):4596-4612.
[17]Mikhailik A, Ford B, Keller J, et al. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling[J]. Mol Cell, 2007, 27(3):486-497.
[18]Lo SC, Hannink M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex[J]. J Biol Chem, 2006, 281(49):37893-37903.
[19]Takeda K, Komuro Y, Hayakawa T, et al. Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1[J]. Proc Natl Acad Sci U S A, 2009, 106(30): 12301-12305.
[20]Wilkins JM, McConnell C, Tipton PA, et al. A conserved motif mediates both multimer formation and allosteric activation of phosphoglycerate mutase 5[J]. J Biol Chem, 2014, 289(36):25137-25148.
[21]Lo S, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria[J]. Exp Cell Res, 2008, 314(8):1789-1803.
[22]Ong S, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease[J]. Antioxid Redox Sign, 2013, 19(4):400-414.
[23]Hall AR, Burke N, Dongworth RK, et al. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease[J]. Brit J Pharmacol, 2014, 8(171):1890-1906.
[24]Wang Z, Jiang H, Chen S, et al. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways[J]. Cell, 2012, 148(1-2):228-243.
[25]de Almagro MC, Vucic D. Necroptosis: pathway diversity and characteristics[J]. Semin Cell Dev Biol, 2015, 39:56-62.
[26]Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation[J]. Nature, 2015, 517(7534):311-320.
[27]Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3[J]. Cell, 2014, 54(1):113-146.
[28]Stephen L, Archer MD. Mitochondrial dynamics: mitochondrial fission and fusion in human diseases[J]. N Engl J Med, 2013, 369(23):2236-2251.
(责任编辑: 林白霜, 罗森)
Phosphoglycerate mutase 5 and necroptosisSHE Lang, PENG Jun
(DepartmentofPharmacology,SchoolofPharmaceuticScience,CentralSouthUniversity,
Changsha410078,China.E-mail:junpeng@csu.edu.cn)
[ABSTRACT]Necroptosis, or programmed cell death, is a type of cell death with a controllable death signaling pathway and the morphological features similar to necrosis. It is mainly mediated by death receptors or pathogen pattern re-cognition receptors. Among them, tumor necrosis factor receptor 1 (TNFR1)-mediated necroptosis is the most well-studied one. Receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3) are the 2 key kinases involved in the formation of complex I & II and necrosome in the process of necroptosis. Phosphoglycerate mutase 5 (PGAM5), a member of phosphoglycerate mutase gene family, lacks PGAM activity and possesses the phosphatase activity. PGAM5 is anchored in the mitochondrial membrane and is also called mitochondrial phosphoglycerate mutase 5. It has been shown that PGAM5 involves in the formation of necrosome during necroptosis and it is able to accelerate the fission of mitochondria by dephosphorylation of dynamin-related protein 1 (DRP1), thus promoting cell necroptosis.
[关键词]坏死样凋亡; 磷酸甘油酸变位酶5; 发动蛋白相关蛋白1; 线粒体分裂; 磷酸酶
[KEY WORDS]Necroptosis; Phosphoglycerate mutase 5; Dynamin-related protein 1; Mitochondrial fission; Phosphatase
doi:10.3969/j.issn.1000- 4718.2016.02.031
[中图分类号]R363
[文献标志码]A
通讯作者△Tel: 0731-82355080; E-mail: junpeng@csu.edu.cn
*[基金项目]国家自然科学基金资助项目(No.91439104; No. 81373409)
[收稿日期]2015- 09- 29[修回日期] 2015- 10- 23
[文章编号]1000- 4718(2016)02- 0377- 04

