人脐带间充质干细胞分泌白细胞介素6促进骨肉瘤细胞增殖和迁移*
胡文龙, 吴平平, 耿书国, 汪建样, 殷 明
(南昌大学1研究生院医学部,2第二附属医院骨科,江西 南昌 330006)
人脐带间充质干细胞分泌白细胞介素6促进骨肉瘤细胞增殖和迁移*
胡文龙1, 2,吴平平1,耿书国1, 2,汪建样1, 2,殷明2△
(南昌大学1研究生院医学部,2第二附属医院骨科,江西 南昌 330006)
[摘要]目的: 探讨人脐带间充质干细胞(hUC-MSCs)对骨肉瘤Saos-2细胞增殖和迁移的作用及分子机制。方法: 组织块贴壁法分离培养hUC-MSCs,流式细胞术鉴定细胞表面标记物;CCK-8法和细胞计数法检测hUC-MSCs条件培养基(CM)、重组人白细胞介素6(rhIL-6)及IL-6中和抗体对Saos-2细胞增殖的作用;ELISA检测hUC-MSCs分泌IL-6的量;RT-PCR检测增殖相关基因增殖细胞核抗原(PCNA)、cyclinD1和survivin的转录水平;Transwell实验检测hUC-MSCs和Saos-2细胞迁移能力的变化。结果: hUC-MSCs可向Saos-2细胞迁移;hUC-MSCs-CM含有高浓度的IL-6,可达(1 835.5±134.1)ng/L;hUC-MSCs-CM和rhIL-6均能促进Saos-2细胞增殖和迁移,IL-6中和抗体能明显削弱hUC-MSCs-CM的促Saos-2细胞增殖和迁移作用;RT-PCR显示hUC-MSCs-CM和rhIL-6均能上调Saos-2细胞增殖相关基因PCNA、cyclinD1和survivin的表达,而IL-6中和抗体则削弱了这一作用。结论: 脐带间充质干细胞能向骨肉瘤Saos-2细胞迁移,并通过分泌IL-6促进其增殖和迁移。
[关键词]人脐带间充质干细胞; 白细胞介素6; 细胞增殖; 细胞迁移
人脐带间充质干细胞(human umbilical cord-derived mesenchymal stem cells,hUC-MSCs)是一类来源于胎儿脐带结缔组织,具有多向分化潜能,能向肿瘤、损伤、炎症等部位趋化,可分泌大量细胞因子的基质干细胞。hUC-MSCs作为MSCs的新生代表,不仅与其它来源的MSCs具有相似的生物学特性和免疫表型,而且具有更强的增殖效率和自我更新能力,来源丰富、伦理争议少,细胞含量丰富、微生物感染率低等优点更使其成为了组织工程和细胞工程最理想的种子细胞。目前,hUC-MSCs移植已成为系统性红斑狼疮[1]、贝克型肌营养不良[2]、糖尿病[3]等难治性疾病的有效治疗手段,并被用作肺癌[4]、卵巢癌[5]等实体瘤基因治疗的载体细胞,发挥抗肿瘤作用,Kalaszczynska等[6]更是将hUC-MSCs称为再生医学的未来。然而,hUC-MSCs与肿瘤的关系却错综复杂,Yang等[7]发现hUC-MSCs的条件培养基能明显抑制胶质瘤细胞的生长并介导其凋亡,而Li等[8]却提供证据表明hUC-MSCs能活化MCF-7和MDA-MB-231乳腺癌细胞的ERK信号通路,从而促进肿瘤的增殖和转移,可见,直接将hUC-MSCs作为肿瘤基因治疗的载体细胞存在较大安全隐患。因此,明确hUC-MSCs与相关肿瘤的关系无疑具有重大意义。骨肉瘤是最常见的骨组织的原发性恶性肿瘤,好发于长骨干骺端,恶性程度高,预后差,以局部高侵袭性和容易向骨、肺部转移为特点[9]。至今为止,hUC-MSCs与骨肉瘤的关系鲜有报道。
为明确hUC-MSCs对骨肉瘤细胞的作用,本实验采用骨肉瘤Saos-2细胞作为研究对象,运用CCK-8、细胞计数、Transwell、抗体中和实验、RT-PCR等方法研究hUC-MSCs对骨肉瘤体外增殖和转移能力的影响及可能的机制。
材料和方法
1标本来源
脐带标本取自九江市妇幼保健院,产妇及胎儿身体健康,营养状况良好。产妇及家属对实验均知情同意,并经医院伦理委员会批准。
2主要试剂与仪器
α-MEM培养基、胎牛血清、0.25%胰蛋白酶-EDTA消化液(Gibco);青、链霉素混合液、0.1% 结晶紫染色液(北京索莱宝科技有限公司);IgG-FITC、CD19-FITC、IgG-PE、CD29-PE、CD90-PE、CD105-PE单抗(Abcam);CCK-8(上海经科化学科技有限公司);重组人白细胞介素6(recombinant human interleukin-6, rhIL-6)酶联免疫吸附测定试剂盒(武汉伊莱瑞特生物科技有限公司);GREENspin细胞RNA快速提取试剂盒(北京庄盟国际生物基因科技有限公司);HiFi-MMLV cDNA逆转录试剂盒(北京康为世纪生物科技有限公司);2×Taq Master Mix(上海欣百诺生物科技有限公司);塑料培养皿、25 cm2塑料培养瓶、6孔板、96孔板、Transwell 24孔板(Corning);倒置相差显微镜 (Nikon);酶标仪(Biotek)。
3实验方法
3.1hUC-MSCs的分离培养及鉴定无菌取得脐带后机械剥离脐动、静脉,将华通氏胶剪碎成体积约3~5 mm3大小的组织块,接种于塑料皿中,加入含10% 胎牛血清的α-MEM培养基,置于5% CO2、饱和湿度培养箱中培养。待细胞融合达80%~90% 时,消化传代,接种于25 cm2塑料培养瓶内,继续以10% 胎牛血清的α-MEM常规贴壁培养,每3 d换液1次,细胞融合达80%~90% 时,按1∶3传代。倒置相差显微镜连续观察细胞形态及塑料贴壁能力。hUC-MSCs细胞传代至第5代,消化离心后加入IgG-FITC、CD19-FITC、IgG-PE、CD29-PE、CD90-PE、CD105-PE单抗孵育后用流式细胞仪检测。
3.2hUC-MSCs条件培养基(conditioned medium, CM)的制备第5代hUC-MSCs融合达80%后,更换无血清α-MEM,培养24 h后收集培养基,1 000×g离心20 min,0.22 μm过滤,加入10%胎牛血清即成hUC-MSCs-CM,-80 ℃冻存备用。
3.3CCK-8法和细胞计数法检测Saos-2细胞增殖情况CCK-8实验取Saos-2细胞按每孔1 000个接种于96孔板,每孔加入200 μL相关培养基,每组设置4个复孔。分别于相应时点取1块板,每孔加入10 μL CCK-8试剂后37 ℃ 孵育2 h,酶标仪测定其450 nm波长处吸光度(A)值;细胞计数实验取Saos-2细胞按每孔20 000个接种于12孔板,每孔加入2 mL相关培养基,每组设置4个复孔。分别于相应时点对各组细胞进行计数。
3.4ELISA检测hUC-MSCs-CM的IL-6浓度及抗体中和实验取第5代hUC-MSCs培养于T25细胞培养瓶内,待细胞融合达80%,PBS漂洗1遍,加入3 mL无血清α-MEM培养24 h,1 000×g离心20 min,0.22 μm过滤。以无血清α-MEM稀释2倍和3倍后按照IL-6 ELISA检测试剂盒使用说明书检测条件培养基中IL-6浓度。根据测定的IL-6浓度及其半数有效浓度,在hUC-MSCs-CM加入适量浓度的IL-6中和抗体以抑制IL-6的活性。
3.5Transwell迁移实验hUC-MSCs迁移实验:Transwell 24孔板,聚碳酸酯膜小孔的孔径为8 μm,在下室加入600 μL含1×105Saos-2细胞或无细胞培养基,上室均种入1×105hUC-MSCs;Saos-2细胞迁移实验:Saos-2细胞种入6孔板内,分别加入完全培养基、含20 μg/L rhIL-6的完全培养基、含或不含20 mg/L IL-6中和抗体的40% hUC-MSCs-CM预处理24 h,无血清α-MEM重悬细胞,调整细胞密度为1×109/L,上室每孔加入相应100 μL细胞悬液,下室均加入600 μL含10% 胎牛血清的α-MEM完全培养基。37 ℃培养12 h后行结晶紫染色,显微镜下随机选取5个视野计数穿过膜的细胞。
3.6RT-PCR检测增殖相关基因表达水平取Saos-2细胞种于6孔板,培养48 h后提取总RNA,逆转录为cDNA后进行PCR扩增。扩增后DNA样本用1% 琼脂糖凝胶电泳20~30 min。SIM凝胶成像系统扫描分析条带灰度值。总RNA的提取、逆转录、PCR反应均按试剂盒说明操作。内参照基因为β-actin,目的基因包括增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)、cyclinD1和survivin,引物序列及产物大小见表1。
4统计学处理
采用SPSS 19.0统计软件进行数据分析。所有实验均最少重复3次,数据采用均数±标准差(mean±SD)表示,组间比较采用单因素方差分析,两两比较采用 Scheffe 检验,计数资料采用非参数秩和检验。以P<0.05为差异有统计学意义。
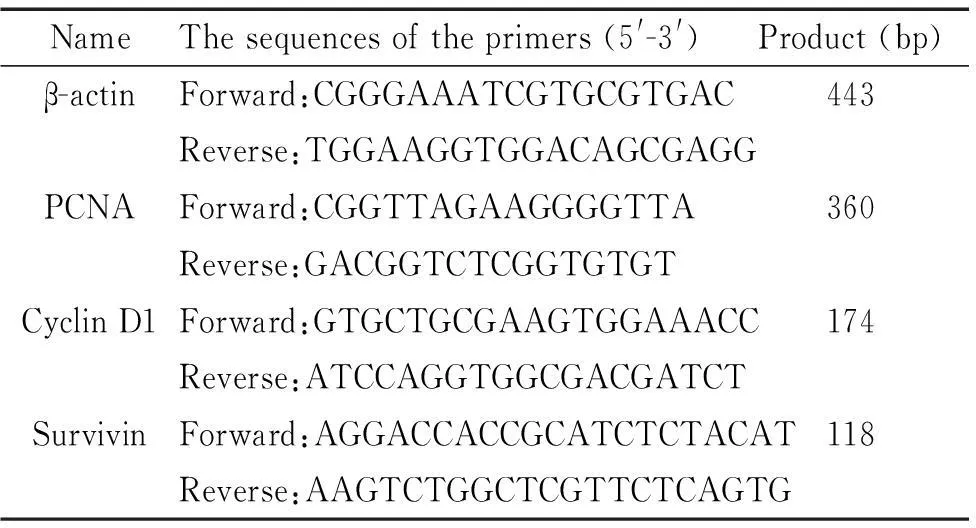
表1 引物序列
结果
1hUC-MSCs的培养及鉴定
脐带组织贴壁培养7 d后,镜下可见少量成纤维样细胞从组织块爬出,此后细胞逐渐增加,增殖迅速,14 d时,细胞融合达90% 以上。传代后细胞形态呈均一的长梭形或纺锤形,呈鱼群样或旋涡状分布。流式细胞表型检测结果显示,hUC-MSCs高表达CD29、CD90、CD105,阳性率均高于97%,CD19阳性率仅为0.1%,符合MSCs鉴定标准,见图1。

Figure 1.The flow cytometry results of cell surface markers on hUC-MSCs.
图1人脐带间充质干细胞表面标记物流式细胞术检测结果
2hUC-MSCs能向Saos-2细胞靶向迁移并通过旁分泌途径促进Saos-2细胞增殖
Transwell迁移实验发现hUC-MSCs能穿过聚碳酸酯膜小孔向培养了Saos-2细胞的下室迁移,而对照组仅有个别细胞穿膜(图2)。为了研究hUC-MSCs分泌的细胞因子对骨肉瘤Saos-2细胞的作用,本实验将Saos-2培养于不同浓度hUC-MSCs-CM,采用CCK-8法绘制其生长曲线并进行了准确的细胞计数。加入培养基后,对照组细胞较长时间处于潜伏期(4 d),20%、40% 和80% CM组细胞经过1~2 d的潜伏期后均开始增殖,20% CM组细胞增殖相对较慢,40% CM和80% CM组细胞增殖迅速,进入对数生长期。接种4 d后,各组细胞均呈对数生长。20% CM和40% CM组细胞均于第10天进入平台期,80% CM组细胞增殖迅速,提前进入平台期(9~11 d),对照组细胞增殖相对缓慢,对数期较长(4~11 d)。3~11 d时,20%、40% 和80% CM组A值均高于对照组,差异有统计学显著性(P<0.05),80%组高于40%组,40%组高于20%组,差异均有统计学显著性(P<0.05)。与CCK-8结果一致,细胞计数实验显示,在细胞接种的第3、6、9天,20%、40% 和80% CM组的细胞平均数量均高于对照组,且具有浓度依赖性,差异均有统计学显著性(P<0.05),见图3。
3hUC-MSCs分泌大量IL-6
ELISA检测发现hUC-MSCs-CM中IL-6的浓度可达(1 835.5±134.1)ng/L(8×105hUC-MSCs/24 h)。
4hUC-MSCs通过IL-6促进Saos-2细胞增殖
分别将5 μg/L、10 μg/L、20 μg/L的rhIL-6加入完全培养基中,CCK-8和细胞计数实验显示IL-6能明显促进Saos-2细胞的活力和增殖,且3种浓度对OS细胞的促增殖效果差异明显,呈现浓度依赖性(P<0.05)。40%的hUC-MSCs-CM促进Saos-2细胞增殖的作用显著,加入IL-6中和抗体后,这种促增殖作用受到明显抑制,见图4。RT-PCR检测发现20 μg/L的rhIL-6和40%的hUC-MSCs-CM均能上调Saos-2细胞增殖相关基因PCNA、cyclinD1和survivin的表达(P<0.05),而在CM中加入IL-6中和抗体后该3种基因的转录水平均出现了明显下调(P<0.05),见图5。

Figure 2.hUC-MSCs migrated to Saos-2 cells. A: complete medium was added to the lower chamber (control); B: complete medium suspended with Saos-2 cells was added to the lower chamber (Saos-2). Mean±SD.n=5.*P<0.05vscontrol.
图2hUC-MSCs向Saos-2细胞定向迁移
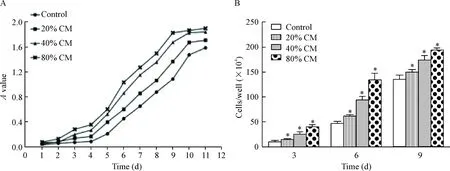
Figure 3.The conditioned medium from hUC-MSCs promoted the proliferation of Saos-2 cells. A: the growth curves of Saos-2 cells cultured with different concentration of conditioned medium of hUC-MSCs were detected by CCK-8; B: the effects of conditioned medium from hUC-MSCs on the proliferation of Saos-2 cells were detected by cell counting. Mean±SD.n=4.*P<0.05vscontrol.
图3hUC-MSCs条件培养基促进Saos-2细胞增殖

Figure 4.hUC-MSCs promoted the proliferation of Saos-2 cells by secreting IL-6. Saos-2 cells in different groups were cultured for 6 d and 9 d, cell proliferation was measured with CCK-8 assay (A) and cytometry (B). Mean±SD.n=4.*P<0.05vscontrol;#P<0.05vsCM.
图4hUC-MSCs分泌IL-6促进Saos-2细胞增殖
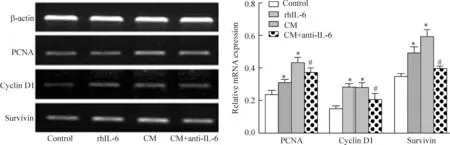
Figure 5.The relative expression of proliferation-related genes detected by RT-PCR.Mean±SD.n=3.*P<0.05vscontrol;#P<0.05vsCM.
图5RT-PCR检测增殖相关基因表达情况
5hUC-MSCs通过IL-6促进Saos-2细胞迁移
20 μg/L的rhIL-6及40% hUC-MSCs-CM分别作用于Saos-2细胞24 h后,细胞的迁移能力明显增强(P<0.05),CM+anti-IL-6组穿膜细胞数量明显比CM组少,且少于对照组细胞(P<0.05),见图6。
讨论
近年来,间充质干细胞与肿瘤的关系备受关注,然而间充质干细胞到底是促进还是抑制肿瘤的发生发展至今尚无定论。大量研究表明MSCs能向损伤、炎症部位定向迁移,分化成结缔组织成分,支持血管生成,分泌细胞因子和生长因子,促进愈合。肿瘤被喻为“不可愈合的损伤”,因而,MSCs对肿瘤细胞的作用可能与其促进伤口愈合的功能相似。MSCs对肿瘤的趋向性是其作为肿瘤基因治疗和细胞治疗的载体细胞最重要的特性[10]。研究表明,进入肿瘤组织的间充质干细胞参与形成肿瘤微环境,进而影响肿瘤的生长、迁移、血管形成等多种生物学行为[11]。间充质干细胞不仅能通过直接接触调节肿瘤细胞活性,还能分泌大量细胞因子、炎症因子调控肿瘤的一系列生物学过程。Matsuzuka等[12]将经过基因修饰高表达IFN-β的hUC-MSCs移植入细支气管肺泡癌

Figure 6.hUC-MSCs promoted the migration of Saos-2 cells by secreting IL-6. Mean±SD.n=4.*P<0.05vscontrol;#P<0.05vsCM.
图6hUC-MSCs分泌IL-6促进Saos-2细胞迁移
裸鼠模型体内,发现该hUC-MSCs能向肿瘤部位迁移并表达IFN-β抑制肿瘤的生长。此外,hUC-MSCs也被作为卵巢癌基因治疗的载体细胞,发挥了显著的抗肿瘤作用[7]。本研究通过Transwell迁移实验证明hUC-MSCs具有强大的向骨肉瘤Saos-2细胞迁移的能力,表明hUC-MSCs有可能成为骨肉瘤基因治疗的重要载体工具。然而进一步的研究却显示hUC-MSCs能通过分泌大量的细胞因子促进Saos-2细胞增殖和迁移,这就为hUC-MSCs应用于骨肉瘤的治疗带来了巨大的安全隐患。因此,有必要进一步研究hUC-MSCs促进骨肉瘤增殖的分子机制,为消除hUC-MSCs的促瘤因素提供依据,使hUC-MSCs尽早安全有效地用于骨肉瘤的治疗,无疑具有重大的临床意义。
IL-6与癌症的发生、发展高度相关,是参与肿瘤进程最重要炎性因子之一,多种恶性肿瘤能分泌大量IL-6,导致转录因子STAT3的持续性活化,进一步促进了IL-6表达上调,这种正反馈调节为肿瘤的生长提供了良好的微环境[13]。 Lin等[14]发现骨肉瘤组织IL-6的表达明显高于正常骨组织,进一步的研究表明IL-6能活化ICAM-1并促进骨肉瘤细胞的迁移。Tzeng等[15]也证明IL-6能活化ASK1通路进而上调血管内皮生长因子,促进骨肉瘤的血管形成。研究表明hUC-MSCs能分泌多种细胞因子和炎症因子,其中IL-6分泌量较高,可达(1 571.0±617.2)ng/L,远远超过骨髓间充质干细胞的(704.0±51.5)ng/L[16-17]。本研究通过ELISA检测发现hUC-MSCs分泌的IL-6高达(1 835.5±134.1)ng/L。为证实IL-6与Saos-2细胞增殖、迁移的关系,本研究将rhIL-6直接作用于Saos-2细胞,发现rhIL-6具有促进其增殖和迁移的作用;加入IL-6中和抗体后,hUC-MSCs-CM的促增殖和迁移作用明显下降,以上数据充分说明了hUC-MSCs能通过分泌IL-6促进Saos-2细胞增殖和迁移。Transwell迁移实验中,IL-6中和抗体预处理过的Saos-2细胞迁移能力明显低于对照组,表明IL-6可能是hUC-MSCs分泌的细胞因子中最主要的促进骨肉瘤转移的因素。进一步的RT-PCR检测发现rhIL-6和hUC-MSCs-CM均能促进Saos-2细胞的增殖相关基因PCNA、cyclinD1和survivin表达,IL-6中和抗体明显削弱了hUC-MSCs-CM对以上基因的上调作用。
综上,本研究表明hUC-MSCs能向骨肉瘤细胞定向迁移并分泌大量的IL-6;hUC-MSCs分泌的IL-6可能通过上调PCNA、cyclinD1和survivin的表达,促进骨肉瘤细胞增殖;hUC-MSCs分泌的IL-6能促进骨肉瘤细胞迁移。本实验明确了hUC-MSCs的促瘤因素,为其基因改造提供了依据,促进了骨肉瘤基因治疗的早日实现。
[参考文献]
[1]Shi D, Wang D, Li X, et al. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus[J]. Clin Rheumatol, 2012, 31(5):841-846.
[2]Li P, Cui K, Zhang B, et al. Transplantation of human umbilical cord-derived mesenchymal stem cells for the treatment of Becker muscular dystrophy in affected pedigree members[J]. Int J Mol Med, 2015, 35(4):1051-1057.
[3]Wang G, Li Y, Wang Y, et al. Roles of the co-culture of human umbilical cord Wharton′s jelly-derived mesenchymal stem cells with rat pancreatic cells in the treatment of rats with diabetes mellitus[J]. Exp Ther Med, 2014, 8(5):1389-1396.
[4]Zhang X, Zhang L, Xu W, et al. Experimental therapy for lung cancer: umbilical cord-derived mesenchymal stem cell-mediated interleukin-24 delivery[J]. Curr Cancer Drug Targets, 2013, 13(1):92-102.
[5]Zhang Y, Wang J, Ren M, et al. Gene therapy of ovarian cancer using IL-21-secreting human umbilical cord mesenchymal stem cells in nude mice[J]. J Ovarian Res, 2014, 7(8):1-10.
[6]Kalaszczynska I, Ferdyn K. Wharton′s jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance[J]. Biomed Res Int, 2015,2015:430847.
[7]Yang C, Lei D, Ouyang W, et al. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell linesinvitro[J]. Biomed Res Int, 2014, 2014:109389.
[8]Li T, Zhang C, Ding Y, et al. Umbilical cord-derived mesenchymal stem cells promote proliferation and migration in MCF-7 and MDA-MB-231 breast cancer cells through activation of the ERK pathway[J]. Oncol Rep, 2015, 34(3):1469-1477.
[9]Jaffe N. Adjuvant chemotherapy in osteosarcoma: an odyssey of rejection and vindication[J]. Cancer Treat Res, 2009, 152(11):219-237.
[10]Compte M, Nunez-Prado N, Sanz L, et al. Immunotherapeutic organoids: a new approach to cancer treatment[J]. Biomatter, 2013, 3(1):e23897.
[11]Hata N, Shinojima N, Gumin J, et al. Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas[J]. Neurosurgery, 2010, 66(1):144-157.
[12]Matsuzuka T, Rachakatla RS, Doi C, et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice[J]. Lung Cancer, 2010, 70(1):28-36.
[13]Chang Q, Daly L, Bromberg J. The IL-6 feed-forward loop: a driver of tumorigenesis[J]. Semin Immunol, 2014, 26(1):48-53.
[14]Lin YM, Chang ZL, Liao YY, et al. IL-6 promotes ICAM-1 expression and cell motility in human osteosarcoma[J]. Cancer Lett, 2013, 328(1):135-143.
[15]Tzeng HE, Tsai CH, Chang ZL, et al. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma[J]. Biochem Pharmacol, 2013, 85(4):531-540.
[16]Friedman R, Betancur M, Boissel L, et al. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation[J]. Biol Blood Marrow Transplant, 2007, 13(12):1477-1486.
[17]Lu LL, Liu YJ, Yang SG, et al. Isolation and characte-rization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials[J]. Haematologica, 2006, 91(8):1017-1026.
(责任编辑: 陈妙玲, 余小慧)
hUC-MSCs promote proliferation and migration of osteosarcoma cells by secreting IL-6HU Wen-long1, 2, WU Ping-ping1, GENG Shu-guo1, 2, WANG Jian-yang1, 2, YIN Ming2
(1MedicineGraduateSchool,2DepartmentofOrthopedics,TheSecondAffiliatedHospital,NanchangUniversity,Nanchang330006,China.E-mail:yinming0791@aliyun.com)
[ABSTRACT]AIM: To investigate the effects of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) on the proliferation and migration of osteosarcoma cells (Saos-2) and the underlying molecular mechanism. METHODS: hUC-MSCs were isolated and cultured by tissue explants adherent method. The cell surface markers on hUC-MSCs were identified by flow cytometry. The effects of conditioned medium (CM) from hUC-MSCs (hUC-MSCs-CM), recombinant human interleukin-6 (rhIL-6) and IL-6 neutralizing antibody on the proliferation of Saos-2 cells were detected by CCK-8 assay and cell counting. IL-6 secretion of hUC-MSCs was assayed by ELISA. RT-PCR was used to assess the transcription level of proliferation-related genes proliferating cell nuclear antigen (PCNA),cyclinD1 andsurvivin. The migration potential of hUC-MSCs and Saos-2 cells was measured by Transwell assay. RESULTS: hUC-MSCs migrated to Saos-2 cells. hUC-MSCs-CM contained a high concentration of IL-6, up to (1 835.5±134.1) ng/L. hUC-MSCs-CM and rhIL-6 promoted the proliferation and migration of Saos-2 cells. Addition of neutralizing antibody against IL-6 in the hUC-MSCs-CM impaired this proliferation and migration of Saos-2 cells. The mRNA expression ofPCNA,cyclinD1 andsurvivinwas up-regulated by hUC-MSCs-CM and rhIL-6, while this effect was dramatically attenuated by treatment with IL-6 neutralizing antibody. CONCLUSION: hUC-MSCs migrate to osteosarcoma cells and promote the proliferation and migration of osteosarcoma cells through secreting IL-6invitro.
[KEY WORDS]Human umbilical cord-derived mesenchymal stem cells; Interleukin-6; Cell proliferation; Cell migration
doi:10.3969/j.issn.1000- 4718.2016.02.002
[中图分类号]R329.2+1; R730.23
[文献标志码]A
通讯作者△Tel: 0792-86301236; E-mail: yinming0791@aliyun.com
*[基金项目]国家自然科学基金资助项目(No. 81160226)
[收稿日期]2015- 08- 10[修回日期] 2015- 12- 03
[文章编号]1000- 4718(2016)02- 0201- 07

