铁催化烯基环丙烷类化合物的三氟甲基化反应
李宗瑞, 邓清海
(上海师范大学 生命与环境科学学院,上海 200234)
铁催化烯基环丙烷类化合物的三氟甲基化反应
李宗瑞, 邓清海
(上海师范大学 生命与环境科学学院,上海 200234)
对铁催化的烯基环丙烷类化合物的自由基三氟甲基化反应进行了研究.以中等到良好的收率获得了含三氟甲基的二氢萘衍生物.方法具有反应条件温和,催化剂廉价易得,反应时间短等优点.同时对反应机理进行了初步研究.
铁催化; 三氟甲基化; 自由基反应; 二氢萘
0 前 言
由于三氟甲基(CF3)具有强吸电子性、亲脂性和稳定的C-F键等特性,将其引入到有机化合物中能够显著改变化合物的酸性、偶极距、极性、亲脂性以及其化学和代谢稳定性.因此含三氟甲基的化合物在医药、农药和材料等领域得到广泛应用[1].几十年来,化学家发展了各种不同类型的向有机分子引入三氟甲基的反应[2],但这些方法存在反应条件苛刻,原料难得及选择差等缺点.近几年来,在金属有机化学研究的推动下,三氟甲基化反应取得了重大突破[3-4].同时,对经典的通过三氟甲基自由基的反应开展了新的探索[5-6].
二氢萘类化合物在有机合成中是一类重要的主体框架,并且在很多具有生物活性的分子中代表一类重要骨架[7].近来,化学家发展了各种不同类型的方法合成此类化合物.但合成含有三氟甲基的二氢萘类化合物的方法报道较少.最近,本课题组报道了用FeCl2催化烯丙基环丙烷类化合物三氟甲基化合成含有三氟甲基的二氢萘类衍生物的方法[5].反应条件温和,有较高的产率(最高达到96%),且底物普适性较好.本文作者在此基础上进一步研究这一反应在一些芳杂环体系和特殊取代基体系的适用性,同时对反应机理进行了初步研究.
1 实验部分
1.1 仪器和试剂
核磁共振采用300,400和600 MHz NMR型核磁共振仪测定;红外光谱采用FI-IR红外光谱仪(KBr压片,4 000~400 cm-1)测定;X-单晶衍射采用Bruker SMART APEX II X-射线单晶衍射仪测定.
实验所需药品均为分析纯.溶剂二氯甲烷用CaH2回流干燥除水,四氢呋喃用钠和二苯甲酮回流干燥除水,柱层析用200~300目硅胶.
1.2 底物的合成
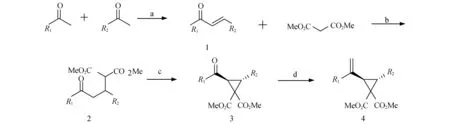
图1 底物4的合成(反应条件:a) NaOH(1eq),MeOH,30 ℃,10 h;b) K2CO3(1eq),MW 130 ℃,15 min;c) I2(1eq),DBU(2eq),EtOH,35 ℃,4 h;d) CH3Ph3PBr(1.5eq),n-BuLi(1.5eq),THF,Ar,RT.,18 h)
1.2.1 查尔酮的制备[8]
取一茄形瓶,加入搅拌子,后将酮加入其中,再加入溶剂甲醇,放在冰浴下搅拌10 min,边搅拌边加入NaOH,再将醛缓慢滴加入其中,恢复室温反应10 h后,向其中滴加稀盐酸溶液,将反应体系的pH调至6~7,后过滤得到固体产物查尔酮1,产率70%~90%.
1.2.2 化合物2的制备[9]
取一个微波反应器专用的反应管(25 mL),将反式查尔酮1与丙二酸二甲酯以及碳酸钾都加入反应管中,盖上塞子放入微波反应器中,调微波反应器的参数,时间为15 min,温度为130 ℃.将所得的反应物用二氯甲烷稀释,用二氯甲烷、乙醇重结晶(冻置),过滤得到固体化合物2,产率56%~75%.
1.2.3 化合物3的制备[10]
化合物2放入一个茄形瓶中,然后向其中加入乙醇作为溶剂,以及DBU和单质碘放入其中,将此混合物在35 ℃下搅拌4 h.待反应结束后,旋蒸除去大部分的乙醇,然后向其中60 mL的冷水,加完冷水后向其中缓慢加入Na2S2O3(边加边搅拌)直到棕色退去为止.用DCM萃取3次,合并有机相,无水硫酸钠干燥,减压蒸馏浓缩,柱层析纯化,得到了化合物3,产率45%~75%.
1.2.4 化合物4的制备[4]
取一个干燥的舒伦克圆底烧瓶,向里面加入搅拌子和甲基三苯基溴化磷,通入氩气,将反应瓶冰盐浴,再加入重蒸的四氢呋喃和正丁基锂,一起搅拌1 h后,再用少量四氢呋喃稀释化合物3,然后用注射器将其加入反应瓶中,后温度慢慢恢复至室温反应18 h.反应结束后用饱和氯化铵淬灭,然后用乙酸乙酯萃取,硫酸镁干燥除水,合并有机相,减压浓缩蒸馏,柱层析纯化,得到化合物4,产率为30%~65%.
1.3 铁催化偶联三氟甲基化反应的一般方法
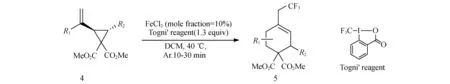
图2 铁催化三氟甲基化
取一个干燥的舒伦克管,将底物4,Togni试剂,FeCl2加入反应管,冲入氩气保护,加入干燥过的溶剂DCM,放入已经预先加热到40 ℃的油浴中搅拌10~30 min,反应完成后用饱和K2CO3水溶液洗,DCM萃取3次,合并有机相,旋蒸除去溶剂后柱层析分离提纯,得到目标产物5,产率17%~94%.
2 实验结果与讨论
2.1 三氟甲基化反应底物拓展
对不同类型的底物进行了三氟甲基化反应的研究(图3).4a两个苯基的底物能较好地进行反应,产率达到82%,4b,4c甲基在对位、间位的底物都能较好地进行反应,分别得到目标产物5b,5c且产率较高.但甲基在邻位的底物4d反应不能发生,可能是邻位有较大位阻的原因.带有硝基强吸电子基团的底物也能较好地进行这个反应,但5e在摩尔分数为10%的氯化亚铁催化下转化率只有23%,催化剂用量增加到70%(摩尔分数)后,产率达到76%,可能是因为底物或者产物对催化剂有一定的毒化作用.又对杂芳香环取代基的底物进行了研究,值得庆幸的是噻吩取代基的底物4g,4 h都能较好地进行这个反应,产率较为理想,但呋喃取代基不能较好地进行这个反应,反应体系比较复杂,可能是底物自身反应活性较高的原因.值得注意的是4k异丙基取代基的底物也可进行这个反应,且产率较高,4l底物虽然能进行这个反应,但产率只有17%.4m底物反应体系也比较复杂,未能得到目标产物.

图3 底物拓展(反应条件:底物4(0.2 mmol),Togni试剂(0.26 mmol),FeCl2(0.01 mmol)DCM(2 mL);产率为分离收率.)
2.2 反应区域选择性的确定
为了证明该反应有较好的区域选择性,以图3(5f)为参考进行分子结构研究,通过X-单晶衍射(图4)分析可知环丙烷结构是在C3和C5之间断裂.目前还不清楚有这么高的区域选择性断裂的原因,可能是FeCl2与两个酯基相互作用促进了C3-C5键的断裂.
2.3 反应机理研究
对三氟甲基化反应的机理进行了研究,设计的对照试验如图5所示,反应步骤:取一个干燥的舒伦克管,将底物4(0.2 mmol,1.0 equiv),Togni试剂(0.26 mmol,1.3 equiv),2,2,6,6-四甲基哌啶氧化物(TEMPO,0.26 mmol,1.3 equiv),FeCl2(0.02 mmol,0.1 equiv)加入反应管,冲入氩气保护,加入干燥过的溶剂DCM,放入已经预先加热到40 ℃的油浴中搅拌10 min,然后恢复至室温后加入三氟甲基苯(0.26 mmol)作为内标,通过氟谱(图6)分析未发现有目标产物5a生成,但得到TEMPO捕捉的化合物6(27%).由此表明反应很可能是自由基反应历程.
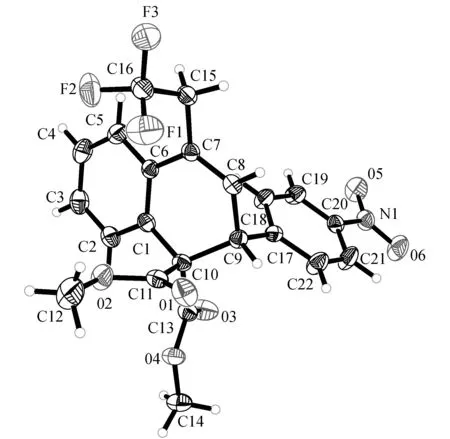
图4 5f X-单晶衍射图
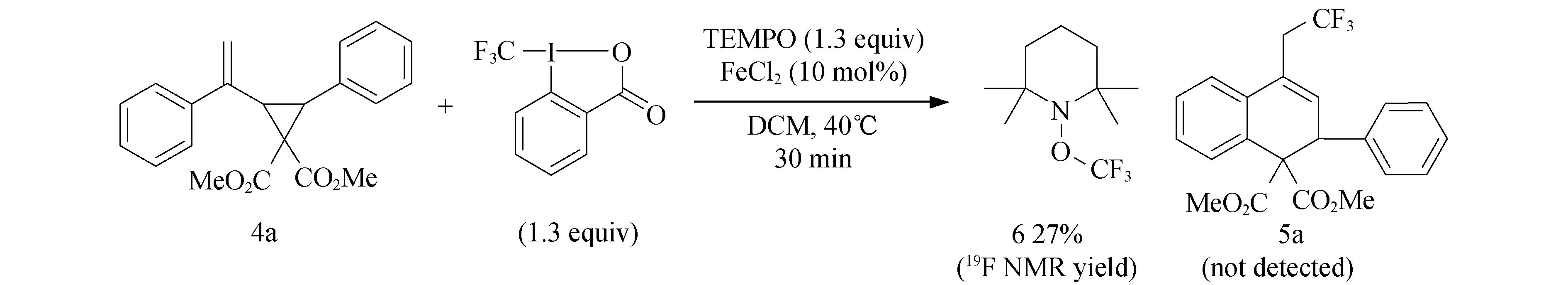
图5 自由基淬灭反应
3 数据表征
3.1 底物数据表征
图7 4a:无色油状物;1H NMR (400 MHz,CDCl3) δ 7.55~7.53 (m,2H),7.33~7.21 (m,8H),5.60 (s,1H),5.20 (s,1H),3.59 (d,J=8.4 Hz,1H),3.54 (d,J=8.4 Hz,1H),3.38 (s,3H),3.35 (s,3H).13CNMR (100 MHz,CDCl3) δ 167.2,167.0,141.1,139.2,134.7,128.7,128.4,128.1,128.0,127.6,126.0,115.1,52.6,52.5,44.9,35.2,34.9.IR (KBr):vmax2 952,1 731,1 453,1 249,1 027 cm-1.
图7 4b:无色油状物;1H NMR (400 MHz,CDCl3) δ 7.46 (d,J=8.4 Hz,2H),7.32~7.24 (m,5H),7.15 (d,J=8.4 Hz,2H),5.63 (s,1H),5.23 (s,1H),3.67 (d,J=8.4 Hz,1H),3.60 (d,J=8.4 Hz,1H),3.47 (s,3H),3.45 (s,3H),2.35 (s,3H);13C NMR (100 MHz,CDCl3) δ 167.2,167.0,140.9,137.7,136.4,134.7,129.1,128.7,128.4,127.5,125.9,114.1,52.6,52.5,44.9,35.1,34.9,21.2;IR (KBr):vmax2 952,1 730,1 435,1 242,1 018 cm-1.
图7 4c无色油状物;1H NMR (400 MHz,CDCl3) δ 7.37 (d,J=7.6 Hz,2H),7.31~7.28 (m,5H),7.22 (d,J=8.0 Hz,1H),7.11 (d,J=8.0 Hz,1H),5.66 (s,1H),5.26 (s,1H),3.66 (d,J=8.4 Hz,1H),3.61 (d,J=8.4 Hz,1H),3.47 (s,3H),3.46 (s,3H),2.36 (s,3H);13C NMR (100 MHz,CDCl3) δ 167.2,167.0,141.2,139.3,137.8,134.7,128.72,128.69,128.4,128.3,127.6,126.7,123.2,114.9,52.6,52.5,45.0,35.2,35.0,21.6;IR (KBr):vmax2 951,1 729,1 435,1 249,1 121 cm-1.
图3 4d无色油状物;1H NMR (600 MHz,CDCl3) δ 7.30~7.23 (m,5H),7.22~7.11 (m,4H),5.46 (s,1H),5.23 (s,1H),3.61 (s,3H),3.57 (d,J=9.0 Hz,1H),3.52 (d,J=9.0 Hz,1H),3.40 (s,3H),2.41 (s,3H);13C NMR (150 MHz,CDCl3) δ 167.1,166.9,142.9,140.9,135.0,134.6,130.4,128.6,128.3,127.44,127.43,1 125.6,118.6,52.5,52.4,45.3,35.8,35.7,20.6.
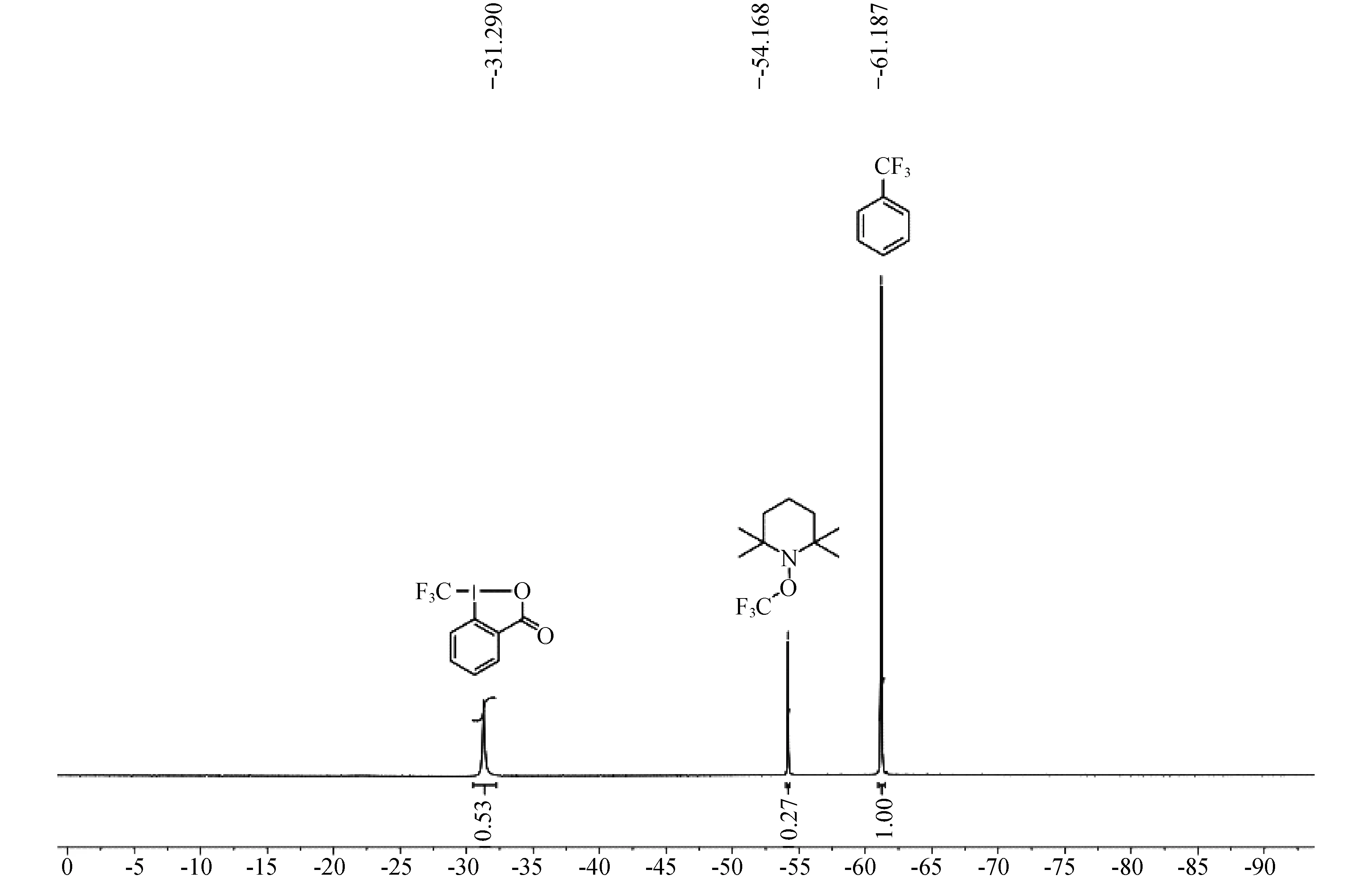
图6 自由基淬灭反应核磁图
图7 反应底物
图7 4e黄色固体;m.p.:110~113 ℃;1H NMR (300 MHz,CDCl3) δ 8.22 (d,J=9.0 Hz,2H),7.74 (d,J=9.0 Hz,2H),7.32~7.28 (m,5H),5.86 (s,1H),5.51 (s,1H),3.68 (d,J=8.4 Hz,1H),3.59 (d,J=8.4 Hz,1H),3.50 (s,3H),3.46 (s,3H).
图7 4f黄色固体;m.p.:100~102 ℃;1H NMR (400 MHz,CDCl3) δ 8.18 (d,J=8.8 Hz,2H),7.54 (d,J=6.8 Hz,2H),7.47 (d,J=8.8 Hz,2H),7.38~7.29 (m,3H),5.69 (s,1H),5.27 (s,1H),3.71 (d,J=8.4 Hz,1H),3.65 (d,J=8.4 Hz,1H),3.52 (s,3H),3.46 (s,3H);13C NMR (150 MHz,CDCl3) δ 166.7,166.4,147.4,142.4,140.6,138.9,129.7,128.5,128.2,126.0,123.7,115.4,53.0,52.8,45.5,35.2,34.5;IR (KBr):vmax2 923,1 729,1 436,1 294,1 143 cm-1.
图7 4g黄色油状物;1H NMR (400 MHz,CDCl3) δ 7.33~7.27 (m,5H),7.25 (dd,J=3.6,1.2 Hz,1H),7.18 (dd,J=5.2,1.2 Hz,1H),6.98 (dd,J=5.2,3.6 Hz,1H),5.62 (d,J=1.2 Hz,1H),5.16 (d,J=1.2 Hz,1H),3.68 (d,J=8.4 Hz,1H),3.61 (d,J=8.4 Hz,1H),3.53 (s,3H),3.48 (s,3H).
图7 4 h黄色固体;m.p.:58~60 ℃;1H NMR (600 MHz,CDCl3) δ 7.55 (d,J=7.2 Hz,2H),7.35~7.33 (m,2H),7.30~7.27 (m,1H),7.20~7.19 (m,1H),6.94~6.93 (m,1H),5.68 (d,J=0.8 Hz,1H),5.29 (d,J=0.8 Hz,1H),3.60 (q,J=0.8 Hz,1H),3.58 (d,J=0.8 Hz,1H),3.58 (s,3H),3.43 (s,3H);13C NMR (150 MHz,CDCl3) δ 166.9,166.6,140.7,139.0,137.9,128.5,128.1,126.9,126.5,126.0,125.2,115.4,52.9,52.6,45.5,36.8,30.1;IR (KBr):vmax2 924,1 730,1 435,1 294,1 118 cm-1.
图3 4i无色油状物;1H NMR (600 MHz,CDCl3) δ 7.56 (d,J=7.2 Hz,2H),7.35~7.33 (m,3H),7.29 (d,J=7.2 Hz,1H),6.31 (q,J=1.8 Hz,1H),6.20 (q,J=3.6 Hz,1H),5.68 (s,1H),5.27 (s,1H),3.62 (s,3H),3.54 (s,2H),3.44 (s,3H).
图3 4j黄色油状物;1H NMR (400 MHz,CDCl3) δ 7.37 (d,J=1.2 Hz,1H),7.33~7.25 (m,5H),6.53 (d,J=3.2 Hz,1H),6.38 (q,J=1.6 Hz,1H),5.74 (s,1H),5.17 (s,1H),3.65 (d,J=8.4 Hz,1H),3.57 (s,3H),3.48 (d,J=8.4 Hz,1H),3.47 (s,3H).
图7 4k无色油状物;1H NMR (400 MHz,CDCl3) δ 7.50 (d,J=3.2 Hz,2H),7.33~7.25 (m,3H),5.56 (s,1H),5.12 (s,1H),3.81 (s,3H),3.35 (s,3H),2.89 (d,J=8.0 Hz,1H),2.16 (dd,J=10.4,8.0 Hz,1H),1.37~1.31 (m,1H),1.11 (d,J=6.4 Hz,3H),1.01 (d,J=6.4 Hz,3H);13C NMR (100 MHz,CDCl3) δ 168.9,167.5,141.9,139.5,128.3,127.8,126.0,114.5,52.7,52.3,42.6,39.1,36.7,28.2,22.4,21.5;IR (KBr):vmax2 956,1 728,1 436,1 293,1 152 cm-1.
图7 4l无色油状物;1H NMR (300 MHz,CDCl3) δ 7.50 (dd,J=8.7,1.2 Hz,2H),7.34~7.25 (m,3H),5.54 (s,1H),5.06 (s,1H),4.30~4.19 (m,2H),4.00~3.84 (m,2H),2.98 (q,J=9.0 Hz,1H),2.02 (dd,J=8.1,4.8 Hz,1H),1.58 (dd,J=5.7,4.8 Hz,1H),1.29 (q,J=7.2 Hz,3H),0.93 (q,J=7.2 Hz,3H);13C NMR (75 MHz,CDCl3) δ 169.9,166.6,141.3,139.8,128.2,127.8,126.1,113.9,61.7,61.3,37.2,31.7,18.3,14.1,13.8;IR (KBr):vmax3 057,2 982,1 724,1 317,1 026 cm-1.
图3 4 m黄色油状物;1H NMR (400 MHz,CDCl3) δ 7.37 (d,J=1.2 Hz,1H),7.33~7.25 (m,5H),6.53 (d,J=3.2 Hz,1H),6.38 (q,J=1.6 Hz,1H),5.74 (s,1H),5.17 (s,1H),3.65 (d,J=8.4 Hz,1H),3.57 (s,3H),3.48 (d,J=8.4 Hz,1H),3.47 (s,3H).
3.2 三氟甲基化产物数据表征
图3 5a收率:82%;白色固体;m.p.:93~94 ℃;1H NMR (400 MHz,CDCl3):δ 7.55 (d,J=7.6 Hz,1H),7.38~7.35 (m,2H),7.33~7.31 (m,1H),7.20~7.15 (m,3H),7.01 (dd,J=8.0,1.6 Hz,2H) 6.26 (d,J=6.0 Hz,1H),4.56 (d,J=6.0 Hz,1H),3.66 (s,3H),3.48 (s,3H),3.41~3.32 (m,1H),3.28~3.17 (m,1H);13C NMR (100 MHz,CDCl3):δ 170.1,169.2,136.8,133.6,132.8,131.8,130.1,129.1,128.6,128.5,128.3,128.1,125.9 (q,J=276 Hz),124.8 (q,J=2.9 Hz),123.3,63.6,53.3,52.4,46.6,36.8 (q,J=29.8 Hz);19F NMR (376 MHz,CDCl3):δ-64.27 (t,J=10.2 Hz);IR (KBr):vmax2 954,1 736,1 647,1 384 cm-1.
图3 5b收率:85%;白色固体;m.p.:156~158 ℃;1H NMR (400 MHz,CDCl3):δ 7.34 (s,1H),7.25~7.14 (m,5H),7.02~6.99 (m,2H),6.18 (d,J=6.0 Hz,1H),4.51 (d,J=6.0 Hz,1H),3.66 (s,3H),3.49 (s,3H),3.41~3.30 (m,1H),3.24~3,13 (m,1H),2.35 (s,3H);13C NMR (150 MHz,CDCl3):δ 170.1,169.3,138.1,137.1,132.5,132.4,130.1,129.7,129.2,129.1,128.4,127.9,125.9 (q,J=276 Hz),124.7 (q,J=2.7 Hz),123.2,63.6,53.2,52.3,46.7,36.8 (q,J=29.7 Hz),21.6;19F NMR (564 MHz,CDCl3):δ-63.29 (t,J=10.2 Hz);IR (KBr):vmax2 953,1 737,1 601,1 454,1 256,1 174,1 133,1 094 cm-1.
图3 5c收率:93%;白色固体;m.p.:126~128 ℃;1H NMR (400 MHz,CDCl3):δ 7.44 (d,J=8.0 Hz,1H),7.20~7.12 (m,5H),7.02(d,J=6.4 Hz,2H),6.24 (d,J=6.0 Hz,1H),4.51 (d,J=6.0 Hz,1H),3.66 (s,3H),3.47 (s,3H),3.40~3.31 (m,1H),3.28~3,19 (m,1H),2.40 (s,3H);13C NMR (150 MHz,CDCl3):δ 170.2,169.4,138.3,137.0,133.5,132.6,131.7,129.1,129.0,128.5,128.0,126.8,126.0 (q,J=276 Hz),124.8 (q,J=2.8 Hz),124.1,63.3,53.3,52.3,46.6,36.8 (q,J=29.9 Hz),21.6;19F NMR (282 MHz,CDCl3):δ-64.66 (t,J=10.4 Hz);IR (KBr):vmax2 954,1 737,1 494,1 434,1 237,1 200,1 134,800 cm-1.
图3 5e收率:76%;黄色固体;m.p.:158~160 ℃;1H NMR (600 MHz,CDCl3):δ 8.02 (d,J=9.0 Hz,2H),7.48 (d,J=7.8 Hz,1H),7.43~7.38 (m,2H),7.35 (t,J=7.2 Hz,1H),7.18 (d,J=9.0 Hz,2H),6.22 (d,J=6.0 Hz,1H),4.66 (d,J=6.0 Hz,1H),3.67 (s,3H),3.55 (s,3H),3.42~3.34 (m,1H),3.29~3,21 (m,1H);13C NMR (150 MHz,CDCl3):δ 168.9,168.2,147.3,138.7,137.9,135.1,131.5,128.9,128.8,128.6,127.5,125.6 (q,J=278 Hz),124.0,123.9 (q,J=3.0 Hz),123.89,63.1,53.8,52.9,46.4,37.0 (q,J=30.4 Hz),29.8;19F NMR (376 MHz,CDCl3):δ-63.45 (t,J=10.5 Hz).
图3 5f收率:94%;黄色固体;m.p.:158~160 ℃;1H NMR (600 MHz,CDCl3):δ 8.02 (d,J=9.0 Hz,2H),7.48 (d,J=7.8 Hz,1H),7.43~7.38 (m,2H),7.35 (t,J=7.2 Hz,1H),7.18 (d,J=9.0 Hz,2H),6.22 (d,J=6.0 Hz,1H),4.66 (d,J=6.0 Hz,1H),3.67 (s,3H),3.55 (s,3H),3.42~3.34 (m,1H),3.29~3,21 (m,1H);13C NMR (100 MHz,CDCl3):δ 169.7,169.0,147.6,144.8,132.4,132.0,131.4,130.8,129.8,129.1,129.0,126.5 (q,J=2.7 Hz),125.8 (q,J=276 Hz),123.64,123.63,63.5,53.5,52.7,46.1,36.8 (q,J=29.9 Hz);19F NMR (282 MHz,CDCl3):δ-63.27 (t,J=9.0 Hz);IR (KBr):vmax2 955,1 737,1 522,1 491,1 349,1 256,1 182,1 135 cm-1.
图3 5g收率:65%;黄色固体;m.p.:87~90 ℃;1H NMR (400 MHz,CDCl3):δ 7.25~7.22 (m,2H),7.21~7.17 (m,3H),7.07~7.05 (m,2H),6.01 (d,J=6.0 Hz,1H),4.59 (d,J=6.0 Hz,1H),3.68 (s,3H),3.42 (s,3H),3.23~3.10 (m,2H);19F NMR (282 MHz,CDCl3):δ-64.9 (t,J=10.4 Hz).
图3 5h收率:90%;白色固体;m.p.:87~89 ℃;1H NMR (600 MHz,CDCl3):δ 7.57 (d,J=7.8 Hz,1H),7.41~7.32 (m,3H),7.07 (d,J=5.4 Hz,1H),6.83~6.82 (m,1H),6.75 (d,J=3.0 Hz,1H),6.32 (d,J=6.0 Hz,1H),4.87 (d,J=6.0 Hz,1H),3.66 (s,3H),3.64 (s,3H),3.39~3.31 (m,1H),3.24~3.16 (m,1H);13C NMR (150 MHz,CDCl3):δ 169.7,168.9,139.4,133.6,132.6,131.3,130.0,128.7,128.4,127.1,126.5,125.8 (q,J=276 Hz),125.7,125.0 (q,J=2.7 Hz),123.1,64.0,53.3,52.7,41.8,36.7 (q,J=29.7 Hz);19F NMR (564 MHz,CDCl3):δ-64.23(t,J=10.2 Hz);IR (KBr):vmax2 954,1 737,1 434,1 255,1 182,1 133,1 109,1 028 cm-1.
图3 5k收率:90%;无色油状;1H NMR (600 MHz,CDCl3):δ 7.42~7.40 (m,1H),7.12~7.09 (m,2H),7.05 (d,J=7.8 Hz,1H),5.93 (d,J=6.0 Hz,1H),3.64 (s,3H),3.42 (s,3H),3.14 (dd,J=6.0,4.2 Hz,1H),3.11~3.02 (m,2H),1.62~1.64 (m,1H),0.81 (d,J=6.6 Hz,3H),0.33 (d,J=6.6 Hz,3H);13C NMR (150 MHz,CDCl3):δ 170.4,170.0,133.0,131.5,131.2,130.3,128.2,127.8,126.6 (q,J=2.7 Hz),126.0 (q,J=276 Hz),123.0,62.3,53.1,52.6,44.6,36.9 (q,J=29.7 Hz),31.4,21.6,16.8;19F NMR (564 MHz,CDCl3):δ-64.70(q,J=10.7 Hz);IR (KBr):vmax2 959,1 736,1 435,1 256,1 225,1 189,1 134,1 104 cm-1.
图3 5l收率:17%;无色油状;1H NMR (400 MHz,CDCl3):δ 7.36~7.33 (m,1H),7.29 (d,J=7.6 Hz,2H),7.19 (d,J=7.6 Hz,1H),6.11 (t,J=4.4 Hz,1H),4.29~4.16 (m,4H),3.23 (q,J=10.4 Hz,2H),3.03 (d,J=4.4 Hz,2H),1.23 (t,J=7.2 Hz,6H);13C NMR (100 MHz,CDCl3):δ 170.9,133.0,132.9,129.0,128.3,128.0,127.41,127.34 (q,J=2.9 Hz),125.8 (q,J=276 Hz),123.4,62.1,59.3,36.8 (q,J=29.7 Hz),30.8,14.1;19F NMR (376 MHz,CDCl3):δ-64.68 (t,J=10.5 Hz);IR (KBr):vmax1 927,1 726,1 628,1 285,1 183 cm-1.
4 结 论
研究了铁催化的烯基环丙烷类化合物的三氟甲基化反应.以商用便宜的FeCl2为催化剂,Togni试剂为三氟甲基化试剂,高区域选择性地获得了一系列三氟甲基取代的二氢萘衍生物.初步的机理研究表明反应为自由基反应历程.
[1] Wang J,Sánchez-Roselló M,Acea J L,et al.Fluorine in pharmaceutical industry:Fluorine-containing drugs introduced to the market in the last decade (2001~2011) [J].Chemical Reviews,2014,114(4):2432-2506.
[2] Ma J A,Cahard D.Update 1 of:Asymmetric fluorination,trifluoromethylation,and perfluoroalkylation reactions [J].Chemical Reviews,2008,108(9):PR1-PR43.
[3] Wang F,Zhu N,Chen P,et al.Copper-catalyzed trifluoromethylazidation of alkynes:Efficient access to CF3-substituted azirines and aziridines [J].Angewandte Chemie International Edition,2015,54(32):9356-9360.
[4] Tomashenko O A,GrushinV V.Aromatic trifluoromethylation with metal complexes [J].Chemical Reviews,2011,111(8):4475-4521.
[5] Zong R L,Xing X B,Qing H D,et al.Iron-catalyzed trifluoromethylation of vinylcyclopropanes:facile synthesis of CF3-containing dihydronaphthalene derivatives [J].Organic Chemistry Frontiers,2016,3(8):934-938.
[6] Zhu Z Z,Chen Kai,Yu L Z,et al.Copper(I)-catalyzed intramolecular trifluoromethylation of methylenecyclopropanes [J].Organic Letters,2015,17(24):5994-5997.
[7] Pape A R,Kaliappan K P,Kündig E P.Transition-metal-mediated dearomatization reactions [J].Chemical Reviews,2000,100(8):2917-2940.
[8] George R F,Fouad M A,Gomaa I E O.Synthesis and cytotoxic activities of some pyrazoline derivatives bearing phenyl pyridazine core as new apoptosis inducers [J].European Journal of Medicinal Chemistry,2016,112:48-59.
[9] Yang G S,Sun Y X,Shen Y.Cis-2,3-disubstituted cyclopropane 1,1-diesters in[3+2]annulations with aldehydes:highly diastereoselective construction of densely substituted tetrahydrofurans [J].Journal of Organic Chemistry,2013,78(11):5393-5400.
[10] Miao C B,Zhang M,Tian Z Y.Base-controlled selective conversion of michael adducts of malonates with enones in the presence of iodine [J].Journal of Organic Chemistry,2011,76(23):9809-9816.
(责任编辑:包震宇,郁 慧)
Iron(II)-catalyzed trifluoromethylation of vinylcyclopropanes
LI Zongrui, DENG Qinghai
(College of Life and Environmental Sciences,Shanghai Normal University,Shanghai 200234,China)
An efficient iron(II)-catalyzed trifluoromethylation of vinylcyclopropanes was developed.A series of CF3-containing dihydronaphthalene derivatives were prepared with a moderate to high yield.This method offers several advantages in terms of its mild condition,readily available and cheap catalyst,as well as short reaction time.Furthermore,the reaction mechanism was investigated preliminarily.
iron(II)-catalyzed; trifluoromethylation; radical reaction; dihydronaphthalene
2016-09-22
国家自然科学基金(21402119);上海高校特聘教授 (东方学者) 岗位计划资助;上海市青年科技启明星计划(16QA1403100).
邓清海,中国上海市徐汇区桂林路100号,上海师范大学生命与环境科学学院,邮编:200234,E-mail:qinghaideng@shnu.edu.cn
O 625.15
A
1000-5137(2016)06-0655-08

