FeCl3 Catalyzed Carbon-Carbon Bond Formation by Nucleophilic Substitution of Hydroxy Groups
BIAN Yongjun,QU Xingyu,LI Jun,LI Jianqing
(Department of Chemistry and Chemical Engineering,Jinzhong University,Jinzhong 030619,China)
N-acyliminium ions as the highly reactive electro⁃philic species were applied intensively in the organic synthesis to introduce some substituents at the α-car⁃bon of an amine[1-4],which are commonly generated in si⁃tu from α-haloalkyl-,α-hydroxyalkyl-,α-alkoxyal⁃kyl-due to their limited stability and high reactivity[5].In addition,the γ-hydroxy lactams could generatedNacyliminium ions through the activation of the hydroxy group as well[6].They are very useful materials in medic⁃inal and pharmaceutical chemistry,substituted prod⁃ucts of hydroxy group of which could form γ-substitut⁃ed amino acid with highly biological activity through the simply hydrolysis[7].For the preparation of theN-ac⁃yliminium ions,some classical Lewis acids(BF3·Et2O,TiCl4,ZnBr2,or SnCl4)are routinely used,and yet their application are also limited due to the sensitive to mois⁃ture or the stoichiometric amounts be required to achieve synthetically useful results.So,the develop⁃ment of compatible processes has been highly desired.Recently,some new catalysts were explored inN-acyli⁃minium ions chemisty.For example,in 2007,Jacobsen and co-workers described that thiourea catalyzed cycli⁃zations through intramolecular nucleophilic additions of indole toN-acyliminium ions[8].Subsequently,they also reported that thiourea catalyzed cyclizations of pyrroles ontoN-acyliminium ions[9].In 2010,Dalla and co-work⁃ers discovered that Sn(NTf2)4could activate the hy⁃droxy of γ-hydroxy lactams to acquire theN-acylimini⁃um ion which was subjected to silyl enol ethers or ac⁃tive methylene derivatives to form the new carbon-car⁃bon bond[10].In 2011,Graham and co- workers achieved a Ni(0)catalyst facilitated efficient C-C bond formation betweenN-acyliminium ions derived from quinoline and isoquinoline with aryl boronic acids[11].Phosphoric acid catalyzed nucleophilic addition toN-acyliminium ions from Tertiary hydroxy lactams us⁃ing indoles as the nucleophilic reagents was reported as well by Rueping and co-workers[12].The reaction need⁃ed long reaction time and only afforded moderate yields.Iron as a kind of cheaper and nontoxic catalyst has attracted more attention[13-14].Herein,we wondered to explore an efficient iron-catalyzed nucleophilic addi⁃tion toN-acyliminium ions by activating C-O bond.
1 Results and discussion
As a starting point,we chose 5-hydroxy-1-p-tol⁃ylpyrrolidin-2-one 1a and indole 2a as standard sub⁃strates to investigate suitable reaction conditions for the desired substitution reaction of hydroxy group.

Condition:1a(0.50 mmol),2a(0.55 mmol),metal cat.(0.025 mmol,5 mol%),solvent(2.0 mL),25℃,5 min.

Tab.1 Optimization of the reaction condition of 5-Hy⁃droxy-1-p-tolylpyrrolidin-2-one(1a)with Indole(2a)a表1 对甲基苯基-5-羟基丁内酰胺(1a)与吲哚(2a)反应条件的优化
Gratifyingly,the desired substituted product 3aa was obtained in 87% yield when FeCl2was used as a catalyst in CH2Cl2during 5 min(Table1,entry 1).Sub⁃sequently,various Fe(III)salts were examined in the reaction(Table1,entries 2- 6).Among them,FeCl3showed the best catalytic activity and provided the de⁃sired product 3aa in 98%yield(Table1,entry 4).More⁃over,when the other strong Lewis acids such as SnCl4or BF3·OEt2were used as the catalysts,only the moder⁃ate yields 3aa was afforded(Table1,entries 7-8).Di⁃chloromethane was the solvent of choice;CH3CN,DCE,and THF afforded unsatisfactory results(Table1,entries 9-11).In addition,the amount of FeCl3was reduced from 5 mol% to 1 mol%,the catalytic result was hardly changed(Table1,compare entry 4 with entry 12).Nota⁃bly,in the absence of an iron catalyst,no desired prod⁃uct 3aa was obtained(Table1,entry 13).
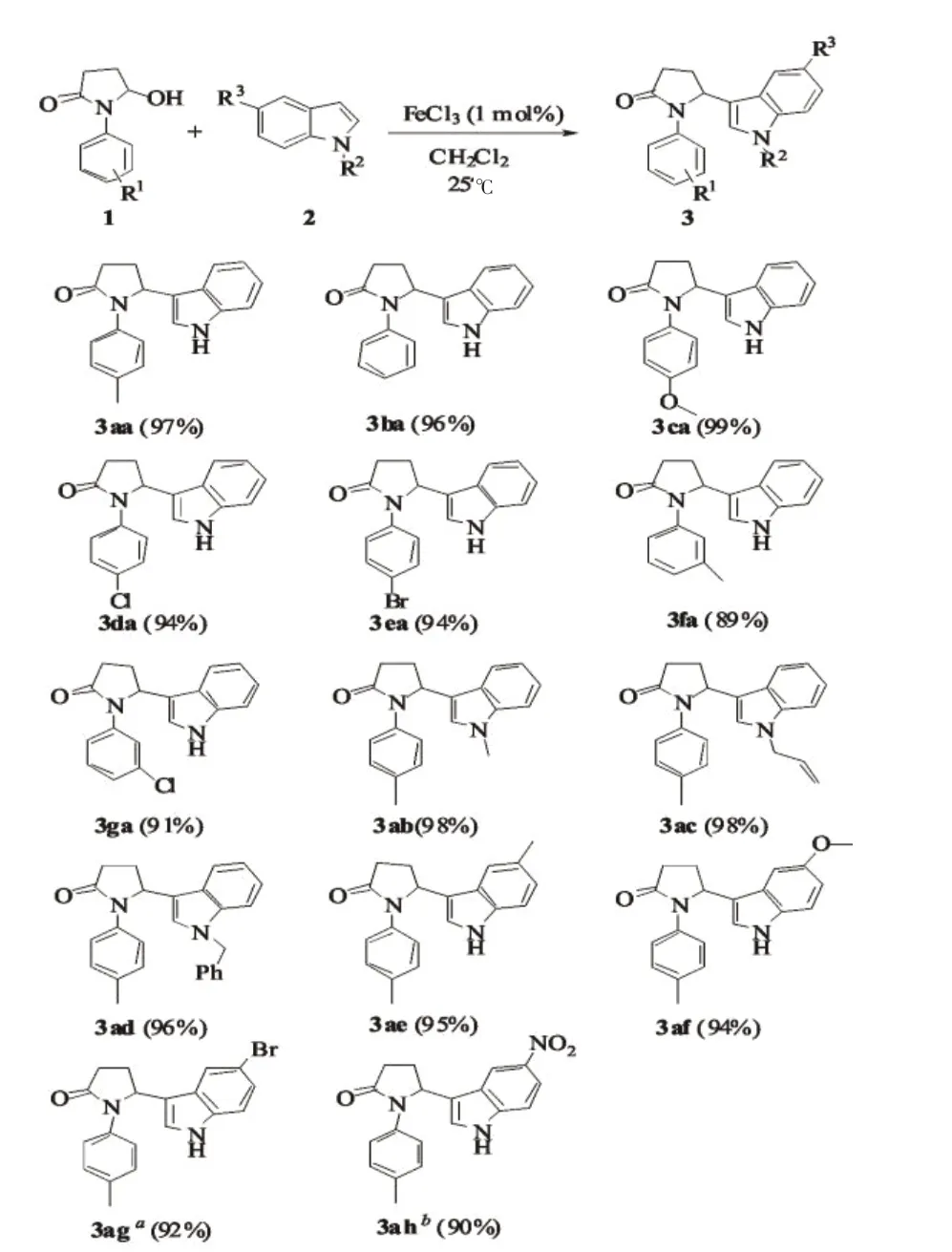
图1 三氯化铁催化的亲核取代反应Fig.1 FeCl3-Catalyzed nucleophilic substitution for the synthesis of the desired products 3
We then assessed the scope and limitations of γhydroxy lactams 1 and indoles 2.The results showed that all reactions could proceed in mild conditions to give the desired products 3 in high yields(Fig 1).Our experiment also demonstrated that the reaction could tolerate various functional groups,such as methoxyl,methoxyl,chloro,bromo,and nitro groups.All substitu⁃tions,no matter what electon-donating groups or ele⁃tron-withdrawing groups,had no obvious effects on the yields of the reaction.Only the reaction time was de⁃layed,when the strong electon-donating groups was used as the substitution(Fig 1,3ag and 3ah).In addi⁃tion,N-protecting groups such as methyl,allyl and ben⁃zyl did not have a significant impact on the reaction(Fig 1,3ab-3ad).

图2 N-苯基-5-羟基丁内酰胺与吲哚的放大反应Fig.2 The substitution reaction of 1b with 2a can be scaled up
Under mild conditions,the reaction could be scaled up(Fig 2;10.0 mmol of 5-hydroxy-1-phenyl⁃pyrro lidin-2-one 1b in the presence of 11.0 mmol of free indole 2a and 0.1 mmol of FeCl3in 40 mL of CH2Cl2).The reaction was completed after stirring for 10 min at room temperature.The product 3ba was puri⁃fied by flash chromatography on silica gel(EA/PE as an eluent)and isolated in 91% yield.The transforma⁃tion is amenable to large scale production.
2 Experimental
2.1 General Remarks
1H NMR and13C NMR spectra were recorded on Bruker ARX-300 or 500 MHz spectrometer with TMS as internal standard.ESI-MS spectra were measured on Thermo Fisher Scientific LCQ FLEET mass spectrome⁃ter.The following abbreviations are used to indicate the multiplicity:s,singlet;d,doublet;dd,double of dou⁃blets;t,triplet;br,broad;m,multiplet.
2.2 Typical Experiment Procedure
A mixture of 1(0.50 mmol),indoles 2(0.55 mmol)and FeCl3(0.005 mmol,1 mol%)in CH2Cl2was stirred at room temperature for 5 min(as for 2b and 2h,the re⁃action time are 30 min and 90 min,respectively).The crude mixture was purified by column chromatography(silica gel,EtOAc/petroleum ether or MeOH/CH2Cl2as eluent)to afford the desired products 3.
2.2.1 5-(1H-indol-3-yl)-1-p-tolylpyrrolidin-2-one 3aa
White solid;m.p.209-210℃;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶8.05 (br s,1 H,-NH),7.63 (d,J=7.8 Hz,1 H),7.37-7.34 (m,3 H),7.25-7.12 (m,2 H),7.02 (d,J=8.4 Hz,2 H),6.96 (d,J=2.4 Hz,1 H),5.56(dd,J=7.5,4.2 Hz,1 H),2.84-2.74(m,1 H),2.69-2.59(m,2 H),2.32-2.24(m,1 H),2.23(s,3 H,-CH3);13C NMR (CDCl3,75 MHz)∶δ (ppm)∶174.8,136.8,135.8,134.7,129.2,125.2,122.43,122.41,122.2,119.8,118.8,115.8,111.6,58.0,31.6,27.2,20.9;MS (ESI)m/z[M+Na]+Calcd for C19H18N2NaO∶313.13,found∶313.25.
2.2.2 5-(1H-indol-3-yl)-1-phenylpyrrolidin-2-one 3ba
oil;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶8.46 (br s,1 H,-NH),7.62 (d,J=7.8 Hz,1 H),7.51-7.48 (m,2 H),7.32 (d,J=7.8 Hz,1 H),7.24-7.12 (m,4 H),7.03(t,J=7.5 Hz,1 H),6.86 (d,J=2.4 Hz,1 H),5.60-5.56(m,1 H),2.87-2.74(m,1 H),2.69-2.54(m,2 H),2.32-2.22 (m,1 H);13C NMR (CDCl3,75 MHz)∶δ (ppm)∶175.0,138.4,136.8,128.6,125.1,125.0,122.39,122.36,122.3,119.8,118.7,115.4,111.7,58.0,31.7,27.5;MS(ESI)m/z[M+H]+Calcd for C18H16N2O∶276.13,found∶276.17.
2.2.3 5-(1H-indol-3-yl)-1-(4-methoxyphenyl)pyr⁃rolidin-2-one 3ca
White solid;m.p.173-174 ℃;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶8.21 (br s,1 H,-NH),7.62 (d,J=7.8 Hz,1 H),7.35-7.30 (m,3 H),7.24-7.11 (m,2 H),6.94(d,J=2.1 Hz,1 H),6.75-6.70(m,2 H),5.51-5.47(m,1 H),3.69 (s,3 H,-OCH3),2.87-2.75 (m,1 H),2.71-2.55 (m,2 H),2.36-2.24 (m,1 H);13C NMR(DMSO,75 MHz)∶δ (ppm)∶174.0,156.6,137.2,131.8,125.5,125.1,124.3,124.2,121.7,119.3,119.0,115.1,113.8,112.3,57.4,55.4,31.7,27.5; MS (ESI)m/z[M+Na]+Calcd for C19H18N2NaO2∶329.13,found∶329.33.
2.2.4 1-(4-chlorophenyl)-5-(1H-indol-3-yl)pyrro⁃lidin-2-one 3da
oil;1H NMR (DMSO,300 MHz)∶δ (ppm)∶10.95(br s,1 H,-NH),7.52-7.45 (m,3 H),7.32 (d,J=7.5 Hz,1 H),7.24-7.22 (m,3 H),7.05 (t,J=7.5 Hz,1 H),6.96 (t,J=7.5 Hz,1 H),5.66 (t,J=6.6 Hz,1 H),2.75-2.52(m,3 H),2.18-2.09(m,1 H);13C NMR(DMSO,75 MHz)∶δ (ppm)∶174.4,137.7,137.2,128.8,128.5,125.2,124.6,124.4,121.8,119.4,118.9,114.5,112.3,57.0,31.8,27.5;MS (ESI)m/z[M + Na]+Calcd for C18H15ClN2NaO∶333.08,found∶333.25.
2.2.5 1-(4-bromophenyl)-5-(1H-indol-3-yl)pyrro⁃lidin-2-one 3ea
White solid; m.p.222-224 ℃;1H NMR (DMSO,300 MHz)∶δ(ppm)∶10.93(br s,1 H,-NH),7.49(d,J=7.8 Hz,1 H),7.43-7.34 (m,4 H),7.30 (d,J=8.1 Hz,1 H),7.24 (d,J=2.4 Hz,1 H),7.04 (t,J=8.1 Hz,1 H),6.95 (t,J=7.5 Hz,1 H),5.67 (t,J=7.2 Hz,1 H),2.74-2.48(m,3 H),2.17-2.05(m,1 H);13C NMR(DMSO,75 MHz)∶δ (ppm)∶174.3,138.2,137.2,131.4,125.2,124.9,124.4,121.8,119.4,118.9,117.0,114.5,112.3,56.9,31.8,27.5;MS (ESI)m/z[M+Na]+Calcd for C18H15BrN2NaO∶377.03,found∶377.07.
2.2.6 5-(1H-indol-3-yl)-1-m-tolypyrrolidin-2-one 3fa
White solid;m.p.223-224 ℃;1H NMR (DMSO,300 MHz)∶δ(ppm)∶10.89(br s,1 H,-NH),7.52(d,J=8.1 Hz,1 H),7.29 (d,J=8.1 Hz,2 H),7.23-7.16 (m,2 H),7.07-7.01 (m,2 H),6.96 (t,J=7.2 Hz,1 H),6.79(d,J=7.5 Hz,1 H),5.67 (t,J=6.6 Hz,1 H),2.73-2.49(m,3 H),2.16 (s,3 H,-CH3),2.11-2.04 (m,1 H); 13C NMR (DMSO,75 MHz)∶δ (ppm)∶174.1,138.9,137.7,137.1,128.4,125.6,125.5,124.2,123.8,121.7,120.3,119.3,119.0,115.1,112.3,57.0,31.8,27.5,21.5; MS(ESI)m/z[M+Na]+Calcd for C19H18N2NaO∶313.13,found∶313.25.
2.2.7 1-(3-chlorophenyl)-5-(1H-indol-3-yl)pyrro⁃lidin-2-one 3ga
White solid; m.p.242-243 ℃;1H NMR (DMSO,300 MHz)∶δ (ppm)∶10.96 (br s,1 H,-NH),7.63 (s,1H),7.51 (d,J=7.8 Hz,1 H),7.37-7.27 (m,3 H),7.20(t,J=8.1 Hz,1 H),7.07-6.94 (m,3 H),5.71 (t,J=7.5 Hz,1 H),2.76-2.53 (m,3 H),2.13-2.04 (m,1 H);13C NMR (DMSO,75 MHz)∶δ (ppm)∶174.5,140.3,137.1,132.9,130.2,125.3,124.5,124.4,122.6,121.8,121.2,119.4,118.9,114.5,112.3,56.9,31.8,27.5; MS (ESI)m/z[M+Na]+Calcd for C18H15ClN2NaO∶333.08,found∶333.25.
2.2.8 5-(1-methyl-1H-indol-3-yl)-1-p-tolypyrro⁃lidin-2-one 3ab
White solid;m.p.143-144 ℃;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶7.62 (dd,J=7.8,0.6 Hz,1 H),7.39(d,J=7.8 Hz,2 H),7.31-7.23 (m,2 H),7.18-7.13 (m,1 H),7.03 (d,J=8.7 Hz,1 H),6.83 (s,1H),5.57-5.53(m,1 H),3.67 (d,J=0.9 Hz,3 H,-CH3),2.87-2.75 (m,1 H),2.69-2.54(m,2 H),2.32-2.21(m,4 H);13C NMR(CDCl3,75 MHz)∶δ (ppm)∶174.7,137.6,136.0,134.5,129.2,126.7,125.7,122.2,122.1,119.4,118.9,114.4,109.7,57.8,32.8,31.6,27.7,20.9; MS (ESI)m/z[M+Na]+Calcd for C20H20N2NaO∶327.15,found∶327.25.
2.2.9 5-(1-allyl-1H-indol-3-yl)-1-p-tolypyrro⁃lidin-2-one 3ac
oil;1H NMR(CDCl3,300 MHz)∶δ(ppm)∶7.65(dd,J=7.8,1.2 Hz,1 H),7.39(d,J=8.4 Hz,2 H),7.31-7.22(m,2 H),7.20-7.14 (m,1 H),7.04 (d,J=8.4 Hz,2 H),6.91 (s,1H),5.97-5.85 (m,1 H),5.55 (dd,J=6.9,4.8 Hz,1 H),5.15 (dd,J=10.2,1.2 Hz,1 H),4.93 (dd,J=17.1,1.2 Hz,1 H),4.60 (d,J=5.4 Hz,2 H),2.89-2.78(m,1 H),2.72-2.56(m,2 H),2.37-2.26(m,1 H),2.25(s,3 H,-CH3);13C NMR (CDCl3,75 MHz)∶δ (ppm)∶174.7,137.0,136.0,134.5,133.2,129.2,126.0,125.9,122.4,122.1,119.6,119.1,117.3,114.8,110.1,58.0,48.7,31.7,27.7,20.9; MS (ESI)m/z[M+Na]+Calcd for C22H22N2NaO∶353.16,found∶353.20.
2.2.10 5-(1-benzyl-1H-indol-3-yl)-1-p-tolypyrro⁃lidin-2-one 3ad
White solid;m.p.140-142 ℃;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶7.66-7.63 (m,1 H),7.34-7.30 (m,2 H),7.25-7.21 (m,4 H),7.19-7.11 (m,2 H),7.02 (d,J=8.1 Hz,2 H),6.93-6.88 (m,3 H),5.54 (dd,J=7.2,4.5 Hz,1 H),5.20 (s,2 H),2.89-2.76 (m,1 H),2.72-2.57(m,2 H),2.39-2.27(m,1 H),2.26(s,3 H,-CH3);13C NMR (CDCl3,75 MHz)∶δ (ppm)∶174.6,137.3,137.2,135.9,134.6,129.2,128.8,127.6,126.7,126.6,126.0,122.8,122.3,119.8,119.1,115.0,110.3,58.0,49.9,31.7,27.6,21.0; MS (ESI)m/z[M+H]+Calcd for C26H24N2NaO∶403.18,found∶403.25.
2.2.11 5-(5-methyl-1H-indol-3-yl)-1-p-tolypyrro⁃lidin-2-one 3ae
White solid;m.p.152-153 ℃;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶8.01 (br s,1 H,-NH),7.39-7.34(m,3 H),7.23 (d,J=8.4 Hz,1 H),7.03 (t,J=8.4 Hz,3 H),6.89 (s,1H),5.54-5.50 (m,1 H),2.86-2.75 (m,1 H),2.69-2.57(m,2 H),2.48(s,3 H,-CH3),2.34-2.25(m,1 H),2.23(s,3 H,-CH3);13C NMR(CDCl3,75 MHz)∶δ (ppm)∶174.8,135.9,135.1,134.6,129.2,129.1,125.4,124.0,122.41,122.36,118.3,115.1,111.3,58.0,31.6,27.4,21.6,20.9; MS (ESI)m/z[M+Na]+Calcd for C20H20N2NaO∶327.15,found∶327.25.
2.2.12 5-(5-methoxy-1H-indol-3-yl)-1-p-tolypyr⁃rolidin-2-one 3af
White solid;m.p.162-164 ℃;1H NMR (CDCl3,300 MHz)∶δ (ppm)∶8.01 (br s,1 H,-NH),7.35 (d,J=8.4 Hz,2 H),7.23 (d,J=8.7 Hz,1 H),7.03-7.01 (m,3 H),6.93-6.85 (m,2 H),5.51 (dd,J=7.2,4.5 Hz,1 H),3.86 (s,3 H,-OCH3),2.84-2.74 (m,1 H),2.70-2.57(m,2 H),2.31-2.25 (m,1 H),2.23 (s,3 H,-CH3);13C NMR (CDCl3,75 MHz)∶δ (ppm)∶174.8,154.1,135.8,134.8,132.0,129.2,125.6,123.0,122.6,115.2,112.4,112.3,100.7,58.0,56.0,31.7,27.4,20.9;MS(ESI)m/z[M+Na]+Calcd for C20H20N2NaO2∶343.14,found∶343.18.2.2.13 5-(5-bromo-1H-indol-3-yl)-1-p-tolypyrro⁃lidin-2-one 3ag
White solid;m.p.179-180 ℃;1H NMR (DMSO,300 MHz)∶δ(ppm)∶11.12(br s,1 H,-NH),7.67(d,J=1.8 Hz,1 H),7.29-7.24 (m,4 H),7.14 (dd,J=8.4,1.8 Hz,1 H),6.99 (d,J=8.1 Hz,2 H),5.64 (t,J=6.9 Hz,1 H),2.71-2.51(m,3 H),2.14(s,3 H,-CH3),2.09-1.98(m,1 H);13C NMR (CDCl3,75 MHz):δ (ppm):174.0,136.3,135.8,134.0,129.1,127.3,125.8,124.2,123.1,121.2,115.0,114.2,111.9,56.7,31.7,27.6,20.9; MS(ESI)m/z[M+Na]+Calcd for C19H17BrN2NaO∶391.04,found∶391.08.
2.2.14 5-(5-nitro-1H-indol-3-yl)-1-p-tolypyrro⁃lidin-2-one 3ah
White solid;m.p.228-230 ℃;1H NMR (DMSO,300 MHz):11.66(br s,1 H,-NH),8.52(d,J=2.1 Hz,1 H),7.94 (dd,J=9.0,2.1 Hz,1 H),7.49-7.45 (m,2 H),7.30 (d,J=8.1 Hz,2 H),6.99 (d,J=8.1 Hz,2 H),5.82-5.77(m,1 H),2.73-2.53(m,3 H),2.13(s,3 H,-CH3),2.11-2.01 (m,1 H);13C NMR (CDCl3,75 MHz):δ(ppm):174.0,140.9 140.2,136.2,134.2,129.2,127.9,124.8,123.1,118.1,117.2,116.2,112.7,56.4,31.6,27.8,20.8;MS (ESI)m/z[M + Na]+Calcd for C19H17N3NaO3:358.12,found:358.29.
3 Conclusions
In summary,we have developed a new iron cata⁃lyzed nucleophilic substitution of hydroxy groups for carbon-carbon bond formation.The γ-hydroxy lactams could be converted stablely into N-acyliminium ions in the presence of iron catalyst to achieve the desired cou⁃pling reaction.The reaction requires only low catalyst loading(1.0 mol%),short reaction time,and affords high yields (up to 99%) in the mild condition.Due to these advantages,it could be applied in industry.Fur⁃ther investigations on chiral iron catalyst catalyzing this nucleophilic substitution are currently underway.
[1]Speckamp W N,Moolenaar M J.New developments in the chemistry of N-Acyliminium ions and related intermediates[J].Tetrahedron,2000,56:3817-3856.
[2]Petrini M,Torregiani E.Recent Advances in Stereoselective Syntheses Using N-Acylimines[J].Synthesis,2007,2,159-186.
[3]Maryanoff B E,Zhang H C,Cohen J H,et al.Cyclizations of N-Acyliminium Ions[J].Chem Rev,2004,104,1431-1628.
[4]Royer J,Bonin M,Micouin L.Chiral heterocycles by imini⁃um ion cyclization[J].Chem Rev,2004,104:2311-2352.
[5]Marson C M.Synthesis via N-acyliminium cyclisations of N-heterocyclic ring systems related to alkaloids[J].Arkivoc,2001:1-16.
[6]Tanis S P,Deaton M V,Dixon L A,et al.Furan-Terminat⁃ed N-Acyliminium Ion Initiated Cyclizatiions in Alkaloid Synthesis[J].J Org Chem,1998,63:6914-6928.
[7]Ordóñez M,Cativiela C.Stereoselective synthesis of amino acids[J].Tetrahedron:Asymmetry,2007,18:3-99.
[8]Raheen I T,Thiara P S,Peterson T E A,et al.Enantioselec⁃tive Pictet-Spengler-type cyclizations of hydroxylactams:H-bond donor catalysis by anion binding[J].J Am Chem Soc,2007,129:13404-13405.
[9]Raheem I T,Thiara P S,Jacobsen E N.Regio and enanti⁃oselective catalytic cyclization of pyrroles onto N-Acylimini⁃um ions[J].Org Lett,2008,10:1577-1580.
[10]Othman R B,Affani R,Tranchant M-J,et al.N-Acyli⁃minium ion chemistry:highly efficient and versatile car⁃bon-carbon bond formation by nucleophilic substitution of hydroxy groups catalyzed by Sn(NTf2)4[J].Angew Chem Int Ed,2010,49:776-780.
[11]Graham T J A,Shields J D,Doyle A G.Transition metalcatalyzed cross coupling with N-acyliminium ions derived from quinolines and isoquinolines[J].Chem Sci,2011,2:980-984.
[12]Rueping M,Nachtsheim B J.Asymmetric brønsted acid catalyzed nucleophilic addition to in situ generated chiral N-Acyliminium ions[J].Synlett,2010:119-122.
[13]Enthaler S,Junge K,Beller M.Sustainable metal catalysis with iron:from rust to a rising star?[J].Angew Chem Int Ed,2008,47:3317-3321.
[14]Sun C L,Li B J,Shi Z J.Direct C-H transformation via iron catalysis[J].Chem Rev,2011,111:1293-1314.

