A 3-fold Interpenetrated lvt Cd(II) Network Constructed from 4-[(3-pyridyl)methylamino]benzoate Acid①
WANG Qing-Wei CHEN Lin QI Xiao-Fei WANG Ya-Nan LIU Bo
A 3-fold Interpenetrated lvt Cd(II) Network Constructed from 4-[(3-pyridyl)methylamino]benzoate Acid①
WANG Qing-Wei②CHEN Lin QI Xiao-Fei WANG Ya-Nan LIU Bo
(136000)
A new three-dimensional (3D) coordination polymer, [Cd(L)2H2O]n·5nH2O1 with 4-[(3-pyridyl)methylamino]benzoate acid (HL),has been synthesized by slow evaporation solvent at room temperature, and structurally characterized by elemental analysis, IR spectrum, thermal analysis, fluorescence spectrum and single-crystal X-ray diffraction. Compound 1 crystallizes in the tetragonal system, space group-4, with= 20.7937(16),= 13.515(2),= 5843.5(11) Å3, C26H34CdN4O10,M= 674.97,D= 1.534 g/cm3,(Mo) = 0.808 mm-1,(000) = 2768,= 8, the final= 0.0276 and= 0.0691 for 5323 observed reflections (> 2()). The result of thermal analysis shows that 1 is stable under290 ℃. Moreover, it exhibits blue photoluminescence at room temperature.
4-[(3-pyridyl)methylamino]benzoate acid, lvt topology, 3-fold interpenetrated, luminescent property
1 INTRODUCTION
2 EXPERIMENTAL
2. 1 General procedures
HL was synthesized by the procedure reported[28],and other reagents and solvents employed were commercially available and used as received.Ele- mental analyses (C, H, N) were carried out with a Perkin-Elmer 2400 CHN elemental analyzer. In- frared (IR) spectra were obtained on a Perkin- Elmer Spectrum One instrument as KBr pellets (4000~400 cm-1). The TG analysis was conducted on a Perkin-Elmer Diamond TG/DTA 6300 thermal ana- lyzer. The solid-state photoluminescent spectra were recorded on a Perkin-Elmer LS-55 Fluorescence Spectrometer at room temperature.
2. 2 Synthesis of [Cd(L)2H2O]n·5nH2O
The title compound was prepared from the following method: Cd(NO3)2·4H2O (0.046 g, 0.15 mmol) and HL (0.068 g, 0.3 mmol) were add to 20 mL water in a beaker. The mixture was stirred until dissolve and NH3·H2O(35 drops) was dropwise added to give a clear solution followed by final filtration. The filtrate was allowed to concentrate slowly by evaporation at room temperature. Several days later, colorless block crystalsof 1 were obtained by filtration and washed with distilled water in 26% yield (based on Cd). Anal. Calcd. (%) forC26H34CdN4O10: C, 46.3; H, 5.1; N, 8.3. Found (%): C, 46.1; H, 4.9; N, 8.2. IR (KBr, cm-1): 3410s, 1608m, 1523m, 1389s, 1323s, 1279s, 1241s, 1141s, 1109m, 1080s, 1051s, 1034s, 861s, 841s, 782m, 706m, 631m.
2. 3 X-ray structure determination
A single crystal of 1with dimensions of 0.40mm´0.37mm´0.34mm was chosenand mounted on a glass fiber. All the data were collected with a Bruker CCD diffractometer equipped with a graphite-mo- nochromatic Mo(= 0.71073 Å) radiation using anscan mode at 293(2) K. In the range of 3.60<2<51.82º, a total of 15877reflections were collected and 5680 were independent withint= 0.0226,of which 5323were observed with> 2(). The correction forfactors was applied. The structure was solved by direct methods with the program SHELXS-97[29]and refined by full-matrix least-squares methods on2with SHELXL-97[30]. Non-hydrogen atoms were refined with anisotropic temperature parameters, and hydrogen atoms of the ligands were refined as rigid groups. The hydrogen atoms associated with water molecules were not located from the difference Fourier maps. The final= 0.0276 and= 0.0691 (= 1/[2(F2) + (0.0438)2+ 0.0000], where= (F2+ 2F2)/3).= 1.053, (Δ)max= 0.379, (Δ)min= –0.222 e/Å3and (Δ/)max= 0.001. The selected bond lengths and bond angles are given in Table 1.

Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for 1
Symmetry transformations used to generate the equivalent atoms: A:+1, –+1, –+2; B:+3/2, –+1/2, –+1/2
3 RESULTS AND DISCUSSION
3. 1 IR spectrum
The COO–is coordinated with its asymmetric and symmetric stretching appearing at 1608 cm–1((OCO)assym) and 1523 cm–1((OCO)sym)[31], re- spectively. The Δ((OCO)assym–(OCO)sym) is 85 cm–1(< 200), showing the presence of bidentate linkage of carboxylates in the dianions. Thus, the carboxylates coordinate to the metal as bidentate ligandsthe carboxylate groups[32]. The absence of characteristic bands around 1700 cm-1in com- pound 1 attributed to the protonated carboxylic group indicates the complete deprotonation of HL ligand upon reaction with the Cd ions[33]. In addition, X-ray diffraction analysis further indicates the bidentate coordinationmanners of carboxylate groups and the deprotonation of HL ligands.
3. 2 Description of the structure
Single-crystal X-ray diffraction analysis reveals that 1crystallizes in tetragonal crystalsystemand space group-4. It exhibits a fascinating 3D poly- meric architecture consisting of three equivalent interpenetratingnets of lvt topology. As illustrated in Fig. 1, the asymmetric unit of 1 contains one Cd ion, two L anions, one coordinated water molecule and five freewater molecules.The seven-coordinated Cd ion is surrounded by four carboxylate oxygen atoms from two different L ligands, two nitrogen atoms from two different L ligands, and one coordinated water molecule, which belongs to distorted penta- gonal-bipyramidal coordination geometry.Topolo- gically,each Cd ion is coordinated by four ligands as a four-connected node, and each ligand links two Cd ions, generating a 3D framework with lvt topology (Point Symbol 4284)[34-43], as shown in Fig. 2(a). In spite of the same Cd ion and L ligand, it is geome- trically and topologically quite different to other reported compounds[28].

Fig. 1. Thermal ellipsoids (40% probability) representation of 1. Hydrogen atoms and free waters were omitted for clarity. Symmetry codes: (A)+1, –+1, –+2; (B)+3/2, –+1/2, –+1/2
Fig. 2. (a) Schematic presentations of a single 3D net of 1:4-connected lvt net. (b) Views of three connected rhombi of a single 3D net in 1
In a single 3D net, each four-connected node generates two types of rhombic cavities; one is a planar eight-membered circuit with a very large cavity (ca. 45.57Å × 20.79Å), and the other is a nonplanar four-membered circuit. The planar square- grids (red and blue) are fused together by sharing metal atoms at the midpoints of the rhombic edges, generating the four-membered rings (Fig. 2(b)). The sizes of the eight-membered circuit are so large that they allow 3-fold interpenetration to occur (Fig. 3). Itis similar to the reported compound [Cu(L)- (DMF)·5/2H2O]n(L = 4,4΄-dicarboxydiphenyl- amine)[44].
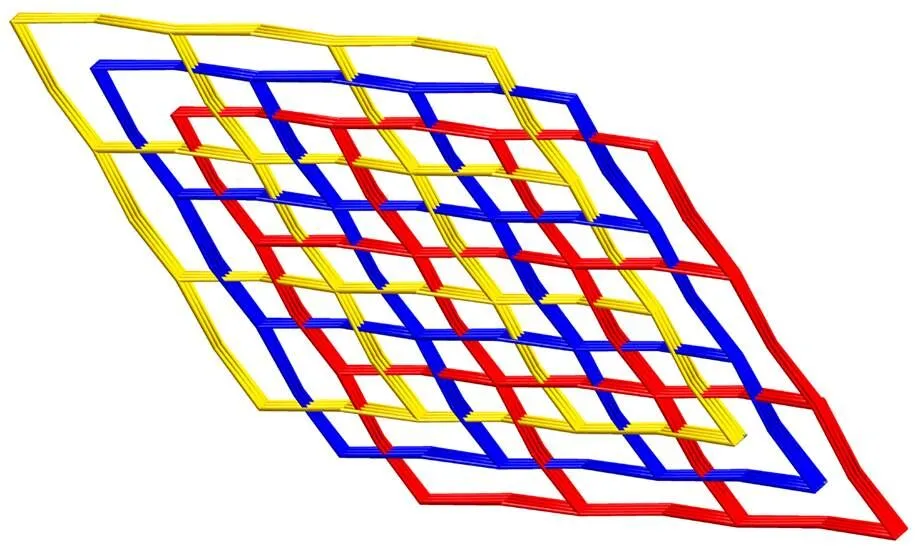
Fig. 3. Schematic view of the 3-fold parallel interpenetration in 1
Hydrogen bonding interactions are usually impor- tant in the synthesis of supramolecular architec- ture[45]. There are persistent N–H···O hydrogen bon- ding interactions between crystal water molecules andnitrogen atom of L ligands (N(4)···O(5W) = 2.982(7) Å, N(4)–H(4A)···O(5W) = 164º), which undoubtedly play an important role in stabilizing compound 1.
首先,通过溴代物与硫代羧酸之间的亲核取代反应合成了两种含羧基结构的双硫酯(1,2),两者的合成路线如图1所示。
3. 3 Thermogravimetric analysis
Thermogravimetric analysis (TGA) was perfor- med to examine the thermal stability of 1. The crushed single crystal sample was heated up to 600 ℃under N2gas at a heating rate of 10 ℃∙min-1. As shown in Fig. 4, the first weight loss of 15.68% occurs between room temperatureand 290 ℃, cor- responding to the removal of water molecules (calcd. 16.01%). In the range of 290~460 ℃, the second weight loss of67.80% can be ascribed to the release of organic ligands (calcd.67.30%). TGA results basically agree with the formula of compound 1. Theabove thermal behaviors may be attributed to the structural features.

Fig. 4. TGA curve of 1 from room temperature to 600 ℃
3. 4 Photoluminescent properties
Recently, CPs with10metal centers have attrac- ted much attention due to there potential applications such as in chemical sensors, photochemistry, and electroluminescent display[46-48]. Hence, the photo- luminescentproperty ofcompound 1 has been in- vestigated in the solid state at room temperature (Fig. 5). Compound 1 exhibits blue fluorescence with maximum emission at 437 nm (ex= 330 nm). The Cdion is difficult to oxidize or to reduce due to the10configuration, so the emission is neither MLCT nor LMCT[49-52]. As a result, the emission can probably be assigned to intraligand transitions.
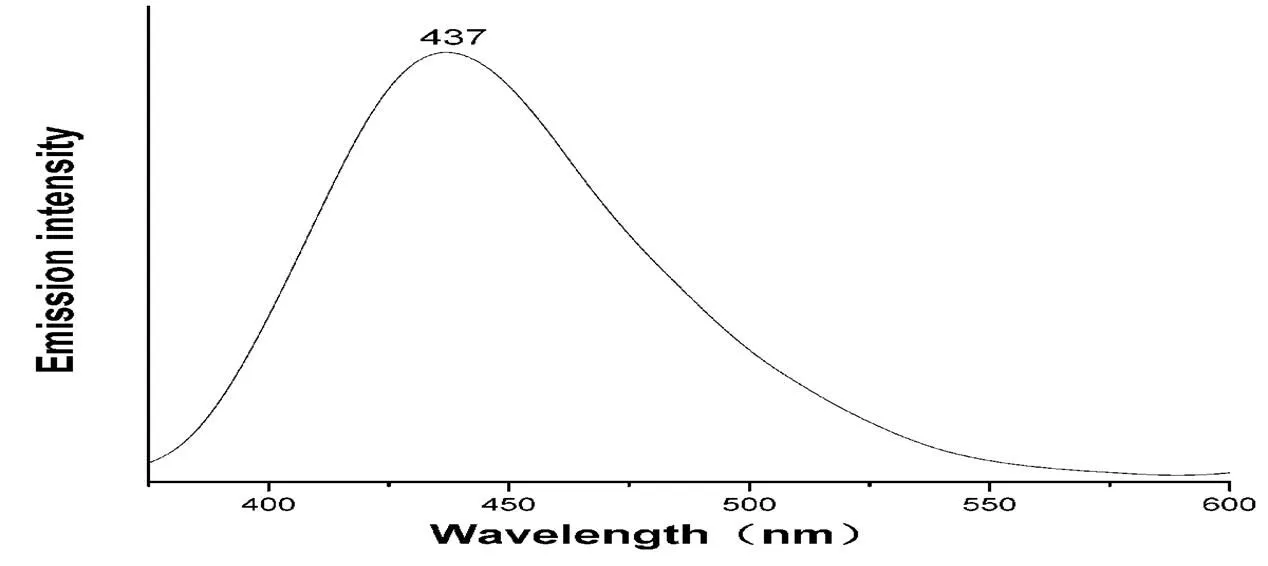
Fig. 5. Solid-state emission spectrum of the title compound at room temperature
(1) Dr, P. D.; Janiak, C.Functional organic analogues of zeolites based on metal-organic coordination frameworks.
1997, 36, 1431-1434.
(2) Li, H.; Eddaoudi, M.; Keeffe, M. O.; Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework.1999, 402, 276-279.
(3) Noro, S.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework engineering by anions and Porous functionalities of Cu(II)/4,4΄-bpy coordination polymers. 2002, 124, 2568-2583.
(4) Kepert, C. J.; Rosseinsky, M. J. A porous chiral framework of coordinated 1,3,5-benzenetricarboxylate: quadruple interpenetration of the (10,3)-a network. 1998, 1, 31-32.
(5) Miller, J. S.; Gatteschi, D.Molecule-based magnets.. 2011, 40, 3065-3066.
(6) Wang, X. Y.; Avendano, C.; Dunbar, K. R.Molecular magnetic materials based on 4and 5transition metals.
2011, 40, 3213-3238.
(7) Ma, L.; Wu, C. D.; Wanderley, M. M.; Lin, W. Single-crystal to single-crystal coss-linking of an interpenetrating chiral metal-organic framework and implications in asymmetric catalysis.. 2010, 49, 8244-8248.
(8) Hwang, Y. K.; Hong, D. Y.; Chang, J. S.; Jhung, S. H.; Seo, Y. K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Ferey, G. Amine grafting on coordinatively unsaturated metal centers of MOFs: consequences for catalysis and metal encapsulation.2008, 47, 4144-4148.
(9) Zhao, D.; Timmons, D. J.; Yuan, D.; Zhou, H. C.Tuning the topology and functionality of metal-organic frameworks by ligand design.. 2011, 44, 123-133.
(10) Dinca, M.; Long, J. R. High-enthalpy hydrogen adsorption in cation-exchanged variants of the microporous metal-organic framework Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2.. 2007, 129, 11172-11176.
(11) Lin, J. B.; Zhang, J. P.; Chen, X. M. Nonclassical active site for enhanced gas sorption in porous coordination polymer.
. 2010, 132, 6654-6656.
(12) Li, J. R.; Kuppler, R. J.; Zhou, H. C.Selective gas adsorption and separation in metal-organic frameworks.. 2009, 38, 1477-1504.
(13) Chen, S. S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G. C.; Sun, W. Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property.. 2011, 47, 4902-4904.
(14) Rocha, J.; Carlos, L. D.; Paz, F. A. A.; Ananias, D. Luminescent multifunctional lanthanides-based metal-organic frameworks.
2011, 40, 926-940.
(15) Liu, S.; Xiang, Z.; Hu, Z.; Zheng, X.; Cao, D. Zeolitic imidazolate framework-8 as a luminescent material for the sensing of metal ions and small molecules.2011, 21, 6649-6653.
(16) Chen, B.; Wang, L.; Xiao, Y.; Fronczek, F. R.; Xue, M.; Cui, Y.; Qian, G. A luminescent metal-organic framework with lewis basic pyridyl sitesfor the sensing of metalions.. 2009, 48, 500-503.
(17) Kondo, M.; Okubo, T.; Asani, A.; Noro, S.; Youshitomi, T.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Seki, K. Rational synthesis of stable channel-like
cavities with methane gas adsorption properties: {[Cu2(pzdc)2(L)]n} (pzdc = pyrazine-2,3-dicarboxylate; L = a pillar ligand).1999, 38, 140-143.
(18) Humphery, S. M.; Wood, P. T. Multiple areas of magnetic bi stability in the topological ferrimagnet [Co3(NC5H3(CO2)2·2,5)2(3-OH)2(OH2)2].2004, 126, 13236-13237.
(19) Gomez-Lor, B.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, M. A.; Ruiz-Valero, C.; Snejko, N. In2(OH3)(BDC01.5(BDC = 1,4-benzendicarboxylate): an In(Ⅲ) supramolecular 3D framework with catalytic activity.. 2002, 41, 2429-2432.
(20) Kitagawa, S.; Kitaura, R.; Noro, S. I. Functional potous coordination polymers..... 2004, 43, 2334-2375.
(21) Fang, Q. R.; Zhu, G. S.; Jin, Z.; Xue, M.; Wei, X.; Wang, D. J.; Qiu, S. L. A multifunctional metal-organic open framework with a bcu topology constructed from undecanuclear clusters.. 2006, 45, 6126–6130.
(22) Bauer, C. A.; Timofeeva, T. V.; Settersten, T. B.; Patterson, B. D.; Liu, V. H.; Simmons, B. A.; Allendorf, M. D. Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks.. 2007, 129, 7136–7144.
(23) Dubbeldam, D.; Walton, K. S.; Ellis, D. E.; Snurr, R. Q.Exceptional negative thermal expansion in isoreticular metal-organic frameworks.. 2007, 46, 4496–4499.
(24) Kesanli, B.; Lin, W. Chiral porous coordination networks: rational design and applications in enantioselective processes.2003, 246, 305–326.
(25) Han, L.; Hong, M. C. Recent advances in the design and construction of helical coordination polymers.. 2005, 8, 406–419.
(26) Xiong, R. G.; Zuo, J. L.; You, X. Z.; Abrahams, B. F.; Bai, Z. P.; Che, C. M.; Fund, H. K. Opto-electronic multifunctional chiral diamondoid-network coordination polymer: bis{4-[2-(4-pyridyl)ethenyl]benzoato}zinc with high thermal stability.2000, 20, 2061–2062.
(27) Lin, W.; Ma, L.; Evans, O. RNLO-active zinc(II) and cadmium(II) coordination networks with 8-fold diamondoid structures.. 2000, 22, 2263–2264.
(28) Liu, B.; Fan, L. J.; Liu, Y. Y.; Yang, J.; Ma, J. F. Syntheses and structures of Cd(II) and Co(II) compounds of 4-[(3-pyridyl)methylamino]benzoate anion.2011, 64, 413-423.
(29) Sheldrick, G. M.. University of Göttingen 2008.
(30) Sheldrick, G. M.. University of Göttingen 2008.
(31) Devereux, M.; Shea, D. O.; Kellett, A.; McCann, M.; Walsh, M.; Egan, D.; Deegan, C.; Kedziora, K.; Rosair, G.; Müller-Bunz, H. J. Synthesis, X-ray crystal structures and biomimetic and anticancer activities of novel copper(II) benzoate complexes incorporating 2-(4΄-thiazolyl)benzimidazole (thiabendazole), 2-(2-pyridyl)benzimidazole and 1,10-phenanthroline as chelating nitrogen donor ligands.2007, 101, 881-892.
(32) Farrugia, L. J.; Wing, X. A.. University of Glasgow, Glasgow, UK 1988.
(33)Fu, Z. Y.; Wu, X. T.; Dai, J. C.; Hu, S. M.; Du, W. X.; Zhang, H. H.; Sun, R. Q. The structure and fluorescence properties of two novel mixed-ligand supramolecular frameworks with different structural motifs.. 2002, 2002, 2730–2735.
(34) Rather, B.; Moulton, B.; Walsh, R. D. B.; Zaworotko, M. J. A new supramolecular isomer of [Zn(nicotinate)2]n: a novel 42.84network that is the result of self-assembly of 4-connected nodes.. 2002, 7, 694-695.
(35) Carlucci, L.; Cozzi, N.; Ciani, G.; Moret, M.; Proserpio, D. M.; Rizzato, S. A three-dimensional nanoporous flexible network of ‘square-planar’copper(II) centres with an unusual topology.. 2002, 13, 1354-1355.
(36) Lu, J. Y.; Fernandez, W. A.; Ge, Z. H.; Abboud, K. A. A novel two-fold interpenetrating 3D 42.84network self-assembled from a new 1D coordination polymer.2005, 29, 434-438.
(37) Du, M.; Zhao, X. J.; Guo, J. H.; Batten, S. R. Direction of topological isomers of silver(I) coordination polymers induced by solvent, and selective anion-exchange of a class of PtS-type host frameworks.. 2005, 38, 4836-4838.
(38) Zhang, J. P.; Lin, Y. Y.; Huang, X. C.; Chen, X. M. Copper(I) 1,2,4-triazolates and related complexes: studies of the solvothermal ligand reactions, network topologies, and photoluminescence properties.2005, 127, 5495-5506.
(39) Wang, X. L.; Qin, C.; Wang, E. B.; Li, Y. G.; Su, Z. M.An unprecedented fivefold interpenetrated lvt network containing the exceptional racemic motifs originated from nine interwoven helices.. 2005, 43, 5450-5452.
(40) Li, Z.; Li, M.; Zhan, S. Z.; Huang, X. C.; Ng, S. W.; Li, D. Pculim nets: topological variation of cyano-bridged copper(I)-tetrazole coordination frameworks caused by Cu2(azole)2-SBU versatility.2008, 10, 978-980.
(41) Xue, D. X.; Lin, J. B.; Zhang, J. P.; Chen, X. M. Syntheses, structures and sorption properties of two framework-isomeric porous copper-coordination polymers.2009, 11, 183-188.
(42) Huang, F. P.; Li, H. Y.; Tian, J. L.; Gu, W.; Jiang, Y. M.; Yan, S. P.; Liao, D. Z. A Three-dimensional (42.84)-lvt topology and a two-dimensional brick-wall network: two pillared supramolecular isomers exploring the use of L-cysteic acid to engineer porous frameworks.. 2009, 9, 3191-3196.
(43) Lin, Q. P.; Zhang, J.; Cao, X. Y.; Yao, Y. G.; Li, Z. J.; Zhang, L.; Zhou, Z. F. Canted antiferromagnetic behaviours in isostructural Co(II) and Ni(II) frameworks with helical lvt topology.2010, 12, 2938-2942.
(44) Yang, Q. X.; Chen, X. Q.; Chen, Z. J.; Hao, Y.; Li, Y. Z.; Lu, Q. Y.; Zheng, H. G. Metal-organic frameworks constructed from flexible V-shaped ligands: adjustment of the topology, interpenetration and porosity via a solvent system.. 2012,48, 10016–10018.
(45) Krische, M. J.; Lehn, J. M. The utilization of persistent H-bonding motifs in the self-assembly of supramolecular architectures.2000, 96, 3–29.
(46) Wu, Q.; Esteghamatian, M.; Hu, N. X.; Popovic, Z.; Enright, G.; Tao, Y.; D’Iorio M.; Wang, S. Synthesis, structure, and electroluminescence of BR2q(R = Et, Ph, 2-naphthyl and q = 8-hydroxyquinolato).. 2000, 12, 79-83.
(47) McGarrah, J. E.; Kim, Y. J.; Hissler, M.; Eisenberg, R. Toward a molecular photochemical device: atriad for photoinduced charge separation based on a platinum diimine bis(acetylide) chromophore.. 2001, 40, 4510-4511.
(48) Santis, G. D.; Fabbrizzi, L.; Licchelli, M.; Poggi, A.; Taglietti, A. Molecular recognition of carboxylate ions based on the metal-ligand interaction and signaled through fluorescence quenching.. 1996, 35, 202-204.
(49) Lin, J. G.; Zang, S. Q.; Tian, Z. F.; Li, Y. Z.; Xu, Y. Y.; Zhu H. Z.; Meng, Q. J. Metal-organic frameworks constructed from mixed-ligand 1,2,3,4-tetra-(4-pyridyl)-butane and benzene-polycarboxylate acids: syntheses, structures and physical properties.. 2007, 9, 915-921.
(50) Wen, L. L.; Li, Y. Z.; Lu, Z. D.; Lin, J. G.; Duan, C. Y.; Meng, Q. J. Syntheses and structures of four d10metal-organic frameworks assembled with aromatic polycarboxylate and bix [bix = 1,4-bis(imidazol-1-ylmethyl)benzene].. 2006, 6, 530-537.
(51) Wen, L. L.; Lu, Z. D.; Lin, J. G.; Tian, Z. F.; Zhu, H. Z.; Meng, Q. J. Syntheses, structures, and physical properties of three novel metal-organic frameworks constructed from aromatic polycarboxylate acids and flexible imidazole-based synthons.. 2007, 7, 93-99.
(52) Liu, H. Y.; Wu, H.; Ma, J. F.; Liu, Y. Y.; Liu, B.; Yang, J. Syntheses, structures, and photoluminescence of zinc(II) coordination polymers based on carboxylates and flexible bis-[(pyridyl)-benzimidazole] ligands.. 2010, 10, 4795-4805.
7 November 2013;
3 May 2014 (CCDC 932708)
① The project was supported by the Science and Technology Development Project of Jilin Provincial Science & Technology Department (201205080) and the Science and Technology Research Projects of the Education Office of Jilin Province (No. 2013. 384)
. Wang Qing-Wei, born in 1961. Tel: 0434-3291973, E-mail: wqw611223@163.com
- 结构化学的其它文章
- Synthesis and Crystal Structure of a New Complex [Cu(C14H9O3)2(C5H5N)2(C2H5OH)2]①
- Synthesis, Structure, and Luminescent Property of a 3-D Strontium Complex [Sr3(pda)2(Hpda)2(H2O)2]n·2nH2O①
- Quantum Chemical Study on the Structural Characteristics and Stability of AlSn±Clusters①
- Structure and Magnetic Properties of a Bipyridine-bridged One-dimensional Ni(II) Coordination Polymer①
- Tuning of NaTaO3 Band Structure through Mn2+ Ion Doping and the Enhanced Visible Light Response①
- Proton Transfer Mechanism of Alanine Induced by Zn2+: a Theoretical Study①

