Inhibitory Effect of Buddlejasaponin IV on Hepatocarcinoma 22(H22)in Mice
Yueyuan CHEN, Yonglin HUANG, Jiejing CHEN, Fenglai LU, Dianpeng LI*
1. Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization, Guangxi Institute of Botany, Chinese Academy of Sciences, Guilin 541006, China;
2. Guangxi Key Laboratory of Metabolic Diseases Research, Guilin 541002, China
Buddlejasaponin IV (BJ-IV) is a triterpenoid saponin containing Δ11-13, 28-epoxy structure.Studies have shown that buddlejasaponin IV has significant anti-inflammatory and analgesic effects, and the mechanisms may be related to inhib ited NO, PGE2 and TNF-α[1]. Buddlejasaponin IV also has anti-hyperchlesterolemia and anti-hyperlipidemia effects, which are equivalent to those of probucol[2].Moreover,buddlejasaponin IV also has anti-liver fibrosis activity in vitro[3]. There are rare reports on inhibitory effect of buddlejasaponin IV on H22tumor in vivo. In this study, the inhibitory effect of buddlejasaponin IV on growth of transplanted H22tumor in mice was investigated. The buddlejasaponin IV was injected into abdominal cavities of mice, and the H22tumorbearing mice were treated as the pharmacodynamic models. The tumor inhibition rate, as well as antioxidant enzyme activities in mice serum, was determined. The possible mechanism of inhibitory effect of buddlejasaponin IV on growth of tumor in vivo was studied,providing pharmacological basis for development of buddlejasaponin IV.
Materials and Methods
Experimental animal, tumor strain and reagents
Total 50 4-6-week-old Kunming mice (SPF,half male and half female)with weight of 18-22 g were randomly divided into five groups,and they were provided by the Animal Experiment Center of Guangxi Medical University(SCXK (G)2003-0003).The H22tumor strain was purchased from the China Center for Type Culture Collection(preservation center of Wuhan University),and its subculture was performed by the Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization.The cyclophosphamide(CTX) for injection (Batch No.03110121) was produced by the Zhangjiagang City Hengrui Pharmaceutical Machinery Co., Ltd. The superoxide dismutase (SOD), malondialdehyde (MDA), gamma glutamyl transpeptidase (GGT) and alkaline phosphatase (AKP) detection kits were purchased from the Nanjing Jiancheng Bioengineering Institute(Batch No. 20101228). The buddlejasaponin IV with purity higher than 99.8% was extracted from Clinopodium by the Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization[4].
Establishment of H22 tumor-bearing mice model
The H22strain was injected into abdominal cavities of mice, and 7 d after the inoculation, appropriate amounts of ascites were sampled from the mice under sterile conditions. The sampled ascites samples were diluted with sterile saline,and then centrifuged at 1 000 r/min for 5 min. The H22cells were washed 3 times and diluted to 5×106cells/ml (percentage of viable cells was higher than 95%). The isolated H22cells were injected to right axillary skins of the mice with inoculation amount of 1×106cells/mouse.
Grouping and treatment
The mice were randomly divided into five groups 24 h after the inoculation, and there were 10 mice in each group. In the model control group,distilled water was given to mice by gavage. In the CTX group, the mice were injected with CTX according to the amount of 20.0 mg/kg.In the high-,middle- and low-dosage BJ-IV treatment groups, the mice were injected with BJ-IV according to the amounts of 1.00, 0.50 and 0.25 mg/kg, respectively once a day. For various groups,the injection was all performed once a day. The injection lasted for 10 consecutive days, and all the mice were sacrificed the next day.The blood was sampled from their eyeballs. Subsequently, the blood samples were centrifuged at 3 000 r/min for 15 min at 4℃, and the serum samples were preserved at -20 ℃for use. The tumor tissues, spleens and thymuses were stripped and weighted by electronic scale. The tumor inhibition rate, thymus index and spleen index were calculated as follows:
Tumor inhibition rate = [(Tumor weight in the control group - Tumor weight in the BJ-IV treatment group) /Tumor weight in the control group]×100%;
Thymus index = Thymus weight(mg)/Mouse body weight(g);
Spleen index = Spleen weight(mg)/Mouse body weight(g).
SOD activity, MDA content, GGT activity and AKP activity in serum of H22 tumor-bearing mice
The SOD activity was determined with xanthine oxidase (hydroxylamine)method; the DMA content was determined with thiobarbituric acid reactive substances assay; the GGT and AKP activities were all determined with chemical colorimetric method.The determination above was all performed in accordance with the instructions of kits produced by the Nanjing Jiancheng Bioengineering Institute. In the determination of SOD activity, MDA content, GGT activity and AKP activity, a UV spectrophotometer (OPTIZEN2120)was used.

Table 1 Inhibitory effect of buddlejasaponin IV on hepatocarcinoma 22(H22)in mice
Data processing and statistics
The results about tumor weight,SOD activity, MDA content, GGT activity and AKP activity were all expressed as mean ± standard deviation. With the ANOVA model of SPSS 13.0, the differences among groups were compared, and the multiple comparisons were performed with the LSD model.
Results and Analysis
Growth situations of tumor and mice
On day 2, grain-sized, irregular and unsmooth tumor nodules were shown in the right armpits of mice.The growth of tumors in mice in the model control group was the fastest,followed by that in the low-dosage buddlejasaponin IV treatment group, and the growth of tumors in mice in the CTX group was the slowest. In terms of feed intake, activity and hair color, the mice in the BJ-IV treatment groups were better than those in the CTX group.
Inhibitory effect of buddlejasaponin IV on H22 tumor in mice
The results of antitumor experiment in vivo showed that the high-,middle- and low-dosage buddlejasaponin IV all showed inhibitory effect on H22tumor.As shown in Table 1,the tumor inhibition rates in the high-,middle- and low-dosage buddlejasaponin IV treatment groups were 56.96% ,50.63% and 35.44%, respectively. In the CTX group, the tumor inhibition rate was 69.49%. There were significant differences in tumor inhibition rate between model control group and the other groups (P<0.01).During the experiment, the weights of mice in various groups were increased in varying degrees. Among the groups, the weight of mice in the CTX group was increased slowest. The thymus index of mice in the CTX group was de-creased (P<0.01), indicating that CTX inhibited the growth of thymuses of mice. Compared with the control group, buddlejasaponin IV showed no significant effects on spleen and thymus weights of mice. It indicated that buddlejasaponin IV had no significant inhibitory effects on spleens and thymuses of mice.
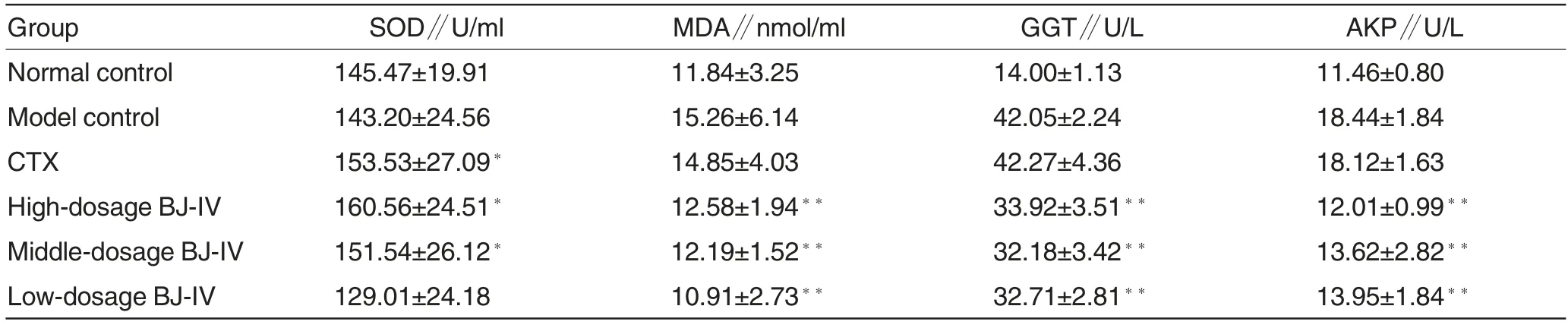
Table 2 SOD activities,MDA contents,GGT activities and AKP activities in serums of mice in all the groups
Effects of buddlejasaponin IV on SOD activity, MDA content, GGT activity and AKP activity in serum of H22 tumor-bearing mice
Compared with those in the model control group, the serum SOD activities in the high- and middle-dosage buddlejasaponin IV treatment groups were significantly increased (P<0.05),and the serum MDA contents in the three buddlejasaponin IV treatment groups were all decreased (P<0.01).Significant differences were observed in serum GGT and AKP activities between the buddlejasaponin IV treatment groups and the model control group(P<0.01).There were no significant differences between the CTX and model control groups except in SOD activity. It suggests that buddlejasaponin IV can scavenge free radicals in tumor-bearing mice, and it can upregulate the tolerance of body to oxidative stress. Moreover, buddlejasaponin IV can also inhibit the sustained damage to liver functions(Table 2).
Discussion
Buddlejasaponin IV (BJ-IV) is a triterpenoid saponin containing Δ11-13,28-epoxy structure. Studies have shown that triterpenoid saponins that have similar structures with buddlejasaponin IV have some anti-tumor activity[5-6], and they can also improve immunomodulatory capacity to some extent[7-8].Cyclophosphamide is a broadspectrum anti-tumor drug, and it can be used to establish immunosuppressed mice models[9]. Therefore, in this study, CTX was selected as the positive control to investigate the inhibitory effect of buddlejasaponin IV on H22tumor in mice.
The guiding principle for antineoplastic pharmacodynamics was as follows: the results of anti-tumor experiment in vivo are an important indicator for evaluating the effectiveness of anticancer drugs[10].Li et al.[11]and Chen et al.[12]considered that the axillarilytransplanted H22tumor-bearing mice are a class of recognized models for studying anti-tumor effects and related mechanisms. Oxidative stress is an important mechanism of tumorigenesis[13]. Currently, it has become a new direction to look for effective antioxidants in vivo in anticancer research.SOD is an important antioxidant enzyme in animal bodies, and it can protect the body from free radical damage. SOD activity can be used to reflect the free radical-scavenging ability of the body.MDA is the end product of cellular lipid peroxides. MDA content can reflect the degree of lipid peroxidation in the body,thus the degree of cell damage can be inferred indirectly.The results of this study showed that the serum SOD activities of mice in the normal control and middle- and highdosage buddlejasaponin IV treatment groups were significantly higher than that of mice in the model control group(P <0.05). Buddlejasaponin IV could significantly up-regulate the SOD activity reduced by growth of H22tumor,improving the immunity of body. The MDA contents in the buddlejasaponin IV treatment groups were significantly lower than that in the model control group (P<0.01),but there was no significant difference in serum MDA content between the normal and model control groups. The reduced serum MDA content meant reduced free radicals and liver damage. Alkaline phosphatase (AKP)is widely distributed in various body organs, but its content is highest in liver.Liver damage and lipid peroxidation caused by free radicals will cause damage to the structure of biofilms and lead to functional inactivation,thereby increasing permeability of cell membrane. Then, the liver intracellular enzymes, such as AKP, will enter blood circulation, resulting in higher AKP level in blood[14-15]. Serum GGT is mainly from the hepatobiliary system.In the onset of liver tumor,intra-hepatic obstruction will occur, inducing the generation of GGT by liver cells;at the same time,tumor cells can also synthesize GGT, resulting in higher GGT content in serum.The results of this study showed that the AKP and GGT contents in the model control group were all significantly higher than those in the normal control group,indicating that the H22tumor-bearing mouse models were successfully established, and the invasion of tumor cells caused damage to cell membrane structure and functional inactivation, thereby resulting in higher AKP content in serum of mice. There were significant differences in serum AKP and GGT contents between the model control and buddlejasaponin IV treatment groups,but there were no significant differences in serum indexes between the model control and CTX groups, indicating that buddlejasaponin IV has certain protective effect on liver cells.
In short,buddlejasaponin IV has a certain anti-hepatoma effect in vivo,and the related mechanism may be associated with regulating the body’s antioxidant capacity. However, where buddlejasaponin IV has inhibitory effects on other kinds of tumors, as well as the related mechanisms, needs tobe studied in depth.
[1]JONG-HEON WON, HO-TAEK IM,YANG-HEE KIM, et al. Anti-inflammatory effect of buddlejasaponin IV through the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via the NF-κB inactivation [J].Bri J Pharm,2006,148(2):216-225.
[2]HYUN-JU JUNG, JUNG-HWAN NAM,HEE-JUHN PARK, et al. 2007. The MeOH extract of Pleurospermum kamtschaticum and its active component buddlejasaponin(IV)inhibits intrinsic and extrinsic hyperlipidemia and hypercholesterolemia in the rat [J]. J Ethno,112(2):255-261
[3]CHEN YY (陈月圆), LI DP (李典鹏),HUANG YL (黄永林), et al. Effects of buddlejasaponin IV on proliferation and activation of hepatic stellate cells (醉鱼草苷Ⅳ对大鼠肝星状细胞增殖? 活化的影响)[J].Chin J Expe Tral Medl Formulae(中国实验方剂学杂志),2011,17(20):203-206.
[4]ELSHARKAWY AM, MANN DA. Nuclear factor-kappa B and the hepatic inflammation -fibrosis -cancer axis [J].Hepamlogy,2007,46(2):590.
[5]MOTOO Y, SAWABU N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma celllines[J].Cancer Lett,1994,86(1):91-95.
[6]YANO H, MIZOGUCHI A, FUKUDA K,et al.The herbal medicine sho-saiko-to inhibits proliferation of cancer celllines by inducing apoptosis and arrest at the G0/G1phase [J]. Cancer Res, 1994, 54(2):448-454.
[7]WU H(吴皓),LIN HS(林洪生),PEI YX(裴迎霞),et al.Effect of ginsenosid Rg3 on the mucosal immunity in tumor -bearing mice treated with cyclophosphamide (人参皂甙Rg3 对荷瘤及环磷酰胺化疗小鼠黏膜免疫力影响) [J].China Cancer (中国肿瘤), 2006, 6:369-371.
[8]MA CL(马春玲),ZHANG XQ(张西强),WANG FQ(王法权),et al.Experimental of research of the effect of South Ernbupleurumon immune functions of mice(南柴胡对小鼠免疫功能影响的实验研究) [J]. Journal of Linyi Medical College(临沂医专学报),1999,21(1):11-13.
[9]QII LJ(齐丽娟),SONG Y(宋雁),WANG W(王伟),et al.Comparison of immunosuppression induced by different doses of cyclophosphamide in normal mice(用环磷酰胺建立小鼠免疫抑制动物模型)[J].J Hygiene Research(卫生研究),2010,39(3):313-315.
[10]GUO J(郭 健),GAO FY(高福云),HU P(胡 萍), et al. Study on the anticancer effects of some Chinese herbs combined with trace element selenium (硒化合物与抗癌中药配伍的抗肿瘤作用)[J].Lab Ani Sci(实验动物科学),2008,25(2):10-12.
[11]LI HY (李海燕), FANG ZQ (方肇勤),LIANG SH (梁尚华).Research on the model of mice hepatoma(H22)and application of the model to experimental study of traditional Chinese medicine(小鼠移植性肝癌(H22)模型的研究及在中医药抗肿瘤中的应用)[J].Chinese J Bas Med TCM (中国中医基础医学杂志),2000,6(1):27-29.
[12]CHEN RT (陈润涛), CHEN B (陈秉),XIA Y(夏源),et al.Sex effect on hepatocarcinoma 22(H22)in mice(性别对荷H22肝癌小鼠肿瘤生长的影响) [J].China Occupa Medic (中国职业医学),2008,35(4):283-285.
[13]LI JF (李俊峰), ZHENG SJ (郑素军),DUAN ZP (段钟平).Liver fibrosis:role of oxidative stress and therapeutic countermeasures (氧化应激在肝纤维化中的作用及治疗对策) [J]. World Chinese Journal of Digestology (世界华人消化杂志), 2013, 21 (17): 1573-1578.
[14]ZHENG RL(郑荣粱).Free Radical Biology&Medicine (自由基生物学)[M].Beijing: Higher Education Press(北京:高等教育出版社),1996.
[15]WENG YC(翁玉椿).Trace determination of cellular and intracellular lipid peroxide(细胞和细胞内过氧化脂质的微量测定)[J]. Chinese Journal of Cell Biology(细胞生物学杂志),1985,17(3):142-145.
 Agricultural Science & Technology2015年10期
Agricultural Science & Technology2015年10期
- Agricultural Science & Technology的其它文章
- Effects of Specific Gravity-based Seed Grading on Seed Germination,Seedling Emergence and Grain Yield of Hybrid Rice
- Effects of NaCl Stress on Seed Germination of Four Canavium album Raeuseh Cultivars
- Application Effects of Ultra-fine Powder Shaped Maize Seed Coating Agent in Spring Sowing areas in northeast China
- Breeding and Application of a Japonic Rice Cytoplasmic Male Sterility Line,E-Jing A
- Effect of Low Temperature and Sparse Light Conditions on Cold Tolerance of Different Rice Lines at Seedling Stage
- Molecular Marker Assisted Selection for Fusarium Wilt Resistance Breeding in Watermelon(Citrullus lanatus)
