Dissolution kinetics of aluminum and iron from coal mining waste by hydrochloric acid☆
Li Cui,Yanxia Guo,Xuming Wang,Zhiping Du,Fangqin Cheng,*
1Institute of Resources and Environment Engineering,Shanxi University,State Environmental Protection Key Laboratory of Efficient Utilization Technology of Coal Waste Resources,Taiyuan 030006,China
2Department of Metallurgical Engineering,College of Mines and Earth Sciences,University of Utah,Salt Lake City 84112,UT,USA
Keywords:
A B S T R A C T
1.Introduction
Coal mining waste(CMW)is the largest industrial solid waste generated from coal preparation plants.Its total discharge in China is estimated at 560 million tons annually.In most cases,CMW contains approximately 18%-32%aluminum oxide(Al2O3),48%-52%silicon dioxide(SiO2),2%-8%iron oxide(Fe2O3),and about 2%other metal compounds.Most of the CMW is used to manufacture building materials or goes directly to land fill mine goafs[1,2].It poses a significant threat to the environment because it contains many potentially hazardous substances[3].One of the most serious environmental problems associated with the unsafe disposal of CMW is the oxidation of the inherent pyrite and consequent generation of acidity[4].
With the increasing demand for metals and decreasing grade of bauxite,extraction of aluminum from CMW is becoming more and more commercially attractive[5-8].Furthermore,amorphous silica could be prepared from the aluminum leaching residue[9].The extraction of aluminum and silicon would provide a new approach to utilize CMW.
The dissolution of Al2O3from untreated CMW is low due to its inactive and stable structure and thus CMW activation is needed.The most common method for CMW activation is the thermal treatment at temperatures from 650 to 800°C[10,11].Al2O3dissolution is improved by 70% using this method under optimum conditions.However,impurities in CMW are more easily introduced into the filtrate in Al2O3dissolution process after thermal activation,particularly for iron[7].The presence of iron in the filtrate is detrimental to the quality of aluminum products,such as aluminum chloride hexahydrate and polyaluminum chloride.In addition,the remaining aluminum and iron in leaching residues will limit the production of silica and increase alkaline consumption in the silicon extraction process[12,13].Therefore,removing iron and promoting aluminum dissolution are greatly significant for fine and step utilization of CMW.It is desirable to understand the dissolution kinetics of aluminum and iron from CMW for further optimizing the leaching process.
Leaching process of CMW with hydrochloric acid can be described as a non-catalytic fluid-solid reaction.The major models developed for these reactions include the first order,shrinking core,shrinking particle,homogeneous and grain models,among which the shrinking core model has been widely accepted in hydrometallurgy for the dissolution kinetics of fluid-solid systems[14-16].
Many studies have been carried out on aluminum dissolution kinetics from coal fly ash,kaolin and clay with shrinking core model[17-19].Most of them concentrate on coal fly ash,which has the same chemical components as CMW but different mineral constitutes,influencing the dissolution behavior.The acid leaching process of coal fly ash roasted with KF as assistant was modeled[14],indicating that the aluminum dissolution rate was controlled by chemical reaction and its dissolution percentage reached 92.46%.However, fluoride ions were easily introduced in the following acid leaching process,which has negative effect on the environment.Seidel and Zimmels investigated aluminum leaching mechanism and kinetics for coal fly ash using sulfuric acid[19].The leaching process followed the shrinking core model.During sulfuric acid leaching process,calcium sulfate formed and precipitated within the pores and on the particle surface,resulting in a selfinhibition effect on mass transfer at the leaching sites.Iron leaching kinetics was also studied.For example,Lee et al.[20]used oxalic acid to dissolve iron oxides from a clay material and found that the dissolution rate increased with oxalate concentrations in the optimum pH range(2.5-3.0).Dissolution of fine pure hematite(105-140μm)followed a diffusion-controlled shrinking core model.Leaching kinetics for the removal of iron from low grade gibbsite bauxite with HCl was studied[21].The dissolution of iron followed the first order equationln(1- α)=kt,with an apparent activation energy of 81.0 kJ·mol-1.Removal of more than 98%iron from hematite was achieved with about 10%aluminum loss from gibbsite phases of the ore by using 4 mol·L-1acid.As a large and prospective aluminum source,the study on CMW leaching mechanism and kinetics is insufficient.Simultaneous dissolution kinetics of Al2O3and Fe2O3from CMW is rarely found,which is needed to optimize aluminum leaching process and purify aluminum products.
The present study is conducted to investigate Al2O3and Fe2O3dissolution kinetics from CMW using HCl as a leaching medium.The influence of acid concentration and leaching temperature on dissolution kinetics are investigated.Based on Al2O3and Fe2O3dissolution characteristics,a process for recovering Al2O3and removal of Fe2O3is proposed and tested experimentally.
2.Experimental
2.1.Materials
CMW was provided by Lu'an Coal Mining Group(Shanxi,China).The XRD analysis of raw material shows that kaolinite(Al2O3·2SiO2·2H2O)and quartz(SiO2)are the major mineral phases(Fig.1).The CMW was crushed and screened to the size fraction of 0.18-0.125 mm and calcined at 700°C for 2 h for activation.The chemical composition of the calcined material based on XRF analysis is listed in Table 1.
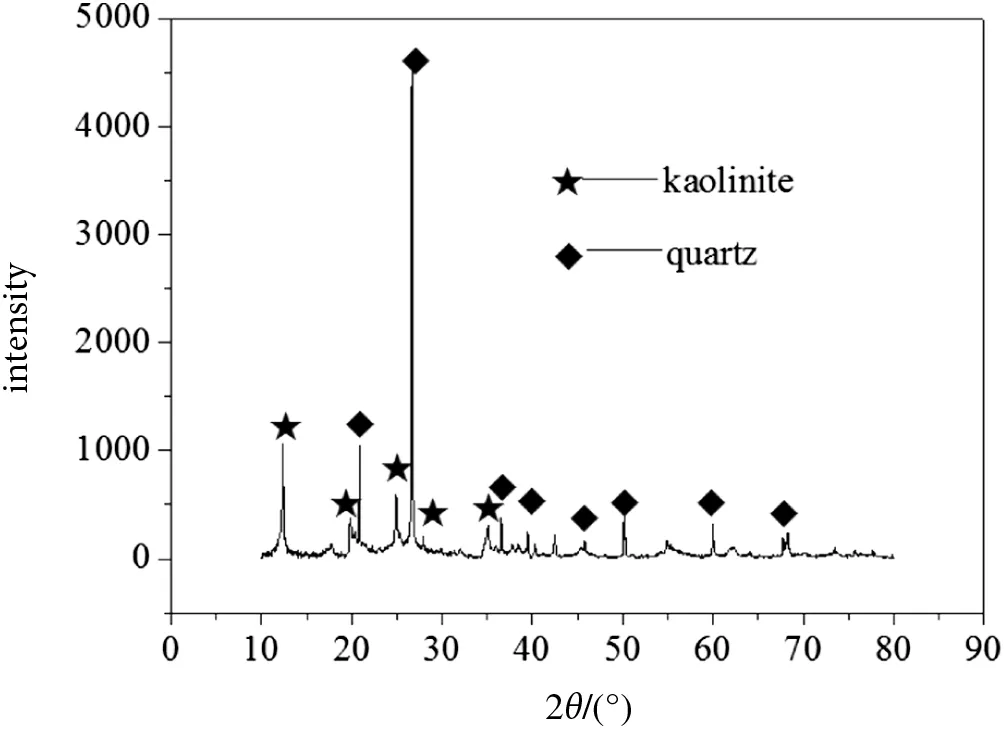
Fig.1.XRD pattern of raw CMW material.

Table 1 Chemical composition of the calcined CMW sample used in the study
Hydrochloric acid(37%,1.18 g·ml-1)was used as the leaching medium.Desired concentrations of HCl were prepared by dilution with de-ionized water for the leaching process.
2.2.Leaching process
CMW dissolution was carried out in a 500 ml four-necked roundbottom flask placed in a thermostatically controlled water bath,as shown in Fig.2.A thermometer was fitted to one of the openings.10 g of calcined CMW was added to 200ml agitated HCl solution(solid/liquid ratio=1/20 g·ml-1)at certain temperature.The reaction mixture was stirred at 500 r·min-1throughout the experiment to avoid diffusion of acid across the solid- fluid boundary layer[21,22].At selected time intervals,10 ml solution sample was taken using a syringe filter fitted with a membrane of 0.45 μm pore size.The filter residue returned to the flask with an addition of 10 ml HCl solution at the same concentration,so that the solid/liquid ratio was maintained constant at 1/20 g·ml-1.The filtrate was analyzed to determine aluminum and iron contents in the solution by the wet chemical method and the dissolution percentages of Al2O3and Fe2O3were calculated.
2.3.Shrinking core model
The shrinking core model assumes that there action products and/or inert matters remaining in the solid phase form a layer of“ash”that encapsulates the unreacted core.The equations of shrinking core model can be expressed as[23]
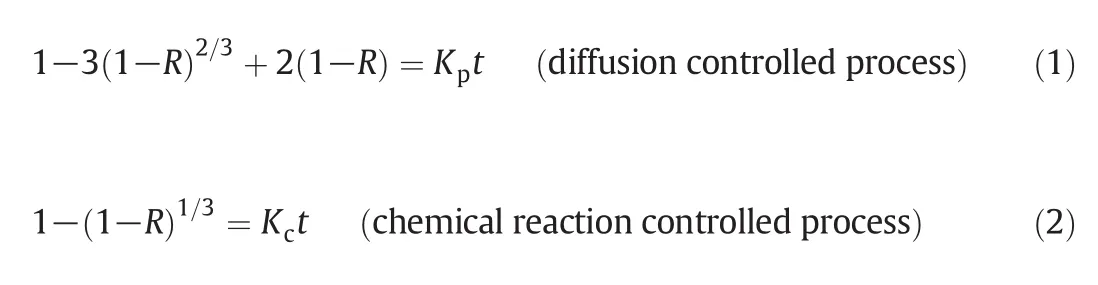
with diffusion rate constantand reaction rate constantwhere R is the fraction of dissolution(%),t is the time(min),D is the diffusion coefficient(cm2·min-1),C0is the initial concentration of HCl solution(mol·L-1),k is the chemical reaction rate constant(cm·min-1),M is the molecular weight of mineral,r0is the initial average radius of particles(cm),ρ is the density of CMW(kg·m-3),and b is the stoichiometric coefficient.
3.Results and Discussion
Leaching of CMW in HCl solution is a complicated process with following chemical reactions:

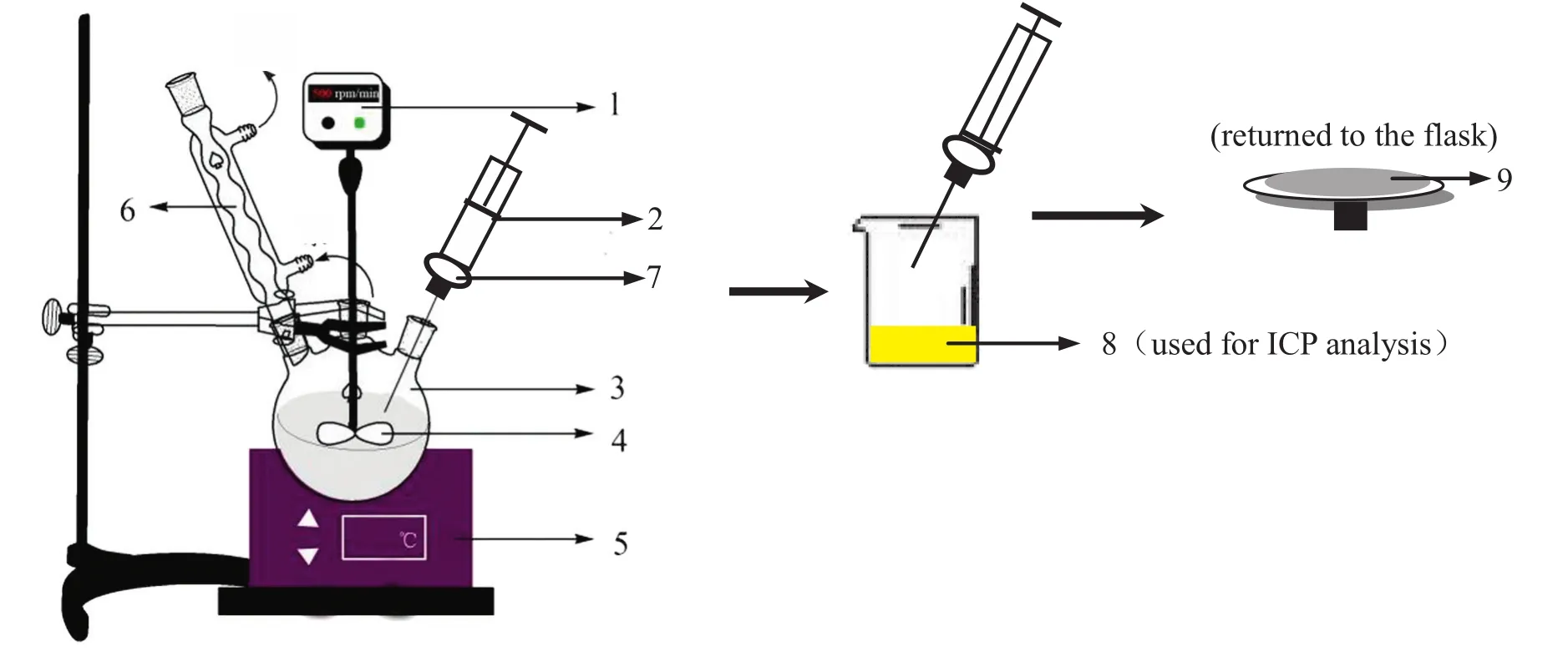
Fig.2.Leaching process and equipment.1—digital stirrer;2—syringe;3— flask,4—stirring stick;5—thermo statically controlled water bath;6—condenser;7— filter with membrane;8— filtrate;9— filter residue.
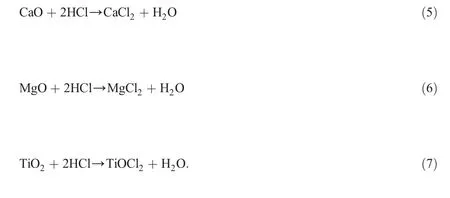
Reactions(3)and(4)are the major reactions in the leaching process.Reactions(5)and(6)occur readily according to previous study[24],in which calcium and magnesium are dissolved at low concentrations of acid.This is also verified in our study but will not be discussed here due to low CaO and MgO contents in the coal waste.Reaction(7)proceeds only to a small extent(less than 1%)based on the results of ion content in the leaching solution as measured by inductively coupled plasma atomic emission spectrography.
3.1.Effect of acid concentration on Al2O3and Fe2O3dissolution
Fig.3 shows the influence of acid concentration on the dissolution of Al2O3andFe2O3at a leaching temperature of 90°C,with all experiments conducted with sufficient acid.The dissolution of Al2O3and Fe2O3increased with time and acid concentration from 3 mol·L-1to 6 mol·L-1.A 63.19%of Al2O3dissolution was achieved at 6 mol·L-1HCl and 120 min.At 7 mol·L-1of HCl,however,Al2O3dissolution increased little after 90 min,leading to a dissolution less than 60%.Compared to Al2O3,Fe2O3dissolution was more sensitive to acid concentration.Dissolution of Fe2O3increased significantly with acid concentration.Fe2O3was nearly completely dissolved after 120 min leaching using 6 mol·L-1HCl solution and after 30 min using 7 mol·L-1HCl solution.The results indicate that the Fe2O3dissolution rate is much faster at higher concentrations of HCl.
Al2O3dissolution rate depends on leaching kinetics,while dissolution tendency relies on thermodynamics.From the perspective of leaching kinetics,the increase of HCl concentration is beneficial for the improvement of Al2O3dissolution rate,but the solubility of aluminum chloride(AlCl3)decreases with the increase of concentration of HCl solution[25,26].When AlCl3is saturated in the reaction system,Al2O3dissolution and precipitation keep a dynamic equilibrium,in which Al2O3dissolution percentage reaches a plateau.This might explain the Al2O3dissolution behavior in 7 mol·L-1HCl solution.Therefore,the optimum concentration of HCl for Al2O3dissolution is 6 mol·L-1.

Fig.3.Effect of acid concentration on dissolution of Al2O3(a)and Fe2O3(b)([HCl]=3-7 mol·L-1,90 °C).
3.2.Effect of leaching temperature on Al2O3and Fe2O3dissolution
Fig.4 shows the effect of leaching temperature on Al2O3and Fe2O3dissolution at acid concentration 6 mol·L-1.The dissolution of Al2O3and Fe2O3increased with time and temperature.The Al2O3dissolution was about 70%after leaching at 106°C for 120 min,which is the azeotropic point of 6 mol·L-1HCl and water.Fe2O3was nearly completely dissolved with 90 min leaching at 100°C.Al2O3dissolution was less sensitive to temperature between 90 °C and 106 °C,which suggests that a diffusion process is likely to be the rate-limiting step because layers may quickly form on the particle surface and prevent acid from reaching the reactive zone at leaching temperature of 90°C and above.

Fig.4.Effect of temperature on the dissolution of Al2O3(a)and Fe2O3(b)(40-106°C,[HCl]=6 mol·L-1).
3.3.Leaching kinetics
3.3.1.Effect of acid concentration
The dissolution experiments provide reliable data for a reaction kinetic model.According to Eqs.(1)and(2),the rate constants calculated at different concentrations are given in Table 2 based on Al2O3dissolution data in Fig.3(a).Results show that the Al2O3dissolution rate is controlled by a diffusion process under acid conditions from 3 mol·L-1to6 mol·L-1at 90 °C.Deviation from linearity is observed for 7 mol·L-1HCl solution with 60 min leaching.

Table 2 Values of Kp,Kcand correlation coefficient for Al2O3dissolution under different acid concentrations(3-7 mol·L-1)at 90 °C for 120 min
The rate constants for the dissolution of Fe2O3are also calculated based on the data in Fig.3(b).A linear relationship between 1-(1-R)1/3and time indicates that the Fe2O3dissolution rate is controlled by a surface chemical reaction at 90°C at the acid concentrations from 3 mol·L-1to 6 mol·L-1in 120 min leaching.The rate constants are listed in Table 3.
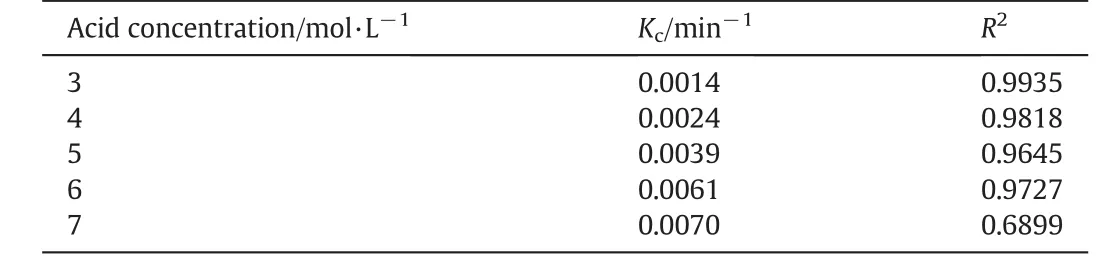
Table 3 Values of Kcand correlation coefficient for Fe2O3dissolution under different acid concentrations(3-7 mol·L-1)at 90 °C for 120 min

Fig.5.Plots of lnKcversus lnc0giving the slope as reaction order.
3.3.2.Effect of temperature
Based on the experimental data in Fig.4(a),a plot of 1-(1-R)1/3versus time for Al2O3dissolution is presented in Fig.6(a)in 6 mol·L-1HCl solution in temperature range from 40 to 80°C.Good linear relationship suggests that the dissolution rate of Al2O3is dominated by a surface reaction in this temperature range.The plot of 1-3(1-R)2/3+2(1-R)as a function of time gives straight lines in the temperature range of 90-106°C in Fig.6(b),showing that the acid diffusion through the product layer to reaction surface is the rate-controlling step.Fig.7 shows the plot of 1-(1-R)1/3as a function of time for the dissolution of Fe2O3.A linear relationship in the leaching temperature 40-100°C indicates that the surface reaction is the rate-controlling step for Fe2O3dissolution under these conditions.
The apparent activation energy can be calculated using the Arrhenius equation

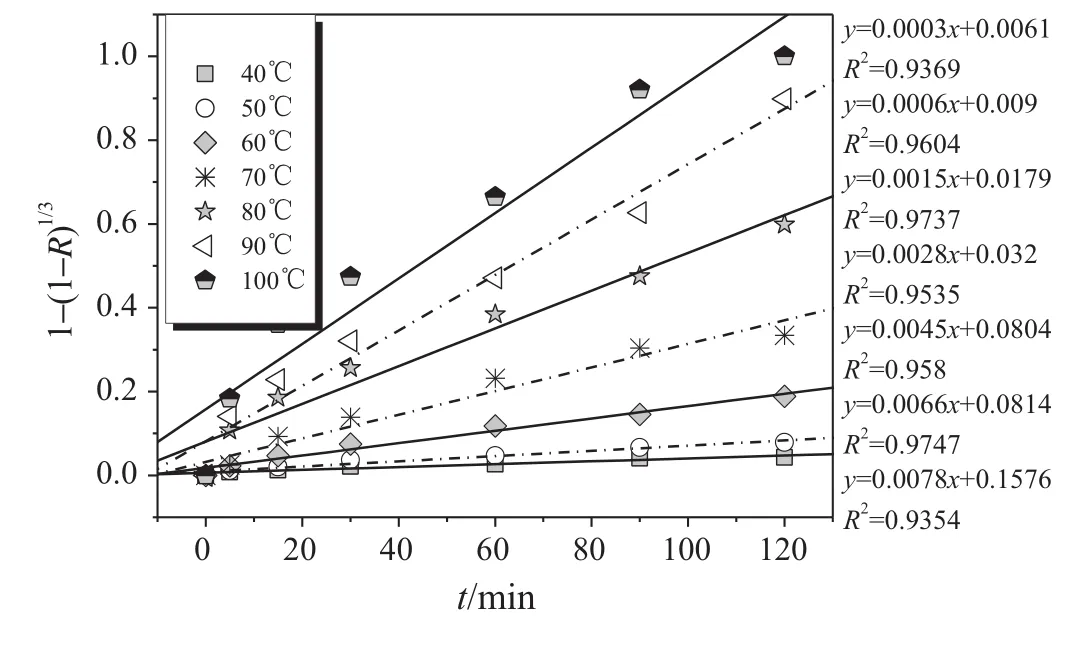
Fig.7.Fe2O3dissolution at[HCl]=6 mol·L-1and temperatures of 40-100 °C.
where K is the rate constant,A is the pre-exponential factor,Ea(kJ·mol-1)is the activation energy,R is the universal gas constant,8.314 × 10-3kJ·mol-1·K-1,and T is the temperature(K).K values can be obtained from the slope of the lines given in Figs.6 and 7 at corresponding temperatures.The plots of lnK versus 1/Tare shown in Figs.8 and 9 for Al2O3and Fe2O3dissolution,respectively.According to Eq.(9)the apparent activation energy can be calculated from the slope of the straight lines.

Fig.8.Arrhenius plot for Al2O3dissolution at leaching temperatures from 40to 106°Cand[HCl]=6 mol·L-1.

Fig.9.Arrhenius plot for Fe2O3dissolution at different temperatures from 40to100°Cand[HCl]=6 mol·L-1.
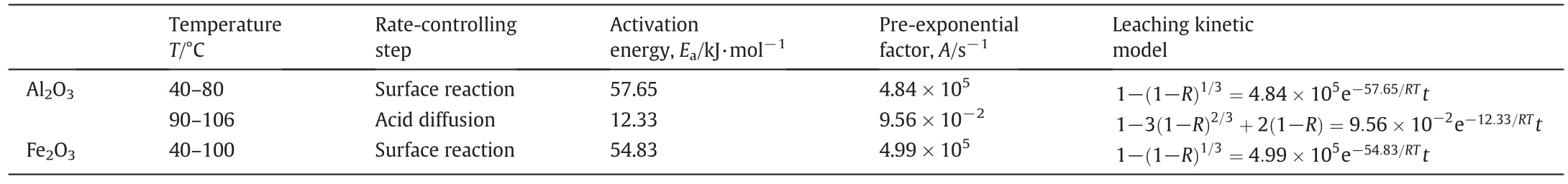
Table 4 Kinetic models and relative parameters for leaching process of Al2O3and Fe2O3
A higher activation energy implies that a surface reaction(~62 kJ·mol-1)dominates the dissolution kinetics,while a lower activation energy suggests that diffusion(~20 kJ·mol-1)is the rate controlling step[27].In the present study,Al2O3dissolution is interface-limited at low temperatures(40-80°C),while the dissolution is diffusion-limited at high temperatures(90-106°C).The results are in accordance with those given by Brantley[27].The activation energy calculated using the Arrhenius equation further confirms the ratecontrolling step.Leaching kinetic model for Al2O3and Fe2O3dissolution from CMW are shown in Table 4.
3.4.Proposed method
3.4.1.Iron removal method
Mineral dissolution kinetics depends on not only the operational conditions but also the mineral properties,such as mineral composition and distribution characteristics[27].XRD analysis of the CMW sample indicates that it mainly contains kaolin and quartz(Fig.1).Al atoms exist primarily in alumina octahedral sheets of kaolin,and small amounts of iron oxides scatter in the CMW.Results of magnetic separation show that most of the iron in CMW is in the form of magnet Fe3O4.XRD analysis of iron slag is shown in Fig.10.Table 5 lists the content of the three main components in CMW samples before and after magnetic separation,along with iron slag.A significant problem in separating iron using the magnetic method is the large loss of alumina entrained in iron slag.

Fig.10.XRD analysis of iron slag after magnetic separation.

Table 5 Content of Al2O3,SiO2,and Fe2O3in the samples before and after magnetic separation and iron slag
Eqs.(3)and(4)indicate that the reactions will be in their thermodynamically equilibrium state when AlCl3and FeCl3are saturated in the reaction system.According to its solubility in HCl solutions at different concentrations[25,28],AlCl3is more soluble in HCl solutions at lower concentrations,while Al2O3dissolution rate is kinetically inhibited.The Al2O3dissolution rate increases with acid concentration,but the dissolution is thermodynamically inhibited at high concentrations of acid due to the low solubility of AlCl3.The Al2O3dissolution is limited by the low solubility of AlCl3at high concentrations of HCl solution.Compared to AlCl3,ferric chloride has a higher solubility at high concentrations of HCl solution[25].Results indicate that the reaction order for the dissolution of Fe2O3with respect to acid concentration is higher than that of the dissolution of Al2O3,suggesting that Fe2O3is more quickly dissolved at higher concentration of HCl solution than Al2O3.In this regard,iron could be first dissolved by using an HCl solution at higher concentration,with a low Al2O3dissolution.In order to validate this statement,several experiments were carried out to investigate iron separation efficiency by leaching the CMW in concentrated HCl solution(12 mol·L-1).Ksp(solubility constant,Ksp(AlCl3)=[Al3+][Cl-]3)values of AlCl3were calculated as 6.40×10-8,1.32× 10-6,4.43× 10-5,and 2.40× 10-3in 12 mol·L-1HCl solution at temperatures 25 °C,45 °C,65 °C,and 85 °C,respectively,based on the thermodynamic data from the previous experiments[26].The results show that 50.2%,56.5%,and 60.8%of iron was dissolved,respectively,after the reaction time of 30,60,and 90 min,with only 1.75%,2.30%,and 3.69%of alumina dissolution at leaching temperature of 45°C.
3.4.2.Aluminum dissolution method
Al exists in CMW mainly in the form of kaolinite.It is critical to break the chemical bonds of kaolinite to improve Al2O3dissolution.Al2O3leaching kinetics demonstrates that acid diffusion through the solid product layer to the reactive surface is the rate-limiting step in 90-106°C,so that a decrease of diffusion resistance can greatly facilitate Al2O3dissolution.Grinding of CMW reduces the diffusion resistance and increases the leaching sites on the surface by decreasing the particle size and diffusion length,so that Al2O3dissolution will be enhanced.In this study,raw materials were grinded for 2,6,12,and 18 h,with corresponding particle sizes of 108.34,94.12,54.17,and 32.66 μm.The grinded CMW was calcined at 700°C for 2 h,and Al2O3dissolution reached 74.54%,80.29%,87.89%,and 94.5%,respectively,after leaching with 6 mol·L-1HCl at 106°C for 2 h,so it is effective to finely grind the raw material before leaching process.Moreover,it creates highly reactive surfaces,reducing the crystalline nature to amorphous phases.The surface defects act as active sites,accelerating the rate of surface reactions and lowering the activation energy required[29],as a result,Al2O3dissolution is promoted.
4.Conclusions
In the present study,the shrinking core model was used to study aluminum and iron dissolution kinetics from CMW using HCl as a leaching medium.The effect of acid concentration and temperature on leaching of Al2O3and Fe2O3from CMW was investigated.Results indicated that the dissolution rate of Fe2O3increased more significantly with acid concentration than that of Al2O3.In addition,Al2O3dissolution was insensitive to temperatures between 90 °C and 106 °C.The apparent activation energies were57.65kJ·mol-1at40-80 °Cand12.33kJ·mol-1at above 90 °C for the leaching of Al2O3;while it was 54.83 kJ·mol-1for the leaching of Fe2O3in temperature range of 40-100°C.The leaching reaction of Al2O3is surface reaction controlled at low temperatures and diffusion controlled at higher temperatures,while the leaching of Fe2O3is only surface reaction controlled under the experimental conditions.
A process of recovering aluminum was proposed based on the characteristics of dissolution kinetics that iron can be separated first at a higher concentration of HCl solution,and then aluminum dissolution can be promoted through the fine grinding method.More than 60%of iron was removed by using 12 mol·L-1HCl after leaching for 90 min at 45°C,with only 3.69%Al2O3loss.Al2O3dissolution achieved 94.5%after fine grinding with particle size of 32.66 μm.The process provides an effective way to recover high quality aluminum from CMW.Furthermore,it is necessary to develop an effective fine grinding technology for the economics of many leaching operations.
 Chinese Journal of Chemical Engineering2015年3期
Chinese Journal of Chemical Engineering2015年3期
- Chinese Journal of Chemical Engineering的其它文章
- Micromixing characteristics in a gas-liquid-solid stirred tank with settling particles☆
- An experimental study of drag reduction by nanofluids in slug two-phase flow of air and water through horizontal pipes☆
- Effect of surfactant type on interfacial area and liquid mass transfer for CO2absorption in a bubble column☆
- Effects of bubbly flow on bending moment acting on the shaft of a gas sparged vessel stirred by a Rushton turbine☆
- Enhanced heat transfer in a heat exchanger square-duct with discrete V- finned tape inserts☆
- A novel purification process for dodecanedioic acid by molecular distillation
